Abstract
The male germ cell-specific fatty acid binding protein 9 (FABP9/PERF15) is the major component of the murine sperm perforatorium and perinuclear theca. Based on its cytoskeletal association and sequence homology to myelin P2 (FABP8), it has been suggested that FABP9 tethers sperm membranes to the underlying cytoskeleton. Furthermore, its upregulation in apoptotic testicular germ cells and its increased phosphorylation status during capacitation suggested multiple important functions for FABP9. Therefore, we investigated specific functions for FABP9 by means of targeted gene disruption in mice. FABP9−/− mice were viable and fertile. Phenotypic analysis showed that FABP9−/− mice had significant increases in sperm head abnormalities (~8% greater than their WT cohorts); in particular, we observed the reduction or absence of the characteristic structural element known as the “ventral spur” in ~10% of FABP9−/− sperm. However, deficiency of FABP9 neither affected membrane tethering to the perinuclear theca nor the fatty acid composition of sperm. Moreover, epididymal sperm numbers were not affected in FABP9−/− mice. Therefore, we conclude that FABP9 plays only a minor role in providing the murine sperm head its characteristic shape and is not absolutely required for spermatogenesis or sperm function.
Keywords: Fatty acid binding protein, Germ cell, Sperm, Testis, Lipid, Perforatorium
Introduction
Mammalian sperm are highly polarized cells with specialized functions restricted to specific regions (Travis and Kopf, 2002). We have previously shown that the plasma membrane overlying the acrosome (APM) is a large domain greatly enriched in sterols and the sphingolipid, GM1, in comparison with the post-acrosomal plasma membrane (PAPM) (Selvaraj et al., 2006). We have also demonstrated that the APM domain is not uniform, but rather contains both membrane raft and non-raft micro-domains of varying compositions; we found no evidence of raft micro-domains in the PAPM (Asano et al., 2009; Selvaraj et al., 2009). The physiological significance of raft segregation to the APM domain is suggested by the functional requirement for sterol efflux in order for sperm to become fertilization competent through the process known as “capacitation” (Austin, 1951, 1952; Chang, 1951; Davis, 1976; Visconti et al., 1999). Our investigations of the mechanism behind the maintenance of this unique micron-scale membrane segregation pointed to membrane interactions with underlying cytoskeletal elements (Selvaraj et al., 2006). This mechanism would be consistent with a membrane compartmentation model of domain segregation (Kusumi and Sako, 1996), but would exist on a larger scale.
On examination of the structural components of the mature sperm head, it has been shown that the perinuclear theca (a dense cytoskeletal network) closely apposes the different membranes of the sperm head (Fawcett, 1970; Lalli and Clermont, 1981; Oko, 1988). It has been hypothesized that a component of the perinuclear theca binds and tethers the different membranes of the sperm head (Fouquet et al., 1992; Oko and Maravei, 1994). The subacrosomal layer of the perinuclear theca appears to anchor the acrosomal vesicle to the nuclear membrane; the post-acrosomal sheath of the perinuclear theca is between the PAPM and the nuclear membrane; the outer peri-acrosomal layer of the perinuclear theca links the APM to the outer acrosomal membrane (Oko and Maravei, 1994; Oko et al., 1990). The major protein present in the perforatorium and associated perinuclear theca, initially named PERF15 (Oko and Clermont, 1988), was identified as testis lipid binding protein (TLBP) or fatty acid binding protein 9 (FABP9) (Oko and Morales, 1994; Schmitt et al., 1994). The presence of FABP9 in the perinuclear theca has so far been reported for rat and mouse sperm (Korley et al., 1997; Oko and Morales, 1994).
