Figure 3.
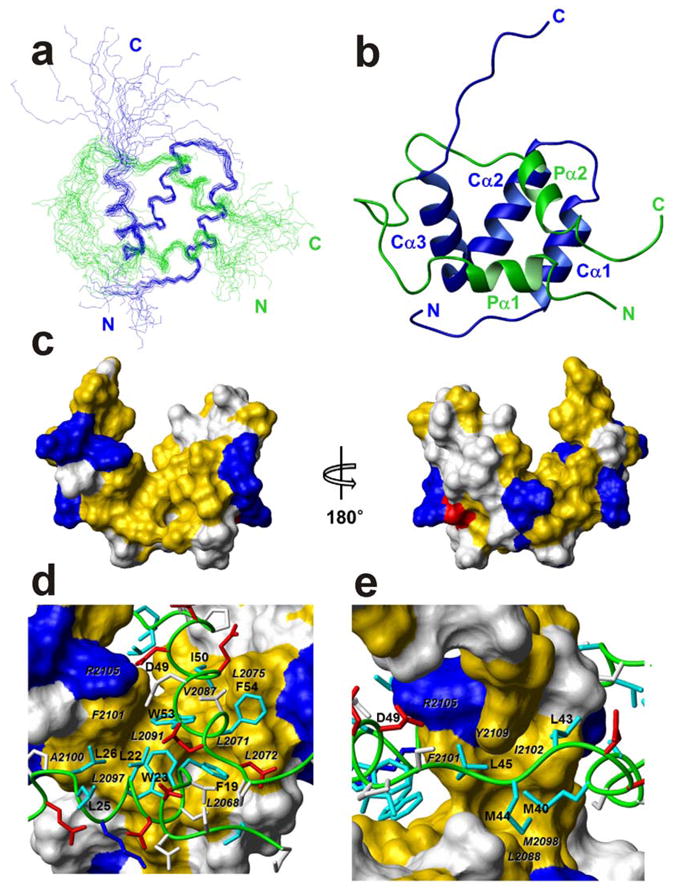
Solution structure of the p53 TAD:NCBD complex. (a) The best 20 structures superposed on backbone heavy atoms in ordered regions. The p53 TAD backbone is shown in green, NCBD in blue, and the N and C termini of each chain are labeled in the corresponding colors. (b) Ribbon representation, in the same orientation and color as (a) Helices Pα1–2 and Cα1–3, and N and C termini are labeled. (c) Surface representation of NCBD domain in complex with p53 TAD. The left and right figures are rotated 180° relative to one another about a vertical axis. Hydrophobic residues (Ala, Met, Leu, Ile, Val, Phe, and Tyr) are colored yellow; positively charged (Arg and Lys) and negatively charged (Asp) residues are colored blue and red, respectively. The other residues are colored white. (d) and (e) Binding site for Pα1 and Pα2 and extended region of p53 TAD (green tube) in the hydrophobic grooves on the surface of NCBD. The surfaces of interacting hydrophobic side chains from NCBD are labeled in black italic characters. The side chains of basic and acidic residues of p53 TAD are shown in blue and red, respectively. The side chains of hydrophobic residues of p53 TAD are shown in cyan and labeled. The side chains of other residues are colored white.
