Abstract
The “F” in FN400 denotes a more frontal scalp distribution relative to the morphologically similar N400 component—a distinction consistent with the hypothesized distinct roles of FN400 in familiarity memory versus N400 in language. However, no direct comparisons have substantiated these assumed dissimilarities. To this end, we manipulated short-term semantic priming during a recognition test. Semantic priming effects on N400 were indistinguishable from memory effects at the same latency, and semantic priming strongly modulated the “FN400,” despite having no influence on familiarity memory. Thus, no evidence suggested either electrophysiological or functional differences between the N400 and FN400, and findings were contrary to the linking of the “FN400” to familiarity. Instead, it appears that semantic/conceptual priming (reflected in the N400) occurs during recognition tests, and is frequently (mis)labeled as FN400 and attributed to familiarity.
Introduction
Familiarity and recollection are two hypothesized processes that contribute to performance in tests of recognition memory (Mandler, 1980; Yonelinas, 2002). Many investigators interpret two distinct ERP effects obtained during recognition tests as unique neural correlates of familiarity and recollection: FN400 old/new effects and late-parietal old/new effects, respectively (Mecklinger, 2000; Paller, Voss, & Boehm, 2007; Rugg & Curran, 2007). The validity of these hypothesized associations is of substantial theoretical importance. For instance, the dissociation between the FN400 and late-parietal ERPs has been widely taken as reification of the constructs of familiarity and recollection, and thus as justification for launching more detailed neuroanatomical studies of these hypothesized processes (e.g., Eichenbaum, Yonelinas, & Ranganath, 2007). Moreover, investigators increasingly cite the strength of associations between the FN400 and familiarity and late-parietal potentials and recollection as justification to infer the involvement of familiarity and/or recollection whenever these potentials are identified; that is, make reverse-inferences of familiarity and recollection based on FN400 and late-parietal potentials (e.g., Czernochowski, Mecklinger, & Johansson, 2009; Ecker, Arend, Bergstrom, & Zimmer, 2009; Klonek, Tamm, Hofmann, & Jacobs, 2009; Mecklinger, Brunnemann, & Kipp, 2010; Nyhus & Curran, 2009; Opitz & Cornell, 2006; Speer & Curran, 2007).
However, as we review below, recent work has called into question the mapping between the FN400 and familiarity and has suggested that the FN400 may not be different from the N400 component. The N400 has been characterized primarily within the domain of language comprehension, but also more broadly, and has been linked to implicit semantic access processes. The FN400 bears striking similarity to the N400 in morphology, timing, and response pattern – indeed, effects that are now referred to as FN400s were originally characterized as N400s in the early literature looking at cognitive electrophysiological responses in memory paradigms. Yet, to our knowledge, no empirical work has attempted to elicit both responses in tandem to directly compare them. We therefore set out to answer fundamental and yet untested questions regarding FN400 potentials: are they in fact N400 potentials present during recognition tests, and are they related to semantic processing rather than to familiarity?
The N400 component was first characterized by Kutas and Hillyard (1980), who found larger negative ERP amplitudes to unexpected relative to expected words completing sentences. Three decades of empirical work (reviewed by Kutas & Federmeier, in press) have shown that the N400 is part of the normal electrophysiological response to not only words in all modalities, but also to pictures, faces, sounds, mathematical symbols, etc. – essentially all meaningful stimuli. Moreover, the amplitude of the N400 is reduced by many variables that have in common that they ease semantic processing. These variables include associative and semantic priming and the fit between the N400-eliciting word and sentence/discourse context information. One notable feature of the N400 is that its latency is remarkably invariant, with a peak at approximately 375 ms in young adults. The distribution of the N400 is broad and, although typically characterized as having a centro-posterior maximum based on the original studies of sentential congruity, is actually variable across input types (as discussed below). It is also notable that the N400 has been found across many paradigms to manifest with high “automaticity”, meaning without the subject's intent to process stimulus meaning. Likewise, the N400 is elicited even when subjects show no awareness for the eliciting stimulus (e.g., Vogel, Luck, & Shapiro, 1998) and N400 modulations occur with repetition even in patients with amnesia who show impaired explicit memory for repetition (e.g., Olichney, et al., 2000). Thus, the N400 has been characterized as a neural manifestation of relatively automatic and implicit semantic processing of a variety of meaningful stimulus categories (Kutas & Federmeier, in press).
Several developments were instrumental in the initial adoption of the N400 as a dependent measure by the memory research community. Repetition of the N400-eliciting stimulus was shown to strongly modulate the amplitude of the N400, with reduced amplitudes (i.e., more positive ERPs) to repeated stimuli (Neville, Kutas, Chesney, & Schmidt, 1986; Paller & Kutas, 1992; Rugg, 1990; Young & Rugg, 1992). In the context of a body of literature delineating the functional sensitivity of the N400 (e.g., Kutas & Van Petten, 1990), these repetition effects could be logically associated with the greater ease/fluency of semantic processing that occurs with repetition, known as conceptual priming when measured in an indirect priming test. At the time these discoveries were being made, the memory research community was substantially influenced by the theories of memory proposed by Craik, Lockhart, and Tulving (Craik & Lockhart, 1972; Craik & Tulving, 1975), which emphasized the central importance of semantic/conceptual processing for recognition. Thus, the N400 seemed promising as a marker of processing relevant to recognition memory.
Besson and colleagues (1992) and Friedman (1990) were among the first to describe N400 effects during recognition tasks. The distribution of the N400 repetition effects observed in the context of recognition memory has been characterized as being more frontal than that for N400 effects in language processing tasks. However, it is important to note that this distributional difference may have been more apparent than real. The distribution of the N400 is broad, and a number of factors have now been found to modulate it, including item type differences: for example, N400 responses are more centro-posterior for abstract words but more frontal for concrete words and pictures (e.g., Ganis, Kutas, & Sereno, 1996; Kounios & Holcomb, 1994). Thus, the distribution of the FN400 typically described in memory tasks is in fact compatible with the distribution of effects observed on the N400 in language processing studies, especially since recognition memory paradigms often use relatively concrete single words or pictures. Furthermore, other ERP effects that overlap in time with N400, such as the late posterior complex (LPC), are elicited during memory tasks, but more rarely occur during language tasks. These contemporaneous effects might also skew the observed distribution of the N400 in studies of memory relative to studies of language.1 Nevertheless, the suggestion of a distributional difference, combined with differences in the types of processes researchers in the memory and language domains set out to characterize with their tasks, led to a split between the literatures, with the FN400 and N400 coming to be treated as functionally distinct entities in studies of memory vs. studies of language.
In particular, Smith (1993) provided early evidence for an association between the FN400 and familiarity-based recognition memory, as distinct from recollection-based recognition. Familiarity and recollection are hypothesized episodic memory processes that differ in the quality of the phenomenological experience associated with recognition and/or with the type of information that is retrieved (Mandler, 1980; Yonelinas, 2002). Recollection entails the retrieval of contextual detail from the prior occurrence, including retrieval of spatiotemporal context or other episodic details, and is associated with the awareness of retrieval or “mental time travel” to the prior event. In contrast, familiarity entails unsubstantiated feelings of familiarity coupled with a lack of retrieval of specific detail from the prior occurrence, as can occur when only item-specific information is retrieved during recognition. Smith showed that the magnitude of the FN400 repetition effect was similar for memory judgments that entailed recollection or only a feeling of familiarity (that is, FN400 effects were observed during memory tasks but were not associated with recollection). Later, in a pivotal study, Curran (2000) investigated FN400 effects using a plurality reversal manipulation that reduced recollection but had little effect on familiarity. This manipulation had no effect on FN400 repetition effects or on familiarity, but did influence recollection as well as late-parietal ERPs (LPC). This was also taken as support for the association between the FN400 and familiarity, and between the LPC and recollection. Many studies followed using similar techniques to associate FN400 potentials with familiarity and later-onset LPC-family potentials with recollection (Rugg & Curran, 2007).
However, several findings present striking challenges to the case for linking the FN400 specifically with familiarity and, correspondingly, for dissociating the FN400 from the N400. When experimental circumstances differentially influence familiarity and semantic processing, FN400 potentials often show properties more characteristic of N400 effects and semantic processing rather than of familiarity. For instance, strong familiarity-based recognition of stimuli – but an absence of corresponding FN400 effects – has been found for a range of stimuli without pre-existing conceptual representations, and therefore little ability to support conceptual/semantic processing. These stimuli include novel faces [(MacKenzie & Donaldson, 2007; Yovel & Paller, 2004), but see (Donaldson & Curran, 2007)], geometrical patterns (Voss & Paller, 2009b), and novel symbols (De Chastelaine, Friedman, Cycowicz, & Horton, 2009)2. Furthermore, at very short delays, when familiarity should be maximal, repetition of stimuli with semantic value produces FN400 effects whereas repetition of stimuli without semantic value does not (Danker, et al., 2008). These results seem to point to a stronger link between FN400 elicitation and meaning-based processing (as would be expected for an N400 modulation) than between the FN400 and familiarity.
Indeed, Paller and colleagues (2007) argued that there is a fundamental and pervasive error in the reasoning that equates FN400 with familiarity. They found that manipulations purportedly linking FN400 to familiarity in over 25 published studies in fact showed instead that the FN400 is not related to recollection – not that it is related to familiarity. For example, the Curran (2000) results showed that FN400 was produced by repetition and was unaffected by a manipulation (plurality reversal) that reduced recollection. This indicates that the FN400 indexes some repetition-related process that is not recollection – but does not show that this process is, in fact, familiarity. Paller et al. (2007) suggested that most evidence linking the FN400 to familiarity (including that cited by Rugg & Curran, 2007) has suffered from the same logical flaw and is, therefore, equally amenable to the interpretation that FN400 effects instead reflect conceptual/semantic priming – and, thus, in fact, the same kind of processing as has been associated with the N400.
Some recent research has endeavored to determine if the FN400 is better characterized as an N400, elicited by repeating meaningful stimuli during recognition tests, rather than as a specific index of familiarity. These studies measured both conceptual priming and familiarity in highly similar circumstances in order to identify ERPs that vary with one versus the other. For instance, Voss and Paller (2006) found that priming the conceptual information associated with celebrity faces led to FN400 effects that covaried with the magnitude of conceptual priming. In contrast, familiarity for the same faces was unrelated to FN400 modulations and was instead associated with late-parietal LPC potentials. A follow-up study using fMRI (Voss, Reber, Mesulam, Parrish, & Paller, 2008) provided further support for the notion that the FN400 reflected conceptual priming, given that the same conceptual priming manipulation found to modulate the FN400 was associated with left inferior prefrontal fMRI response reductions characteristic of the control processes associated with conceptual priming (Schacter, Wig, & Stevens, 2007).