Fatty acid-binding proteins (FABPs) belong to the conserved multigene family of intracellular lipid-binding proteins (Chmurzynska, 2006). Ten different FABPs (Fig. 1A) showing tissue-specific expressions have been characterized in several species: FABP1 (liver; L-FABP), FABP2 (intestine; I-FABP), FABP3 (heart; H-FABP), FABP4 (adipocyte; A-FABP), FABP5 (epidermis; E-FABP), FABP6 (ileum; IL-FABP), FABP7 (brain; B-FABP), FABP8 (peripheral nervous system; M-FABP/myelin P2), FABP9 (testis/germ cell; T-FABP) and the newly discovered FABP12 (retina and testis) (Liu et al., 2008; Zimmerman and Veerkamp, 2002). Transcripts of human homologs for all the FABPs have been sequenced except for FABP9. The different FABPs have a 22–73% sequence similarity with highly conserved three-dimensional structures (Chmurzynska, 2006; Zimmerman and Veerkamp, 2002). Several functions have been demonstrated for FABPs, including the promotion of cellular uptake of fatty acids (Schurer et al., 1994; Stremmel et al., 1985; Trotter et al., 1996), intracellular fatty acid trafficking (Falomir-Lockhart et al., 2006; Storch and Thumser, 2000), fatty acid metabolism (Hotamisligil et al., 1996; Shaughnessy et al., 2000), signal transduction (Graber et al., 1994; Widstrom et al., 2001), cell growth (Burton et al., 1994), differentiation (Aponte, 2002; Borchers et al., 1997; Yang et al., 1994), and regulation of gene expression (Bernlohr et al., 1997; Wolfrum et al., 2001). Because of the significant sequence similarity of FABP9 to the membrane-associated FABP8 (myelin P2; 60% sequence homology), which is observed on the cytoplasmic face of the myelin sheath of peripheral neurons (Eylar et al., 1980; Trapp et al., 1984), it has been suggested that FABP9 in the perinuclear theca could be the component that tethers the sperm membranes to the perinuclear theca (Oko and Morales, 1994).
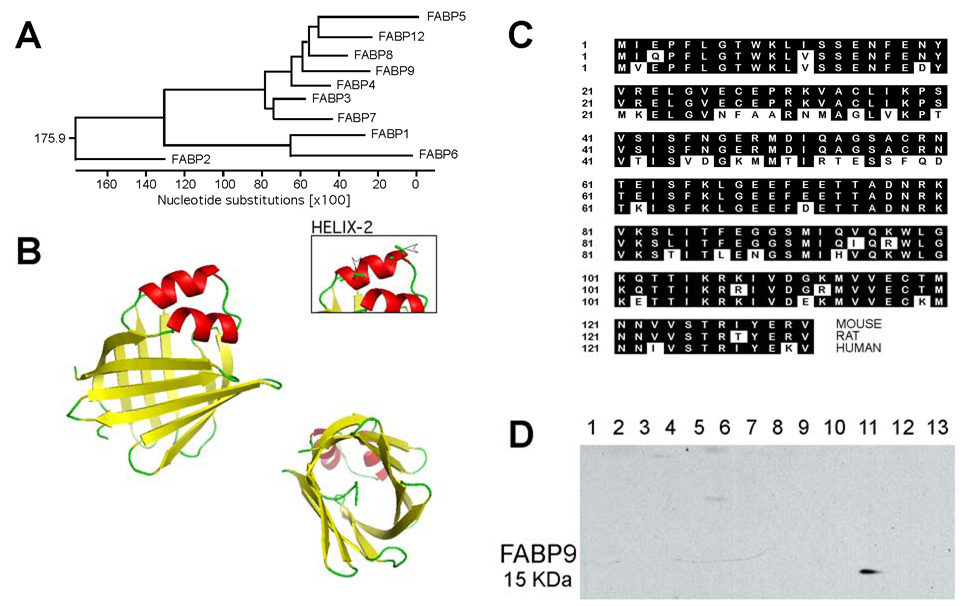
Fig. 1. Structure, sequence conservation and tissue specific expression of FABP9.
(A) A dendrogram showing relative nucleotide substitutions between the different murine FABP genes. Myelin P2 (FABP8) is the closest relative to FABP9 (60% sequence homology), both diverging from Epidermal-FABP (FABP5). (B) Three dimensional structure for murine FABP9 constructed based on homology to bovine FABP8, satisfying spatial restraints. The structure is comprised of two α-helices and one β-barrel. The beta barrel forms a hydrophobic pocket for accepting/binding lipids. Note the two cysteine residues in one of the α-helices that might play a role in disulfide bonding of FABP9 protein to structural elements (Arrowheads in inset). (C) Sequence alignments for mouse, rat and human FABP9 proteins showing conserved amino acids (shaded black). (D) Multiple tissue immunoblot showing specific expression of FABP9 in the testis. Lanes: 1. brain, 2. heart, 3. lung, 4. liver, 5. kidney, 6. spleen, 7. intestine, 8. muscle, 9. ovary, 10. uterus, 11. testis, 12. skin, 13. fat.
Another functional role for FABP9 in regulating programmed cell death in germ cells has been suggested (Kido and Namiki, 2000). In their study, FABP9 was shown to localize to germ cells undergoing apoptosis. Supporting this observation, transgenic mice overexpressing FABP9 showed an increase in multinucleate symplasts indicative of apoptosis in elongated spermatids, and a reduction in the number of sperm carrying the transgene (Kido et al., 2005). However, fertility remained unaffected in these mice. These studies indicate that FABP9 could be involved in several regulatory processes during germ cell development.
Moreover, a recent mass spectrometric study reported that FABP9 was the only protein showing a transition from a completely non-phosphorylated state in non-capacitated sperm to a Ser3 phosphorylated state in capacitated sperm (Platt et al., 2009). This also suggested an unknown but functional property of potential importance for FABP9 in mature sperm.
In this study, we examined the functional phenotype resulting from the loss of FABP9 by the targeted disruption of the FABP9 gene in mice. Based on these reports, we hypothesized that defects might be observed in germ cell development, interactions of the cytoskeleton and membranes in the sperm head, and/or capacitation or fertilization. Of interest, our results showed that FABP9 was not absolutely required for successful spermatogenesis and sperm function.
Materials and Methods
Reagents and animals
All reagents were purchased from Sigma (St. Louis, MO), unless otherwise noted. Commercial antibodies used were against α–tubulin (Calbiochem, San Diego, CA) and phosphorylated tyrosine (clone 4G10, Millipore, Lake Placid, NY). The EGFP-conjugated domain 4 of perfringolysin O (PFO-D4) used for localizing focal enrichments of sterols was synthesized as described previously (Selvaraj et al., 2009). Polyclonal antisera against murine FABP9 were raised in two rabbits at Quality Controlled Biochemicals (Hopkinton, MA) using the antigenic epitope [Ser40-Cys58] specific for FABP9. Lipid standards were from Matreya LLC (Pleasant Gap, PA). Pregnant mare serum gonadotropin (PMSG; Calbiochem) and human chorionic gonadotropin (hCG; Calbiochem) were used for superovulation. Mice of the following strains, C57BL/6, 129Sv and B6D2F1 were purchased from the Jackson Laboratories (Bar Harbor, ME).
Protein modeling
Three dimensional homology models for FABP9 were constructed by means of comparative protein structure modeling by satisfying spatial restraints (Fiser et al., 2000; Sali and Blundell, 1993). Bovine FABP8 [PDB ID: 1pmp; (Cowan et al., 1993)] and equine FABP8 [PDB ID: 1yiv; (Hunter et al., 2005)] that had the highest sequence homologies (63.4% and 61.8% respectively) and whose crystal structures were known were used. Structural modeling was carried out using the automated alignment algorithms of Modeller 9v2 (http://www.salilab.org/modeller/) (Eswar et al., 2003; Marti-Renom et al., 2000) to derive a homology-based model. The output was visualized using the graphical interface of PyMOL v1.0 (DeLano Scientific, Palo Alto, CA).
Human FABP9
A human testis large-insert cDNA library (BD biosciences, Palo Alto, CA) was used to clone and sequence the human FABP9 (hFABP9). Based on results from the NCBI-Gnomon gene prediction method, primers (5’ATGGTTGAGCCCTTCTTGGGAAC and 5’TCACACCTTTTCGTAGATTCTGGTG) were designed to amplify the putative hFABP9. The resulting PCR product was then cloned using a Topo TA cloning kit (Invitrogen) according to the manufacturer’s instructions and sequenced.
Testicular expression of FABP9
For examining developmental expression of FABP9 in the postnatal testis, samples were collected every two days starting from postnatal day 2 to postnatal day 36 and stored in liquid nitrogen. Testis lysates were prepared by homogenization in RIPA buffer (25 mM TrisHCl pH 7.6, 150 mM NaCl, 1% NP-40, 1% sodium deoxycholate, 0.1% SDS) and protein concentration was estimated using a bicinchoninic acid protein assay kit (Thermo Scientific, Rockford, IL). Equal concentrations of protein (150 µg) from each sample were separated by SDS-PAGE, transferred to PVDF membrane and immunoblotted for the presence of FABP9. For examining spermatogenic stage-specific expression of FABP9, murine testes were decapsulated and incubated in PBS containing 0.75 mg/ml collagenase in a shaking water bath at 33°C for 30 minutes. The resultant preparation of seminiferous tubules was then washed to remove collagenase and interstitial cells. The separated tubules were visualized under a Nikon SMZ microscope (Melville, NY) and regions containing different stages of the cycle of the seminiferous epithelium (stages I–III, IV–VI, VII–IX and X–XII) were microdissected and collected as described (Kotaja et al., 2004). Lysates were made by homogenization and boiling in sample buffer. Following protein assay, 75 µg of proteins from each segment were separated by SDS-PAGE, transferred to PVDF membrane and immunoblotted for the presence of FABP9.