Other, related studies have dissociated ERP correlates of conceptual priming and familiarity by examining variations in both during priming and recognition tests. For instance, abstract geometric shapes vary in the extent to which individuals find them to be meaningful (as in the Rorschach test), and thus in the extent to which they can support conceptual priming with repetition. Repeating them also produces different amounts of familiarity, similar to repeating the kind of word stimuli that are more typically used in recognition memory tasks. Voss & Paller (2007) found that repeating relatively meaningful abstract shapes produced conceptual priming, whereas repeating relatively meaningless shapes did not. During a recognition test, meaningful and meaningless shapes were both recognized with equal levels of familiarity, which was associated with LPC potentials for both meaningfulness levels. However, only meaningful shapes produced FN400 effects, indicating that it is the potential for conceptual priming, not for familiarity, that can be associated with the FN400. A follow-up study substantiated this finding by indicating that the magnitude of conceptual priming for meaningful shapes is strongly correlated with the size of FN400 effects (Voss, Schendan, & Paller, 2010). Furthermore, obscure words were also found to vary in their ability to produce conceptual priming during a recognition test, and these variations in conceptual priming were linked to the FN400 (Voss, Lucas, & Paller, 2009). These studies collectively argue against the functional distinctions that have motivated the differentiation of the FN400 from the N400. Instead, they indicate that the semantic processing reflected in the N400 is also active during recognition memory tasks and can be modulated by repetition, meaning that N400 variations may be incorrectly attributed to familiarity memory if not exhaustively characterized (Voss & Paller, 2008a).
Although this recent work has built a case for reintegrating the FN400 with the more general N400 literature, a stronger test of the notion that the FN400 is actually an N400 would be to conduct a direct, within-subjects comparison of the two effects, by jointly manipulating factors associated with each. To that end, in the current study we manipulated short-term semantic priming within a recognition test. Preceding a target word with a semantically related prime (e.g., doctor-nurse) is an established as a means of facilitating (reducing) N400 amplitude (Kutas & Federmeier, in press), presumably because prior access to shared/linked information eases semantic access for the target concept. We therefore repeated “target” words during a continuous recognition paradigm and preceded each target by either a semantically related prime or an unrelated word. Subjects explicitly made recognition memory decisions, and reported on phenomenological feelings of familiarity, recollection, and confidence. Thus, ERP correlates of familiarity-based recognition in the absence of semantic priming could be identified by focusing on repetition of targets with unrelated preceding words (as in a standard recognition memory paradigm). Likewise, ERP correlates of semantic priming could be identified by focusing on targets with related primes, independent from repetition (as in a standard semantic priming paradigm).
The hypothesis that FN400 potentials are distinct from N400s and are unique correlates of familiarity would predict an association between familiarity-based recognition and FN400 effects, separable from N400 effects associated with semantic priming. That is, an anterior FN400 effect should correlate with familiarity and a more posterior N400 effect should correlate with semantic priming. In contrast, on the hypothesis that FN400 effects and N400 effects share a common function and neural source, then we would expect to find nearly identical effects for familiarity-based recognition and for semantic priming. Furthermore, this hypothesis would predict the FN400/N400 effect to old items to be sensitive to semantic priming – but not strongly yoked to familiarity-based recognition.
We also predicted that recognition would be associated with late-parietal old/new effects, on what is known as the late-positive complex or “LPC.” LPC effects have been associated with self-reported recollection, accurate source memory, and the amount of information retrieved during recollection, and therefore are widely accepted as ERP correlates of processes that support recognition memory (Friedman & Johnson, 2000; Mecklinger, 2000; Paller, Voss, & Boehm, 2007; Rugg & Curran, 2007; Voss & Paller, 2008a, 2008b). Some evidence indicates that LPC effects can be fractionated, with left-lateralized LPC effects signaling recollection and more widespread LPC effects signaling confidence (e.g., Vilberg & Rugg, 2009). We therefore predicted that LPC effects would be associated with recognition, and that this relationship would not be influenced markedly by semantic priming. We also included direct tests of laterality to examine whether the LPC effects we observe are lateralized.
Methods
Subjects
Behavioral and electrophysiological data are reported for 16 right-handed, native speakers of English (ages 18-27, 9 female) recruited from the University of Illinois Urbana-Champaign community. All subjects had normal or corrected-to-normal vision and were native speakers of English. The experiment was terminated early for an additional three subjects because of failure to control eye blinking, and these data were discarded. All procedures were approved by the IRB of the University of Illinois Urbana-Champaign. Subjects were paid $30 for their participation.
Stimuli
Verbal stimuli included 600 common English words (3-8 letters in length) that were divided into 200 triplets. Each triplet comprised a semantically related word pair [e.g., doctor-nurse; forward and backward free association strengths range=0.08-0.25 according to University of South Florida Free Association Norms, (Nelson, McEvoy, & Schreiber, 1998)], and the third word was unrelated to the pair (e.g., kettle, not listed as an associate in the University of South Florida Free Association Norms). Unrelated words were matched, on average, for length and written frequency (Kucera & Francis, 1967) to related words. Words in 70% of the triplets described common objects, and normalized concreteness ratings for all words was greater than 400 (on a scale of 100-700). An additional 100 common English words served as filler stimuli, and were chosen to be as unrelated as possible to all words in the 200 triplets. Words were presented at the center of a computer monitor and subtended average approximate visual angles of 0.5°×5.0°. A central fixation cross was present during interstimulus intervals (ISI).
Behavioral Procedures
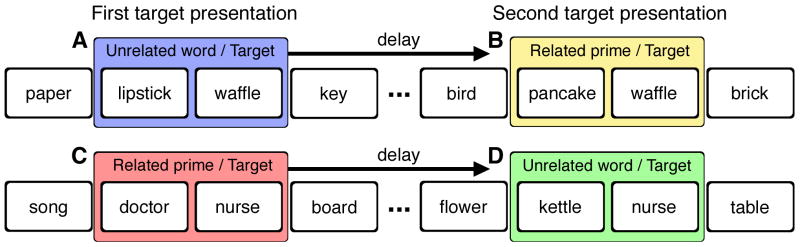
Subjects performed a continuous recognition test for words. Targets were one member of each of the 200 semantically related word pairs. Each target was shown twice, with the second presentation at a delay of 15-25 intervening targets from the first presentation (mean=20, delay range = 83-138 seconds). Each target trial was preceded by either its semantically related prime (e.g., doctor-nurse) or by the corresponding semantically unrelated word (e.g., kettle-nurse). Half of the targets were preceded by the related prime on first presentation and then by the unrelated word on second presentation, and vice versa for the other half of the targets (Figure 1). The other 100 filler words were interspersed in order to maintain the average delay between consecutive presentations of a target. Order of presentation of the pairs (i.e. Figure 1AB vs. Figure 1CD) was counterbalanced across subjects, as was the word from each semantically related pair that served as the target item.
Figure 1. Schematic diagram of the experiment design.
Half of the targets in a continuous recognition paradigm were presented for the first time following semantically unrelated words (A) and were presented again after a delay following semantically related primes (B). The other half of the targets were presented first following semantically related primes (C) and then again after a delay following unrelated words (D). The coloration of these conditions is used for reference in subsequent figures.
During each trial, one word was presented for 500 ms. Subjects were instructed to immediately make a judgment about the word's emotional valence, in order to encourage semantic processing. The subject pressed one button to indicate if the word was of positive valence and another button to indicate if the word was of neutral or negative valence. No words had actual strong affective valence. At a delay of 2,500 ms from word onset, a recognition prompt appeared on the screen (the letter “R”). This prompt signaled to the participant to make a memory judgment, whereby the participant attempted to indicate if the word was old or new. The memory judgment was made using a modified remember/know procedure (Gardiner & Java, 1991; Tulving, 1985b) that captured recollection, familiarity, and confidence. Subjects pressed one button to indicate “remember old”, another button to indicate “know old”, another button to indicate “guess old”, and another button to indicate “new.” Remember, know, and guess responses were only correct for the second presentation of a target. The correct response to all other stimuli was “new”. The next trial immediately followed, with a randomized 2,500-3,500 ms ISI between the recognition prompt and the onset of the next word. The semantic judgment response and the recognition memory judgment response were made with different hands, alternated across subjects. To become familiarized with the experiment, subjects completed a practice session with 50 additional targets (25 preceded by related primes and 25 preceded by unrelated words), which were not used again during the main experiment.
ERP Procedures
EEG was sampled continuously from 64 scalp locations conforming to the extended International 10-20 positioning system (Chatrian, Lettich, & Nelson, 1988) using a BioSemi Active II system (BioSemi Instrumentation, Amsterdam). Four additional channels were used to monitor horizontal and vertical eye movements and two additional channels recorded from left and right mastoids. The digitization rate was 1024 Hz. The recording bandpass was of 0.01 to 120 Hz, and no additional filtering was performed. Recordings were rereferenced offline to averaged mastoids. ERPs time-locked to the onset of each word were calculated for each condition of interest in 900 ms epochs, beginning 100 ms prior to stimulus onset. Baseline correction was performed using the mean amplitude of the prestimulus interval. Epochs contaminated by artifacts were discarded. ERPs were averaged over latency intervals and electrode clusters for statistical assessment, determined based on a priori hypotheses regarding FN400 and late-posterior potentials. Latency intervals included 300-500 ms and 600-800 ms. Four electrode clusters included mid-frontal (F1, Fz, F2, FC1, FCz, FC2, C1, Cz, C2), mid-posterior (CP1, CPz, CP2, P1, Pz, P2, POz), left-posterior (TP7, CP5, CP3, P7, P5, P3, PO7, PO3), and right-posterior (TP6, CP6, CP4, P8, P6, P4, PO8, PO4). ERP values averaged over clusters were used for statistical assessment. Repeated-measures ANOVAs incorporated Geisser-Greenhouse correction when appropriate. Only trials with correct recognition memory responses were included in ERP averages (i.e., remember, know, and guess responses for old targets, and new responses for new targets and all primes). The mean number of trials per condition, after excluding trials contaminated by artifact and trials with incorrect behavioral responses, was 64 (range 61-69).
Results
Behavioral findings
Hit rates for the recognition responses and response times (RTs) for the valence judgments are summarized in Table 1. It is clear that RTs for the valence judgments did not vary reliably for any two conditions, and no pairwise comparisons were significant. Therefore, the inclusion of valence judgments to each word for the intent of encouraging semantic analysis of words did not introduce RT differences between conditions that could potentially confound ERP comparisons.
Table 1.
| Stimulus type | Valence judgment RT (ms) | Recognition response type (hit rate) | |||
|---|---|---|---|---|---|
| Remember old | Know old | Guess old | New | ||
| Targets | |||||
| 1st Presentation (new) | |||||
| Related prime | 692 (55) | .03 (.01) | .03 (.01) | .07 (.02) | .87 (.03) |
| Unrelated prime | 697 (76) | .03 (.01) | .03 (.01) | .06 (.02) | .88 (.03) |
| 2nd Presentation (old) | |||||
| Related prime | 691 (56) | .57 (.07) | .21 (.04) | .09 (.02) | .13 (.02) |
| Unrelated prime | 702 (58) | .57 (.07) | .23 (.04) | .08 (.03) | .12 (.03) |
| Primes | |||||
| 1st Target presentation | |||||
| Related target | 703 (57) | .02 (.01) | .02 (.01) | .06 (.02) | .90 (.03) |
| Unrelated target | 675 (55) | .02 (.01) | .02 (.01) | .05 (.02) | .91 (.03) |
| 2nd Target presentation | |||||
| Related target | 682 (57) | .05 (.01) | .05 (.01) | .09 (.02) | .81 (.04) |
| Unrelated target | 660 (58) | .02 (.01) | .03 (.01) | .08 (.03) | .87 (.03) |
Provided for each stimulus type is the mean valence judgment response time (RT) and the mean hit rate for each recognition response type. Targets are classified according to old/new status and according to whether the target was preceded be a semantically related or unrelated prime (Figure 1). Primes are classified according to the old/new status of the immediately following target and by the semantic relatedness to the target. No primes were repeated (all were “new”). Parentheses indicate SEM.
It is also clear from Table 1 that subjects succeeded at correctly rejecting related primes and unrelated words that preceded targets. False alarm rates for both conditions were uniformly low for remember, know, and guess responses, and correct rejection rates were uniformly high. There was a tendency for slightly more false alarm responses to primes that preceded the second presentations of targets (Figure 1B) compared to unrelated words that preceded the second presentation of targets (Figure 1D) [Condition by response type interaction F(3,45)=19.5, p<0.001]. This tendency was most pronounced for remember responses [t(15)=4.2, p<0.001], but was also evident for know responses [t(15)=2.3, p=0.04] and guess responses [t(15)=2.2; p=0.05]. Because these primes were semantically related to the target, and the target had been viewed previously (Figure 1AB), this indicates that subjects occasionally mistook primes to second target presentations as the targets themselves and therefore endorsed them as old. Given the very small number of trials comprising this bias, it is reasonable to conclude that subjects rarely made this error.3
Subjects successfully discriminated repeat targets (old) from first-seen targets (new), both for targets preceded by related primes and for targets preceded by unrelated words [repetition (old/new) by recognition response type (remember/know/guess/new) interactions F(3,45)=109.7, p<0.001 and F(3,45)=109.9, p<0.001, respectively]. For targets preceded by related primes, hits to old items significantly outnumbered false alarms to new items for remember responses [t(15)=8.3, p<0.001] and know responses [t(15)=4.4, p<0.001]. Likewise, correct rejections for new items significantly outnumbered misses for old items [t(15)=17.8, p<0.001]. Guess responses did not significantly discriminate between old and new items [t(15)=1.0, p=0.34]. A similar pattern was identified for targets preceded by unrelated words [remember hits vs. false alarms t(15)=8.4, p<0.001; know hits vs. false alarms t(15)=4.5, p<0.001; correct rejections vs. misses t(15)=16.8, p<0.001; guess hits vs. false alarms t(15)=1.9, p=0.07]. Collapsing across remember, know, and guess response types, the mean discrimination sensitivity (d′) score was 2.5 for targets following related primes [t(15)=12.3, p<0.001 vs. chance] and was 2.7 for targets following unrelated words [t(15)=11.5, p<0.001 vs. chance].
An important finding shown in Table 1 is that the semantic priming manipulation had no discernable influence on measures of familiarity-based recognition [priming status by repetition by recognition response type interaction F(3,45)=1.5, p=0.23]. That is, for targets following related primes versus targets following unrelated words, response rates were virtually identical for remember responses [t(15)=0.2, p=0.86], know responses [t(15)=.7, p=0.49], guess responses [t(15)=0.8, p=0.43], and new responses [t(15)=0.5, p=0.62]. Some findings indicate that the remember/know procedure measures gradations in memory strength rather than separate recollection and familiarity processes (Rotello & Zeng, 2008; Wixted, 2007, 2009).4 Nonetheless, treating remember, know, guess, and new responses as a confidence scale (high to low) yields the same conclusions regarding the lack of influence of semantic priming on familiarity. The virtually matched prevalence of each response type would indicate highly matched distributions of memory strength for targets following related primes versus targets following unrelated words, and we are aware of no models of familiarity memory that would predict different levels of familiarity despite highly matched memory strength distributions (Wixted, 2007; Yonelinas, 2002).
Semantic priming ERP effects for new targets
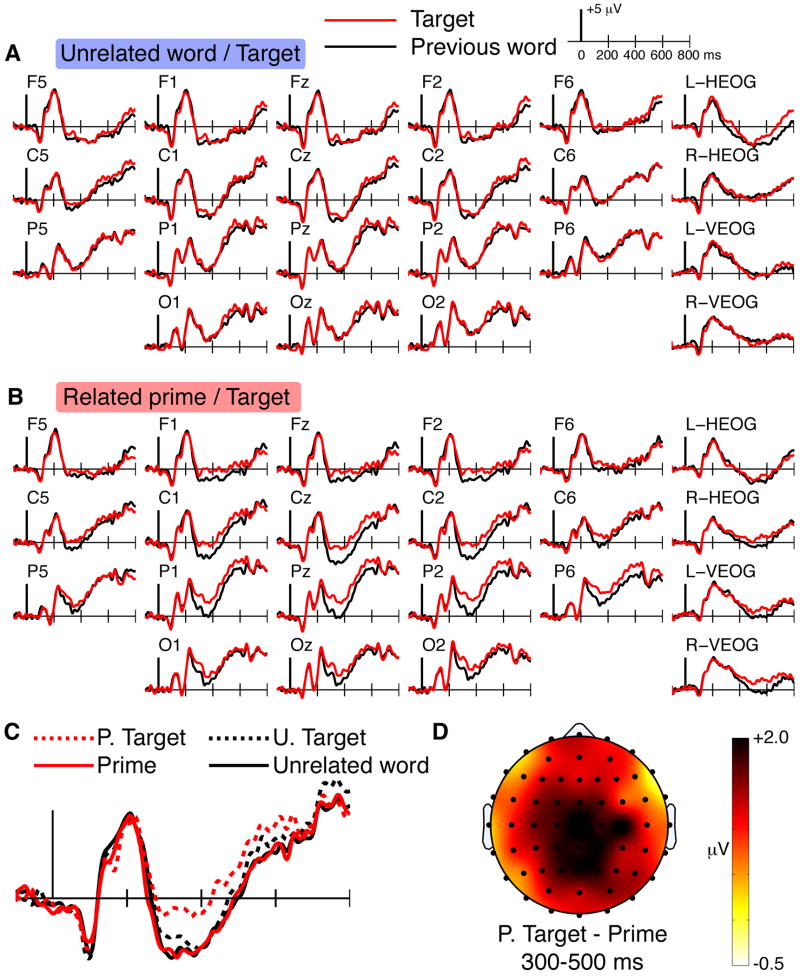
The first set of ERP analyses concerned differences between targets and the immediately preceding words, as a function of whether the preceding words were related primes versus unrelated words. To identify effects of semantic priming without repetition (Figure 1C), ERPs are shown for two conditions that vary in the degree to which they produce semantic priming but are matched in repetition: (1) the first presentation of targets (new) and preceding unrelated words (Figure 2A), and (2) the first presentation of a targets and preceding related primes (Figure 2B). Figure 2A shows that there were virtually no ERP differences between targets and their unrelated preceding words for any electrodes or for any latencies. In contrast, Figure 2B shows robust semantic priming effects on the N400 for targets preceded by related primes. Target ERPs were more positive than prime ERPs between 300 and 500 ms post-stimulus onset, especially over central electrode sites.
Figure 2. ERPs for the first presentation of targets.
ERPs are shown for targets presented for the first time as well as for the words immediately preceding these targets. A ERPs for targets preceded by unrelated words and for the unrelated words. B ERPs for targets preceded by primes and for the primes. C Overlay of all four conditions at electrode Cz. Primed targets (P. Targets) are those that followed primes. Unprimed targets (U. Targets) are those that followed unrelated words. ERPs were matched except for primed targets, which showed positive N400 shifts. D The topography of ERP difference between primed targets and their primes is shown for the N400 interval, with difference amplitude values indicated by coloration.
Statistical assessment of the 300-500 ms latency interval confirmed that target ERPs differed reliably from related prime ERPs for the mid-frontal, mid-posterior, left-posterior, and right-posterior electrode clusters [Condition main effect F(1,15)=12.8, p=0.003]. A marginal Condition-by-Cluster interaction [F(1.8,27.6)=3.5, p=0.05] substantiated the impression that the prime/target ERP differences were slightly more pronounced at mid-frontal and mid-posterior electrode clusters (mean=1.8 and 1.6 μV, respectively) versus left-posterior and right posterior electrode clusters (mean=0.9 and 1.2 μV, respectively). Indeed, the 300-500 ms related prime/target difference was significantly more positive for the mid-frontal and mid-posterior electrode clusters relative to the left-posterior and right-posterior clusters (pairwise p values < 0.05), but did not differ for the mid-frontal and mid-posterior clusters (p=0.22). Figure 1C shows that ERPs for targets following unrelated words, for the unrelated words preceding these targets, and for primes are all well-aligned, indicating that the relative positivity associated with semantic priming was selective for targets that were preceded by related primes. ERP effects occurring after 500 ms are described below in the last section of the Results.
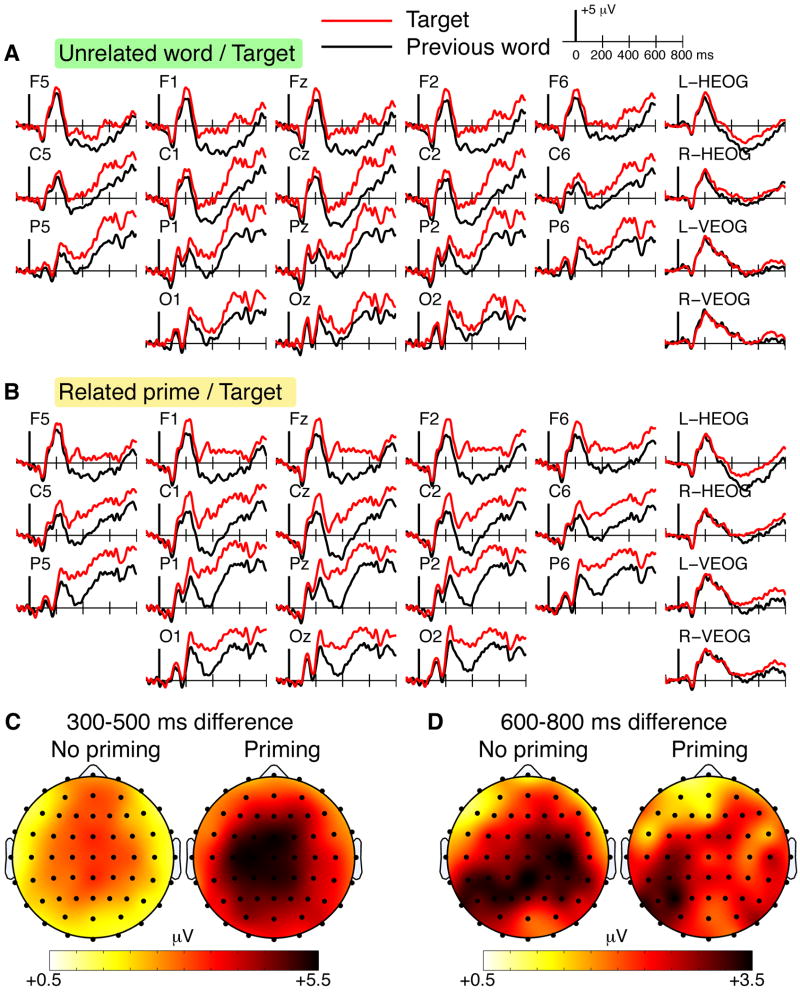
Semantic priming and recognition ERP effects for repeated targets
Figure 3 shows ERPs for the second presentations of targets (old) and also for the words immediately preceding the target, as a function of whether the preceding words were related primes versus unrelated words (as in Figure 1BD). Comparing target ERPs to preceding unrelated word ERPs identified reductions in negativity between 300-500 ms (FN400) and later posterior effects (Figure 3ACD), both of which are commonly associated with recognition memory. For the 300-500 ms latency interval, target ERPs were more positive than ERPs to unrelated preceding words, with significant variation across electrode clusters [Condition main effect F(1,15)=15.2, p=0.001; Condition-by-Cluster interaction F(1.7,24.9)=9.4; p=0.002]. This 300-500 ms positive difference was significantly greater for the mid-frontal and mid-posterior electrode clusters relative to the left-posterior and right-posterior clusters (pairwise p values < 0.03), but did not differ for the mid-frontal and mid-posterior clusters (p=0.46).
Figure 3. ERPs for the second presentation of targets.
ERPs are shown for targets presented for the second time as well as for the words immediately preceding these targets. A ERPs for targets preceded by unrelated words and for the unrelated words. B ERPs for targets preceded by primes and for the primes. C Topographic maps are shown for the N400 interval for the ERP difference between targets and their preceding words, as a function of whether the preceding words were unrelated (no priming) or whether they were related primes (priming). D Topographic maps are provided using the same format for the later LPC interval.
For the 600-800 ms latency interval, targets were more positive than unrelated preceding words, with an apparent left-posterior topography (Figure 3D). However a main effect of Condition [F(1,15)=15.0, p=0.002] and non-significant Condition-by-Cluster interaction [F(3,45)=0.47; p=0.71) indicated that the magnitude of the positive effect did not differ across the electrode clusters. Based on a priori hypotheses that the late-posterior effect would be left-lateralized, we directly compared the left-posterior and right-posterior clusters, and found a trend towards left-lateralization of the positive difference between targets and unrelated preceding words [t(15)=2.2, p=0.05].
Very similar target/prime ERP effects were identified for old targets following related primes, but the 300-500 ms effects were strikingly more pronounced than for old targets following unrelated words (Figure 2BCD). For the 300-500 ms interval, a main effect of Condition [F(1,15)=40.9, p<0.001] and Condition-by-Cluster interaction [F(1.5,23.1)=10.4; p=0.001] were due to reliably more positive prime/target differences for the mid-frontal and mid-posterior electrode clusters relative to the left-posterior and right-posterior clusters (all pairwise p values < 0.02), with no significant difference between the mid-frontal and mid-posterior clusters (p=0.70). For the 600-800 ms interval, a main effect of condition [F(1,15)=8.1, p=0.01] and non-significant Condition-by-Cluster interaction [F(3,45)=1.6, p=0.20] indicated that target ERPs were more positive than prime ERPs, but did not differ across electrode clusters. Again, direct comparison between the left-posterior and right-posterior clusters indicated significant left laterality [t(15)=2.7, p=0.02].
The primary difference between prime/target ERP effects and ERP effects for targets following unrelated words thus appeared to be markedly greater positivity from 300-500 ms for prime/target pairs (Figure 2CD). We therefore directly tested the magnitude of the ERP differences between targets minus primes and targets minus preceding unrelated words. Indeed, prime/target ERP differences were significantly more positive than differences between targets and unrelated preceding words from 300-500 ms [Condition main effect F(1,15)=5.9, p=0.03; nonsignificant Condition-by-Cluster interaction F(3,45)=0.2, p=0.87], but such differences were not reliable for the 600-800 ms interval [nonsignificant Condition main effect F(1,15)=0.3, p=0.61; nonsignificant Condition-by-Cluster interaction F(3,45)=0.9, p=0.45].
To summarize, ERP effects for old targets compared to the immediately preceding word included 300-500 ms mid-frontal/mid-posterior positivities and 600-800 ms left-posterior positivities. These effects were identified both for targets following unrelated words and for targets following related primes. Semantic priming modulated the magnitude of the 300-500 ms effect, which was greater for prime/target pairs than for target/unrelated-word pairs. In contrast, left-lateralized effects from 600-800 ms did not differ significantly for targets following primes versus targets following unrelated words. Semantic priming also did not modulate behavioral indices of familiarity memory (no effects on recognition accuracy or remember/know/guess response rates). The aforementioned analyses are thus consistent with an association between 300-500 ms effects at mid-frontal and mid-posterior sites and semantic priming and between 600-800 ms left-lateralized posterior effects and recognition. Indeed, a very similar 300-500 ms semantic priming effect was also identified for the first presentation of a target following a related prime (Figure 2), as discussed later.
ERP old/new effects
In the previous analyses, the distribution of the 600-800 ms ERP effects for second presentations of targets appeared to be more broadly distributed for the comparison involving related primes than that with unrelated words (Figure 3D). One possible cause for this difference is that ERPs might have differed for words immediately preceding the targets, which served as the comparison condition for the ERP effects. Indeed, the behavioral results indicated that subjects showed significantly more false alarms to the primes preceding targets presented for the second time (Figure 1B) than to unrelated words preceding targets presented for the second time (Figure 1D). In this section, therefore, we assessed old/new ERP effects, partly in order to provide a different baseline against which to compute repetition ERP effects immune to these small effects on prime ERPs.
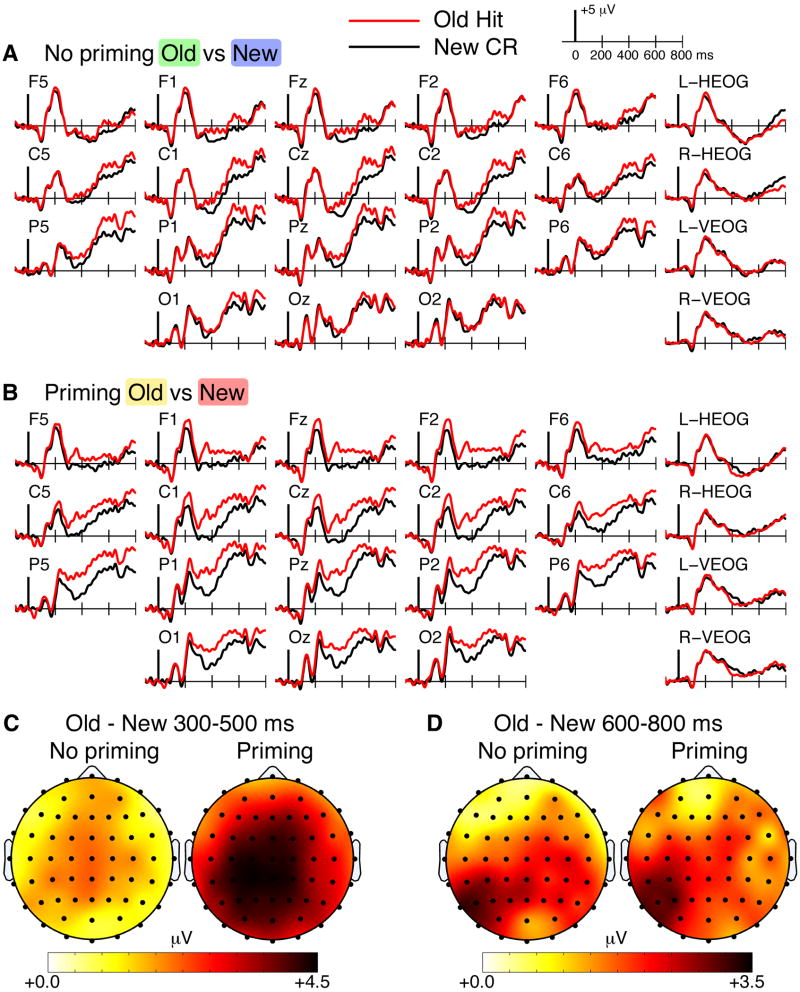
Figure 4 shows ERPs to old and new targets, split by whether the targets were preceded by related primes or unrelated words. That is, targets were compared for Figure 1A (unrelated new) versus Figure 1D (unrelated old), and for Figure 1C (related new) versus Figure 1B (related old). The ERP old/new difference (Figure 4) is highly similar to the comparison between targets and preceding words (Figure 3). One exception is that the distribution of the 600-800 ms old/new effects were clearly left-lateralized both for targets following related primes and for targets following unrelated words (Figure 4D), unlike 600-800 ms effects in Figure 3 which may have shown slight ERP differences due to choice of baseline conditions, as discussed above.
Figure 4. ERP old/new effects for targets with and without semantic priming.
ERPs are shown for new targets correctly rejected as new (new CR) and old targets correctly identified as old (old hit). A ERPs for old targets and new targets without semantic priming on either presentation, as targets were preceded by unrelated words on both occasions. B ERPs for old targets and new targets with semantic priming on both presentations, as targets were preceded by related primes on both occasions. C Topographic maps of the old/new ERP differences are shown for the N400 interval for the targets preceded by unrelated words on both occasions (no priming) and for targets preceded by related primes on both occasions (priming). D Topographic maps are provided using the same format for the later LPC interval.
Statistical assessment of the 300-500 ms latency interval indicated that old ERPs were significantly more positive than new ERPs for targets following unrelated words [Condition main effect F(1,15)=5.0, p=0.04; nonsignificant Condition-by-Cluster interaction F(3,45)=1.7, p=0.17] and for targets following related primes [Condition main effect F(1,15)=37.5, p<0.001; marginal Condition-by-Cluster interaction F(1.9,28.4)=3.2, p=0.06]. For targets following related primes, old/new effects were marginally more positive for mid-frontal and mid-posterior clusters relative to left-posterior and right-posterior clusters (pairwise p values <= 0.05), but did not differ for mid-frontal and mid-posterior clusters (p=0.13).
For the 600-800 ms latency interval, old ERPs were significantly more positive than new ERPs for targets following unrelated words [Condition main effect F(1,15)=6.7, p=0.02; nonsignificant Condition-by-Cluster interaction F(3,45)=2.2, p=0.10] and for targets following related primes [Condition main effect F(1,15)=5.6, p=0.03; marginal Condition-by-Cluster interaction F(2.1,32.2)=3.0, p=0.06]. Assessments of posterior lateralization showed significantly greater ERP old/new differences for left-posterior versus right-posterior electrode clusters both for targets following unrelated words [t(15)=2.8, p=0.01] and for targets following related primes [t(15)=3.9, p=0.001].
The aforementioned analyses converged in indicating that positive shifts in ERP potentials from 600-800 ms with a left-lateralized posterior distribution were associated with recognition memory. These potentials were sensitive to repetition, in that they were greater for old targets relative to the immediately preceding words (Figure 3ABD) and for old targets relative to new targets (Figure 4ABD). Furthermore, recognition memory strength and accuracy were matched for targets following related primes versus targets following unrelated words, and the magnitude of the 600-800 ms ERP effect likewise was matched for these conditions. Left-lateralized 600-800 ms ERP effects and recognition memory were therefore both related to repetition, and both insensitive to semantic priming. There were distinct effects on potentials from 300-500 ms showing a mid-frontal/central distribution, which appeared to be more anterior than 600-800 ms effects. Moreover, these 300-500 ms effects were related to repetition, in that they were evident for old targets following unrelated words, relative both to the unrelated words (Figure 3AC) and to the first presentation of the targets (i.e., old/new effects, Figure 4AC). However, it appeared that the same 300-500 ms effect was strongly related to semantic priming, in that it was much greater for targets following related primes, both when targets were old (Figure 3BC, Figure 4BC) and when they were new (Figure 2BCD).
ERP topographic comparisons
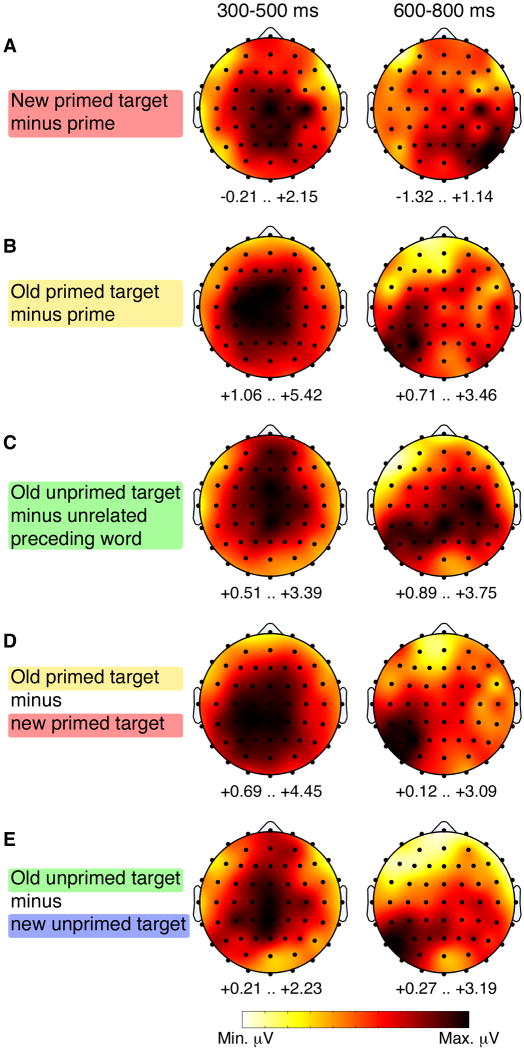
We next report a series of analyses to determine how topographically distinct 300-500 ms effects were from 600-800 ms effects, and if there were any topographical differences across the 300-500 ms effects for any of the various conditions. Topographical/distributional comparisons were made using the range normalization approach (McCarthy & Wood, 1985) to remove overall amplitude differences between the conditions being compared. In the first set of analyses, all electrodes were included. Overall distributional differences between two conditions would be indicated by a significant Condition-by-Electrode interaction (although such differences do not necessarily implicate distinct neuroelectric generators, see (Urbach & Kutas, 2002; Wilding, 2006)). Topographical plots that correspond to these distributional analyses (that is, scaled individually according to minimum and maximum amplitude values such that distributions can be compared visually across plots) are provided in Figure 5.
Figure 5. Range-scaled topographic maps.
Topographic maps are provided for the difference ERPs for the given conditions. Map coloration is based on the range of values for each condition, and therefore corresponds to the topographic analyses described in Results. Primed targets are those preceded by semantically related primes. Unprimed targets are those preceded by unrelated words. Note that the same ERP topographic maps are shown without range scaling in Figures 2-4.
The first set of comparisons sought to determine if 300-500 ms effects were topographically distinct from 600-800 ms effects for each condition separately. The analyses thus compared each plot in the left column of Figure 5 to the corresponding plot in the right column of Figure 5. Significant Condition-by-Electrode interactions were obtained for every comparison [respectively for the five conditions shown in Figure 5A-E: F(4.7,70.2)=3.3, p=0.01; F(3.8,56.9)=4.0, p=0.007; F(4.0,60.1)=3.4, p=0.01; F(3.9,58.5)=4.1, p=0.005; F(4.3,64.8)=3.0, p=0.02], thus substantiating the impression that 300-500 ms effects were more anterior and central than later effects. This distinction between 300-500 ms and later effects is consistent with the criteria generally used to distinguish so-called “FN400” effects from late-posterior effects.
We next sought to determine if the 300-500 ms semantic priming effect obtained by preceding a target with a related prime differed for the first time a target was viewed versus the second time a target was viewed. Comparing the related prime/target effect for the first presentation of a target (Figure 5A) to the related target/prime effect for the second presentation of the target (Figure 5B) yielded a nonsignificant interaction [F(4.0,59.7)=1.6; p=0.20]. Likewise, comparing the prime/target effect for the first presentation of a target (Figure 5A) to the old/new effect for the second presentation of targets following related primes (Figure 5D) yielded a nonsignificant interaction [F(5.1,76.2)=1.3, p=0.26]. These results indicate that target repetition did not significantly alter the influence of semantic priming on ERP effects from 300-500 ms.
The next set of comparisons sought to determine if 300-500 ms repetition effects differed topographically for targets following related primes compared to targets following unrelated words. The first comparison was made for the prime/target effect (Figure 5B) versus the effect for targets minus unrelated preceding words (Figure 5C), and yielded a nonsignificant interaction [F(4.1,62.1)=1.9, p=0.12]. The second comparison was made for the old/new effect for targets following related primes (Figure 5D) versus targets following unrelated words (Figure 5E), and yielded a nonsignificant interaction [F(1.0,15.0)=2.1, p=0.16].
To summarize, 300-500 ms ERP effects were always significantly more anterior than 600-800 ms ERP effects. However, there was no evidence to indicate that ERP correlates semantic priming differed spatially as a function of repetition (Figure 5A versus 5BD), nor was there evidence to indicate that ERP correlates of repetition differed as a function of semantic priming (Figure 5B vs. 5C and 5D vs. 5E).
Semantic priming ERPs versus recognition ERPs
The primary goal of the current study was to directly compare ERP correlates of semantic priming to ERP correlates of familiarity-based recognition in the same subjects and using the same experimental parameters. The final set of analyses therefore concerned: (1) prime/target ERP effects for the first presentation of a prime/target pair (Figure 1C), and (2) old/new ERP effects for new targets preceded by unrelated words (Figure 1A) compared to old targets preceded by unrelated words (Figure 1D). Thus, the analysis of semantic priming ERPs was entirely independent of repetition, and therefore independent of familiarity-based recognition, as in standard semantic priming paradigms. Likewise, the analysis of ERP old/new effects was entirely independent of semantic priming, as in standard recognition memory paradigms. The ERP effects for semantic priming without recognition are shown in Figure 2BCD and in Figure 5A, and the ERP effects for recognition without semantic priming are shown in Figure 4ACD and in Figure 5E. Note that topographic comparisons made in the previous section provided evidence that 300-500 ms ERP effects for both conditions were more anterior than corresponding ERP effects from 600-800 ms.
We first sought to compare the topographies of the two effects for the 300-500 ms interval using the range normalization method described above for all electrodes (Figure 5A versus 5E). A nonsignificant condition-by-cluster interaction [F(63,945)=0.8, p=0.83] indicated no reliable topographic difference. We then conducted a more specific test of any potential anterior “FN400” topographic differences for a restricted set of frontal electrodes. Only frontal electrodes near the midline were included (Fp1, Fpz, Fp2, AF3, AFz, AF4, F2, F1, F2, FC3, FC1, FCz, FC2, and FC4), such that the anterior/posterior extent of the frontal topographies could be compared across conditions with high sensitivity. The condition-by-cluster interaction for this more sensitive test of anterior distributional differences was also strikingly nonsignificant [F(15,225)=0.6, p=0.90].
Another sensitive test of any possible anterior/posterior differences between the distributions of the two effects was then conducted. For this analysis, we included nine clusters each comprised of three electrodes, each of which included the three electrodes that straddled the midline ranging from the most anterior (Fp1, Fpz, Fp2) to the most posterior (O1, Oz, O2) (also: Af3, Afz, and Af4; F1, Fz, and F2; FC1, FCz, and FC2; C1, Cz, and C2; CP1, CPz, and CP2; P1, Pz, and P2, PO3, POz, and PO4). The condition-by-cluster interaction on range normalized values from these nine clusters was nonsignificant [F(8,120)=0.3, p=0.96], indicating that there were no discernable anterior/posterior differences between the distributions of the two effects.
We next sought to compare the topographies of the two effects from 600-800 ms, which appeared to be right-lateralized for semantic priming without repetition (Figure 5A) and left-lateralized for repetition without semantic priming (Figure 5E). A significant condition-by-electrode interaction on range-normalized values substantiated this impression [F(4.4, 66.2)=2.9, p=0.03]. As discussed above, recognition was associated with left-lateralized parietal effects from 600-800 ms for all conditions involving repetition (Figure 5B-E). Moreover, left-lateralized 600-800 ms effects were not modulated by semantic priming, just as behavioral indicators of recognition were not modulated by semantic priming. The current topographic dissociation further supports the association with recognition by indicating that these left-lateralized ERP correlates of recognition could be dissociated from effects of semantic priming on 600-800 ms ERPs.
These tests collectively provided no evidence consistent with a dissociation between N400 correlates of semantic priming and FN400 correlates of recognition, given that both modulated topographically indistinguishable ERPs from 300-500 ms. Furthermore, the significant topographic dissociation for the 600-800 ms interval as well as the topographic dissociations between the 300-500 ms interval and the 600-800 ms interval reported above indicate that the tests did not simply lack the sensitivity required to identify topographic differences across conditions.
Discussion
Repetitions of target words in the absence of semantic priming (thus, similar to standard recognition memory paradigms) were associated with ample familiarity-based recognition and elicited ERP old/new effects (Figure 4ACD and Figure 5E). In particular, in addition to left-lateralized late-parietal effects, these old/new effects included reductions in negativity between 300 and 500 ms with a more anterior distribution, consistent with extant descriptions of “FN400” old/new effects (Paller, et al., 2007; Rugg & Curran, 2007). There were at least two possible causes of these FN400 effects: (1) familiarity-based recognition due to word repetition, and (2) semantic/conceptual priming due to word repetition.
The hypothesis that FN400 potentials index familiarity (Rugg & Curran, 2007) would predict correlations of this response primarily with familiarity (i.e., as opposed to semantic priming per se) and would also predict that semantic priming would be associated with distinct, posterior N400 repetition effects. By examining ERP prime/target effects for semantically related word pairs seen for the first time (Figure 2ACD and Figure 5A), we were able to isolate ERP correlates of semantic priming independent from recognition memory within the same experiment. Contrary to what would be predicted by the hypothesis that FN400 potentials are distinct from N400 potentials, semantic priming without recognition elicited ERP effects that were indistinguishable in timecourse or distribution from those evident for familiarity-based recognition without semantic priming. In contrast, ERP effects from 600-800 ms were dissociated for these conditions, with left-lateralized ERP correlates of recognition memory distinct from less left-lateralized ERP correlates of semantic priming. To our knowledge, no previous study has directly compared “FN400” and N400 effects; instead, the hypothesized electrophysiological distinction between the two responses has been based on visual comparisons of ERP topographies obtained from different subjects participating in different experimental paradigms, often in different laboratories using different analysis strategies. Our results indicate that when these factors are controlled, there is no reason to conclude that “FN400” correlates of memory are distinct from N400 correlates of semantic priming.
Other aspects of our results provide additional evidence for this conclusion. Preceding an old target with a semantically related prime had no discernable effects on recognition memory strength, accuracy, confidence, or self-reported familiarity, relative to old targets preceded by unrelated words (and therefore not primed). However, old targets primed by related words were associated with strikingly larger modulations of anterior N400 (“FN400”) effects than were old targets preceded by unrelated words. It is difficult to reconcile the hypothesis that FN400 old/new effects are a general index of familiarity when two conditions that are highly matched in familiarity nevertheless differ so markedly in FN400 amplitude.
Instead, it was LPC effects that seemed to be more yoked to recognition than to semantic priming, consistent with the larger literature linking LPC amplitudes to recognition measures (Friedman & Johnson, 2000; Mecklinger, 2000; Paller, et al., 2007; Rugg & Curran, 2007; Voss & Paller, 2008a, 2008b). The magnitude of left-lateralized LPC repetition effects did not vary across any of the conditions we tested involving repetition (that is, were completely insensitive to doctor/nurse semantic priming). Likewise, behavioral indicators of recognition memory and memory-strength distributions were matched for these conditions.5
Collectively, our data support the hypothesis that effects in recognition memory paradigms labeled as “FN400” are actually N400 effects, and thus share both the electrophysiological characteristics (such as temporal stability) and functional sensitivity of the N400. As shown by the present findings and buttressed by the established literature on the N400 (see, e.g., Kutas and Federmeier, in press), this N400 response is sensitive to short-term conceptual priming arising from shared semantic features and/or association as well as to semantic facilitation that occurs when a word is repeated after a delay. This conclusion is consistent with findings that ERP correlates of recognition for semantically impoverished stimuli do not include N400-like effects (De Chastelaine, et al., 2009; MacKenzie & Donaldson, 2007; Voss & Paller, 2009b; Yovel & Paller, 2004) even at very short delays (Danker, et al., 2008), and that N400-like effects in recognition memory paradigms covary with the magnitude of conceptual/semantic priming and with the ability for relatively low-meaning stimuli to support conceptual priming (Voss, et al., 2009; Voss & Paller, 2006, 2007; Voss, et al., 2010). More generally, our results thus suggest that semantic/conceptual priming operates during recognition memory testing whenever meaningful stimuli are repeated and N400-like (“FN400”) effects are observed (Paller, et al., 2007; Voss & Paller, 2008a).
Some recent findings appear to provide evidence counter to our conclusion that FN400 effects during memory testing are actually N400 correlates of semantic/conceptual priming rather than signals association with familiarity-based recognition (Woodruff, Hayama, & Rugg, 2006; Yu & Rugg, 2010). In these studies using words and nameable pictures, self-reports of familiarity strength (confidence ratings for “know” judgments using the remember/know procedure) did show correlations with the magnitude of “FN400” effects, with higher familiarity strength associated with larger FN400 effects. Importantly, however, these studies did not attempt to dissociate familiarity confidence from variations in semantic facilitation, which we would argue are the actual basis of the observed correlation. Why might confidence decisions and semantic/conceptual priming be correlated for words, thus creating an apparent association between “FN400” effects and familiarity? Below we explicate one possible theoretical account that draws from the notion that decisions during a memory test are made on the basis of multiple, contemporaneous brain processing events.
We start by pointing out that confidence ratings often provide valid measures of memory “strength” or accuracy (Heathcote, 2003; Yonelinas, 2001). However, confidence and memory strength/accuracy are not perfectly correlated, and can be dissociated (Chandler, 1994; Dobbins, Kroll, & Liu, 1998; Heathcote, Freeman, Etherington, Tonkin, & Bora, 2009; Tulving, 1981; Voss, Baym, & Paller, 2008; Voss & Paller, 2009a; Wilimzig, Tsuchiya, Fahle, Einhauser, & Koch, 2008). Some evidence indicates that confidence ratings may be particularly sensitive to signals of perceptual and conceptual fluency (Jacoby & Whitehouse, 1989; Keane, Orlando, & Verfaellie, 2006; Verfaellie & Cermak, 1999; Whittlesea & Williams, 2000; Wolk, et al., 2005). Indeed, all experiments referenced above as supporting dissociations between confidence and accuracy achieved these dissociations through manipulations of fluency. The influence of fluency on confidence does not necessarily need to derive from an object's “oldness”—that is, fluency need not be related to repetition during a memory test. For instance, Verfaellie and colleagues have shown that fluency can influence memory confidence, even for items that were never studied previously during the experiment (Verfaellie & Cermak, 1999). Thus, when episodic memory signals are weak, as is the case for items that garner “know” responses rather than “remember” responses, perceptual or conceptual fluency might exert stronger influences on confidence decisions than when memory is relatively strong. Indeed, some evidence indicates that fluency has little, if any, influence on memory decisions made with high confidence or strength—that is, fluency and confident recognition can be convincingly dissociated in many circumstances (e.g., Conroy, Hopkins, & Squire, 2005; Stark & Squire, 2000; Wagner, Gabrieli, & Verfaellie, 1997).
Following this logic, the reason that confidence ratings for “know” items with relatively weak memory signals might covary with N400 signals of semantic/conceptual priming (mistaken as familiarity-based “FN400”) is that these signals vary across items and influence confidence decisions accordingly. This kind of semantic/conceptual variation is plausible for many reasons. For instance, there is likely considerable variation across items in terms of how “deeply” words are semantically elaborated during encoding, and variations in semantic elaboration would be expected to correlate with the degree of later conceptual/semantic priming (Roediger, 1990). Furthermore, words vary in fluency and conceptual priming based on factors such as the frequency with which they are encountered (Yap, Tse, & Balota, 2009), and how associated they are to other concepts stored in semantic memory (Griffiths, Steyvers, & Firl, 2007). Our proposal is simply that subjects use these signals when making confidence decisions, especially when memory is relatively weak (i.e., when “know” responses are registered; consistent with findings of Jacoby & Whitehouse, 1989; Keane, et al., 2006; Verfaellie & Cermak, 1999; Wolk, et al., 2005). Thus, just as fluency biases decisions in many circumstances (Alter & Oppenheimer, 2009), conceptual fluency biases recognition judgments, leading to covariation between confidence ratings and N400 correlates of semantic/conceptual priming.
Our results can thus be taken as evidence consistent with multiple memory-systems theories that propose that memory behaviors are determined by the output from several, co-active memory systems, including a semantic system distinct from an episodic memory system (Henson & Gagnepain, in press; Tulving, 1985a). Confidence ratings can be sensitive to the output from different systems in different circumstances, and manipulations such as those used in the present experiment are necessary to determine the dissociations and associations between these systems in producing a memory decision. In other words, relying on confidence ratings alone will fail to accurately characterize neural substrates of memory, given that additional information is needed to determine which processes in which systems produced the ratings.
In conclusion, then, we found evidence consistent with the notion that conceptual priming occurs during recognition memory tests and is the source of N400 old/new effects during these tests. These potentials have been (mis)labeled as “FN400” old/new effects and assumed to have a specific, separable functional sensitivity and electrophysiological signature, despite having never been dissociated convincingly or empirically from N400 potentials. Rather than merely serving as a cautionary note in the search for ERP correlates of memory, we take our results as support for a multiple-memory systems account of recognition, whereby a relatively implicit, automatic semantic system assesses all incoming information and thus produces semantic/conceptual fluency signals that can influence memory behaviors and decisions along with the output from other systems. This semantic analysis does not always provide accurate memory decisions, given that semantic fluency need not stem from true “oldness” or repetition, but we speculate that it provides an unobtrusive and reasonably accurate means of adapting to environmental stimuli based on recent experiences.
Acknowledgments
We thank Brian Gonsalves for providing access to his EEG equipment, Ashley Galvan for help collecting data, and Ken Paller for commenting on an earlier draft of this manuscript. Research support was provided by a Beckman Institute Postdoctoral Fellowship award, and by National Institutes of Health grants K99-NS069788-01 to J.L.V. and R01-AG026308 to K.D.F.
Footnotes
It is important to note, as discussed in more detail below, that the strong hypothesis linking FN400 to familiarity (e.g., Rugg & Curran, 2007) does not propose that FN400 effects are just slight anterior shifts in the observed distribution of the N400, but rather that these are distinct effects that would not be expected to manifest with mostly overlapping distributions.
De Chastelaine et al. (2009) also found little evidence to associate FN400 with semantic/conceptual processing, but their analyses were limited, as they were based on ratings of semantic content collected after subjects finished the lengthy experiment, which, therefore were likely not well-yoked to the level of conceptual processing engaged on a trial-by-trial basis during the experiment. Furthermore, the validity of the ad hoc semantic ratings was not assessed by including, for example, tests of conceptual priming analyzed according to the ratings. However, trial-by-trial estimates of recognition were straightforward and convincing, and unequivocally showed no FN400 effects despite familiarity-based recognition.
ERP correlates of false alarm trials included positive shifts in frontal N400 waveforms similar to those reported below for other experimental conditions, but we do not report these ERP effects in detail given that it is unclear whether they were related to semantic priming or to familiarity. That is, because the semantically related target appeared previously, ERPs could conceivable reflect either semantic priming due to the target or episodic familiarity for the target. The analyses described below were suitable for separately assessing these possible influences on ERPs.
Indeed, correlations across subjects between remember response rates to old and to new items showed that subjects who made more remember responses to old items also made more remember responses to new items [r(14)=0.49, p=0.03 for targets following related primes and r(14)=0.46, p=0.04 for targets following unrelated words). These correlations are consistent with the hypothesis that subjects varied in terms of the threshold that was adopted for reporting recollection, rather than the hypothesis that remember responses indicate a qualitatively unique state of phenomenological awareness of memory (Wixted & Stretch, 2004). That is, remember responses would be registered for old items if they truly reflected veridical recollection of specific details, and the correlation instead suggests that some subjects were more likely to make remember responses than other subjects, even to new items for which recollection should not be possible.
Of course, direct evidence for the link between recognition memory and LPC effects would require manipulations that affect both in a similar way, but establishing this relationship was not the goal of the current study.
References
- Alter AL, Oppenheimer DM. Uniting the tribes of fluency to form a metacognitive nation. Personality and Social Psychology Review. 2009;13:219–235. doi: 10.1177/1088868309341564. [DOI] [PubMed] [Google Scholar]
- Besson M, Kutas M, Van Petten C. An event-related potential (ERP) analysis of semantic congruity and repetition effects in sentences. Journal of Cognitive Neuroscience. 1992;4:132–149. doi: 10.1162/jocn.1992.4.2.132. [DOI] [PubMed] [Google Scholar]
- Chandler CC. Studying related pictures can reduce accuracy, but increase confidence, in a modified recognition test. Memory & Cognition. 1994;22:273–280. doi: 10.3758/bf03200854. [DOI] [PubMed] [Google Scholar]
- Chatrian GE, Lettich E, Nelson PL. Modified nomenclature for the “10%” electrode system. Journal of Clinical Neurophysiology. 1988;5:183–186. [PubMed] [Google Scholar]
- Conroy MA, Hopkins RO, Squire LR. On the contribution of perceptual fluency and priming to recognition memory. Cognitive, Affective & Behavioral Neuroscience. 2005;5:14–20. doi: 10.3758/cabn.5.1.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craik F, Lockhart R. Levels of processing: A framework for memory research. Journal of Verbal Learning & Verbal Behavior. 1972;11:671–684. [Google Scholar]
- Craik F, Tulving E. Depth of processing and the retention of words in episodic memory. Journal of Experimental Psychology: General. 1975;104:268–294. [Google Scholar]
- Curran T. Brain potentials of recollection and familiarity. Memory & Cognition. 2000;28:923–938. doi: 10.3758/bf03209340. [DOI] [PubMed] [Google Scholar]
- Czernochowski D, Mecklinger A, Johansson M. Age-related changes in the control of episodic retrieval: An ERP study of recognition memory in children and adults. Developmental Science. 2009;12:1026–1040. doi: 10.1111/j.1467-7687.2009.00841.x. [DOI] [PubMed] [Google Scholar]
- Danker JF, Hwang GM, Gauthier L, Geller A, Kahana MJ, Sekuler R. Characterizing the ERP Old-New effect in a short-term memory task. Psychophysiology. 2008;45:784–793. doi: 10.1111/j.1469-8986.2008.00672.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Chastelaine M, Friedman D, Cycowicz YM, Horton C. Effects of multiple study-test repetition on the neural correlates of recognition memory: ERPs dissociate remembering and knowing. Psychophysiology. 2009;46:86–99. doi: 10.1111/j.1469-8986.2008.00754.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobbins IG, Kroll NE, Liu Q. Confidence-accuracy inversions in scene recognition: A remember-know analysis. Journal of Experimental Psychology: Learning, Memory, and Cognition. 1998;24(5):1306–1315. doi: 10.1037//0278-7393.24.5.1306. [DOI] [PubMed] [Google Scholar]
- Donaldson DI, Curran T. Potential (ERP) studies of recognition memory for faces. Neuroimage. 2007;36:488–489. doi: 10.1016/j.neuroimage.2006.12.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ecker UK, Arend AM, Bergstrom K, Zimmer HD. Verbal predicates foster conscious recollection but not familiarity of a task-irrelevant perceptual feature--an ERP study. Consciousness & Cognition. 2009;18:679–689. doi: 10.1016/j.concog.2009.04.005. [DOI] [PubMed] [Google Scholar]
- Eichenbaum H, Yonelinas AP, Ranganath C. The medial temporal lobe and recognition memory. Annual Review of Neuroscience. 2007;30:123–152. doi: 10.1146/annurev.neuro.30.051606.094328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman D. ERPs during continuous recognition memory for words. Biological Psychology. 1990;30:61–87. doi: 10.1016/0301-0511(90)90091-a. [DOI] [PubMed] [Google Scholar]
- Friedman D, Johnson R., Jr Event-related potential (ERP) studies of memory encoding and retrieval: A selective review. Microscopy Research and Technique. 2000;51:6–28. doi: 10.1002/1097-0029(20001001)51:1<6::AID-JEMT2>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- Ganis G, Kutas M, Sereno M. The search for common sense: An electrophysiological investigation of the semantic analysis of words and pictures in sentences. Journal of Cognitive Neuroscience. 1996;8:89–106. doi: 10.1162/jocn.1996.8.2.89. [DOI] [PubMed] [Google Scholar]
- Gardiner JM, Java RI. Forgetting in recognition memory with and without recollective experience. Memory & Cognition. 1991;19:617–623. doi: 10.3758/bf03197157. [DOI] [PubMed] [Google Scholar]
- Griffiths TL, Steyvers M, Firl A. Google and the mind: Predicting fluency with PageRank. Psychological Science. 2007;18:1069–1076. doi: 10.1111/j.1467-9280.2007.02027.x. [DOI] [PubMed] [Google Scholar]
- Heathcote A. Item recognition memory and the ROC. Journal of Experimental Psychology Learning, Memory, and Cognition. 2003;29:1210–1230. doi: 10.1037/0278-7393.29.6.1210. [DOI] [PubMed] [Google Scholar]
- Heathcote A, Freeman E, Etherington J, Tonkin J, Bora B. A dissociation between similarity effects in episodic face recognition. Psychonomic Bulletin & Review. 2009;16:824–831. doi: 10.3758/PBR.16.5.824. [DOI] [PubMed] [Google Scholar]
- Henson RN, Gagnepain P. Predictive, interactive multiple memory systems. Hippocampus. doi: 10.1002/hipo.20857. in press. [DOI] [PubMed] [Google Scholar]
- Jacoby LL, Whitehouse K. An illusion of memory: False recognition influenced by unconscious perception. Journal of Experimental Psychology: General. 1989;118:126–135. [Google Scholar]
- Keane MM, Orlando F, Verfaellie M. Increasing the salience of fluency cues reduces the recognition memory impairment in amnesia. Neuropsychologia. 2006;44:834–839. doi: 10.1016/j.neuropsychologia.2005.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klonek F, Tamm S, Hofmann MJ, Jacobs AM. Does familiarity or conflict account for performance in the word-stem completion task? Evidence from behavioural and event-related-potential data. Psychology Research. 2009;73:871–882. doi: 10.1007/s00426-008-0189-8. [DOI] [PubMed] [Google Scholar]
- Kounios J, Holcomb PJ. Concreteness effects in semantic processing: ERP evidence supporting dual-coding theory. Journal of Experimental Psychology: Learning, Memory, and Cognition. 1994;20:804–823. doi: 10.1037//0278-7393.20.4.804. [DOI] [PubMed] [Google Scholar]
- Kucera H, Francis WN. Computational analysis of present-day American English. Providence: Brown University Press; 1967. [Google Scholar]
- Kutas M, Federmeier K. Thirty years and counting: Finding meaning in the N400 component of the event-related brain potential (ERP) Annual Review of Psychology. doi: 10.1146/annurev.psych.093008.131123. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kutas M, Hillyard SA. Reading senseless sentences: Brain potentials reflect semantic incongruity. Science. 1980;207:203–205. doi: 10.1126/science.7350657. [DOI] [PubMed] [Google Scholar]
- Kutas M, Van Petten C. Electrophysiological perspectives on comprehending written language. Electroencephalography & Clinical Neurophysiology Supplement. 1990;41:155–167. doi: 10.1016/b978-0-444-81352-7.50020-0. [DOI] [PubMed] [Google Scholar]
- MacKenzie G, Donaldson DI. Dissociating recollection from familiarity: Electrophysiological evidence that familiarity for faces is associated with a posterior old/new effect. Neuroimage. 2007;36:454–463. doi: 10.1016/j.neuroimage.2006.12.005. [DOI] [PubMed] [Google Scholar]
- Mandler G. Recognizing: The judgment of previous occurrence. Psychological Review. 1980;87:252–271. [Google Scholar]
- McCarthy G, Wood CC. Scalp distributions of event-related potentials: An ambiguity associated with analysis of variance models. Electroencephalography and Clinical Neurophysiology. 1985;62:203–208. doi: 10.1016/0168-5597(85)90015-2. [DOI] [PubMed] [Google Scholar]
- Mecklinger A. Interfacing mind and brain: A neurocognitive model of recognition memory. Psychophysiology. 2000;37:565–582. [PubMed] [Google Scholar]
- Mecklinger A, Brunnemann N, Kipp K. Two processes for recognition memory in children of early school age: An event-related potential study. Journal of Cognitive Neuroscience. 2010 doi: 10.1162/jocn.2010.21455. in press. [DOI] [PubMed] [Google Scholar]
- Nelson DL, McEvoy CL, Schreiber TA. The University of South Florida word association, rhyme, and word fragment norms. 1998 doi: 10.3758/bf03195588. Retrieved February 15, 2010: http://www.usf.edu/FreeAssociation/ [DOI] [PubMed]
- Neville H, Kutas M, Chesney G, Schmidt AL. Event-related brain potentials during initial encoding and recognition memory of congruous and incongruous words. Journal of Memory & Language. 1986;25:75–92. [Google Scholar]
- Nyhus E, Curran T. Semantic and perceptual effects on recognition memory: Evidence from ERP. Brain Research. 2009;1283:102–114. doi: 10.1016/j.brainres.2009.05.091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olichney JM, Van Petten C, Paller KA, Salmon DP, Iragui VJ, Kutas M. Word repetition in amnesia. Electrophysiological measures of impaired and spared memory. Brain. 2000;123:1948–1963. doi: 10.1093/brain/123.9.1948. [DOI] [PubMed] [Google Scholar]
- Opitz B, Cornell S. Contribution of familiarity and recollection to associative recognition memory: Insights from event-related potentials. Journal of Cognitive Neuroscience. 2006;18:1595–1605. doi: 10.1162/jocn.2006.18.9.1595. [DOI] [PubMed] [Google Scholar]
- Paller KA, Kutas M. Brain potentials during memory retrieval provide neurophysiological support for the distinction between conscious recollection and priming. Journal of Cognitive Neuroscience. 1992;4:375–391. doi: 10.1162/jocn.1992.4.4.375. [DOI] [PubMed] [Google Scholar]
- Paller KA, Voss JL, Boehm SG. Validating neural correlates of familiarity. Trends in Cognitive Sciences. 2007;11:243–250. doi: 10.1016/j.tics.2007.04.002. [DOI] [PubMed] [Google Scholar]
- Roediger HL. Implicit memory. Retention without remembering. American Psychologist. 1990;45:1043–1056. doi: 10.1037//0003-066x.45.9.1043. [DOI] [PubMed] [Google Scholar]
- Rotello CM, Zeng M. Analysis of RT distributions in the remember-know paradigm. Psychonomic Bulletin & Review. 2008;15:825–832. doi: 10.3758/pbr.15.4.825. [DOI] [PubMed] [Google Scholar]
- Rugg MD. Event-related brain potentials dissociate repetition effects of high- and low-frequency words. Memory & Cognition. 1990;18:367–379. doi: 10.3758/bf03197126. [DOI] [PubMed] [Google Scholar]
- Rugg MD, Curran T. Event-related potentials and recognition memory. Trends in Cognitive Sciences. 2007;11:251–257. doi: 10.1016/j.tics.2007.04.004. [DOI] [PubMed] [Google Scholar]
- Schacter DL, Wig GS, Stevens WD. Reductions in cortical activity during priming. Current Opinion in Neurobiology. 2007;17:171–176. doi: 10.1016/j.conb.2007.02.001. [DOI] [PubMed] [Google Scholar]
- Smith ME. Neurophysiological manifestations of recollective experience during recognition memory judgments. Journal of Cognitive Neuroscience. 1993;5:1–13. doi: 10.1162/jocn.1993.5.1.1. [DOI] [PubMed] [Google Scholar]
- Speer NK, Curran T. ERP correlates of familiarity and recollection processes in visual associative recognition. Brain Research. 2007;1174:97–109. doi: 10.1016/j.brainres.2007.08.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stark CE, Squire LR. Recognition memory and familiarity judgments in severe amnesia: No evidence for a contribution of repetition priming. Behavioral Neuroscience. 2000;114:459–467. doi: 10.1037//0735-7044.114.3.459. [DOI] [PubMed] [Google Scholar]
- Tulving E. Similarity relations in recognition. Journal of Verbal Learning and Verbal Behavior. 1981;20:479–496. [Google Scholar]
- Tulving E. How many memory systems are there? American Psychologist. 1985a;40:385–398. [Google Scholar]
- Tulving E. Memory and consciousness. Canadian Journal of Psychology. 1985b;26:1–12. [Google Scholar]
- Urbach TP, Kutas M. The intractability of scaling scalp distributions to infer neuroelectric sources. Psychophysiology. 2002;39:791–808. doi: 10.1111/1469-8986.3960791. [DOI] [PubMed] [Google Scholar]
- Verfaellie M, Cermak LS. Perceptual fluency as a cue for recognition judgments in amnesia. Neuropsychology. 1999;13:198–205. doi: 10.1037//0894-4105.13.2.198. [DOI] [PubMed] [Google Scholar]
- Vilberg KL, Rugg MD. Functional significance of retrieval-related activity in lateral parietal cortex: Evidence from fMRI and ERPs. Human Brain Mapping. 2009;30:1490–1501. doi: 10.1002/hbm.20618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogel EK, Luck SJ, Shapiro KL. Electrophysiological evidence for a postperceptual locus of suppression during the attentional blink. Journal of Experimental Psychology: Human Perception and Performance. 1998;24:1656–1674. doi: 10.1037//0096-1523.24.6.1656. [DOI] [PubMed] [Google Scholar]
- Voss JL, Baym CL, Paller KA. Accurate forced-choice recognition without awareness of memory retrieval. Learning & Memory. 2008;15:454–459. doi: 10.1101/lm.971208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voss JL, Lucas HD, Paller KA. Conceptual priming and familiarity: Different expressions of memory during recognition testing with distinct neurophysiological correlates. Journal of Cognitive Neuroscience. 2009 doi: 10.1162/jocn.2009.21341. in press. [DOI] [PubMed] [Google Scholar]
- Voss JL, Paller KA. Fluent conceptual processing and explicit memory for faces are electrophysiologically distinct. Journal of Neuroscience. 2006;26:926–933. doi: 10.1523/JNEUROSCI.3931-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voss JL, Paller KA. Neural correlates of conceptual implicit memory and their contamination of putative neural correlates of explicit memory. Learning & Memory. 2007;14:259–267. doi: 10.1101/lm.529807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voss JL, Paller KA. Brain substrates of implicit and explicit memory: The importance of concurrently acquired neural signals of both memory types. Neuropsychologia. 2008a;46:3021–3029. doi: 10.1016/j.neuropsychologia.2008.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voss JL, Paller KA. Neural substrates of remembering: Electroencephalographic studies. In: Byrne J, editor. Learning and memory: A comprehensive reference. Vol. 3. Oxford: Elsevier; 2008b. pp. 79–97. [Google Scholar]
- Voss JL, Paller KA. An electrophysiological signature of unconscious recognition memory. Nature Neuroscience. 2009a;12:349–355. doi: 10.1038/nn.2260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voss JL, Paller KA. Remembering and knowing: Electrophysiological distinctions at encoding but not retrieval. Neuroimage. 2009b;46:280–289. doi: 10.1016/j.neuroimage.2009.01.048. [DOI] [PubMed] [Google Scholar]
- Voss JL, Reber PJ, Mesulam MM, Parrish TB, Paller KA. Familiarity and conceptual priming engage distinct cortical networks. Cerebral Cortex. 2008;18:1712–1719. doi: 10.1093/cercor/bhm200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voss JL, Schendan HE, Paller KA. Finding meaning in novel geometric shapes influences electrophysiological correlates of repetition and dissociates perceptual and conceptual priming. Neuroimage. 2010;49:2879–2889. doi: 10.1016/j.neuroimage.2009.09.012. [DOI] [PubMed] [Google Scholar]
- Wagner AD, Gabrieli JD, Verfaellie M. Dissociations between familiarity processes in explicit recognition and implicit perceptual memory. Journal of Experimental Psychology: Learning, Memory, and Cognition. 1997;23:305–323. doi: 10.1037//0278-7393.23.2.305. [DOI] [PubMed] [Google Scholar]
- Whittlesea BW, Williams LD. The source of feelings of familiarity: The discrepancy-attribution hypothesis. Journal of Experimental Psychology: Learning, Memory, and Cognition. 2000;26:547–565. doi: 10.1037//0278-7393.26.3.547. [DOI] [PubMed] [Google Scholar]
- Wilding EL. On the practice of rescaling scalp-recorded electrophysiological data. Biological Psychology. 2006;72:325–332. doi: 10.1016/j.biopsycho.2005.12.002. [DOI] [PubMed] [Google Scholar]
- Wilimzig C, Tsuchiya N, Fahle M, Einhauser W, Koch C. Spatial attention increases performance but not subjective confidence in a discrimination task. Journal of Vision. 2008;8:7 1–10. doi: 10.1167/8.5.7. [DOI] [PubMed] [Google Scholar]
- Wixted JT. Dual-process theory and signal-detection theory of recognition memory. Psychological Review. 2007;114:152–176. doi: 10.1037/0033-295X.114.1.152. [DOI] [PubMed] [Google Scholar]
- Wixted JT. Remember/Know judgments in cognitive neuroscience: An illustration of the underrepresented point of view. Learning & Memory. 2009;16:406–412. doi: 10.1101/lm.1312809. [DOI] [PubMed] [Google Scholar]
- Wixted JT, Stretch V. In defense of the signal detection interpretation of remember/know judgments. Psychonomic Bulletin & Review. 2004;11:616–641. doi: 10.3758/bf03196616. [DOI] [PubMed] [Google Scholar]
- Wolk DA, Schacter DL, Berman AR, Holcomb PJ, Daffner KR, Budson AE. Patients with mild Alzheimer's disease attribute conceptual fluency to prior experience. Neuropsychologia. 2005;43:1662–1672. doi: 10.1016/j.neuropsychologia.2005.01.007. [DOI] [PubMed] [Google Scholar]
- Woodruff CC, Hayama HR, Rugg MD. Electrophysiological dissociation of the neural correlates of recollection and familiarity. Brain Research. 2006;1100:125–135. doi: 10.1016/j.brainres.2006.05.019. [DOI] [PubMed] [Google Scholar]
- Yap MJ, Tse CS, Balota DA. Individual differences in the joint effects of semantic priming and word frequency: The role of lexical integrity. Journal of Memory & Language. 2009;61:303. doi: 10.1016/j.jml.2009.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yonelinas AP. Consciousness, control, and confidence: The 3 Cs of recognition memory. Journal of Experimental Psychology: General. 2001;130:361–379. doi: 10.1037//0096-3445.130.3.361. [DOI] [PubMed] [Google Scholar]
- Yonelinas AP. The nature of recollection and familiarity: A review of 30 years of research. Journal of Memory & Language. 2002;46:441–517. [Google Scholar]
- Young MP, Rugg MD. Word frequency and multiple repetition as determinants of the modulation of event-related potentials in a semantic classification task. Psychophysiology. 1992;29:664–676. doi: 10.1111/j.1469-8986.1992.tb02044.x. [DOI] [PubMed] [Google Scholar]
- Yovel G, Paller KA. The neural basis of the butcher-on-the-bus phenomenon: when a face seems familiar but is not remembered. Neuroimage. 2004;21:789–800. doi: 10.1016/j.neuroimage.2003.09.034. [DOI] [PubMed] [Google Scholar]
- Yu SS, Rugg MD. Dissociation of the electrophysiological correlates of familiarity strength and item repetition. Brain Research. 2010 doi: 10.1016/j.brainres.2009.12.071. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]