Sperm collection and handling
Murine sperm were collected from the cauda epididymides by a swim-out procedure as described previously (Travis et al., 2001b) in a modified Whitten’s medium [MW; 22 mM HEPES, 1.2 mM MgCl2, 100 mM NaCl, 4.7 mM KCl, 1 mM pyruvic acid, 4.8 mM lactic acid hemi-calcium salt, 5.5 mM D-glucose, pH 7.35; (Travis et al., 2001a)]. All steps of collection and washing were performed at 37°C, using large orifice pipette tips when handling sperm to minimize membrane damage.
Sperm biochemical fractionation
Membranes from sperm were collected with or without the use of detergents by methods previously described (Travis et al., 2001b) with minor modifications as described below. Sperm (10 × 106 cells) were subjected to 20 bursts of sonication for 10 seconds each at output 6 using a Branson Sonifier 450 (Branson Ultrasonics Corporation, Danbury, CT) in the presence of 5x protease inhibitors (Roche Applied Science, Indianapolis, IN). Care was taken to avoid frothing and to keep the sample cool. The cell lysate was then centrifuged at 10,000 ×g for 10 minutes and the supernatant was carefully collected; the remaining coarse pellet of cellular debris formed the P10 fraction. The supernatant was again centrifuged at 100, 000 ×g for 1 hour. The supernatant (S100 fraction) was carefully separated and the resulting pellet of sperm membrane vesicles formed the P100 fraction. Soluble proteins in the S100 fraction were precipitated using 4% trichloro-acetic acid (v/v) followed by neutralization with Tris-buffer. Proteins from the different fractions were boiled in sample buffer, separated by SDS-PAGE, transferred to a PVDF membrane and immunoblotted for the presence of FABP9.
Histology and indirect immunofluorescence
Testes fixed in Bouin’s solution were processed, embedded in paraffin and sectioned at 4 µm slice thickness. For routine histological examination, sections were stained with hematoxylin and eosin. For localization of FABP9 in testis sections, slides were de-paraffinized, hydrated and then subjected to antigen retrieval in 0.01 M citrate buffer. Nonspecific binding of antibodies was blocked using 5% normal goat serum (NGS), and then samples were incubated with anti-FABP9 antibody (1:100). After incubation, slides were washed in PBS and incubated with an Alexafluor-555-conjugated secondary antibody (1:500). Fluorescent nuclear counterstaining was carried out using Sytox® Green (1 µM final concentration; Invitrogen) for 10 minutes. Slides were then washed in PBS and mounted using Prolong-Gold mountant (Invitrogen).
For localization of FABP9 in epididymal sperm, cells collected in MW medium were allowed to attach to coverslips and fixed/permeabilized using either 1:1 acetone:methanol mixture for 60 seconds at −20°C, or 4% formaldehyde (30 minutes) and 0.1% TX-100 (2 minutes). Coverslips were air-dried, rehydrated in PBS and blocked for 30 minutes with 5% NGS in PBS. Samples were incubated with anti-FABP9 antibody (1:100 dilution). They were then washed with PBS and incubated with Alexafluor-488-conjugated secondary antibody (1:500 dilution). Coverslips were then rinsed and mounted on slides with a GVA-based mountant.
FABP9 targeting construct
Murine bacterial artificial chromosome clone bMQ75a12 (Genome Research Ltd., Wellcome Trust Sanger Institute, Cambridge, UK) containing the 129S7 strain genomic locus of FABP9 was transformed into a recombineering competent strain of bacteria [EL350; (Liu et al., 2003)]. A 15 Kb genomic sequence (~6 Kb upstream and downstream of the FABP9 locus) including the FABP9 locus was retrieved into plasmid PL253. PL253 carried a mc1-driven thymidine kinase cassette for negative selection of ES cells. Plasmid PL452 with a neo cassette flanked by loxP sites (loxP-Pgk-em7-Neo-loxP) was used to recombineer and replace exons 1 and 2 of the FABP9 gene in PL253 (Liu et al., 2003). Bacteria EL350 and plasmids PL253 and PL452 were generous gifts from Neal Copeland, Institute of Molecular and Cellular Biology, Singapore.
Targeting, selection and microinjection of ES cells
TC-1 embryonic stem cells (Deng et al., 1996) were electroporated with SalI-linearized construct and selected in media containing G418 and FIAU as described previously (Weiss et al., 2000). Genomic DNA from 85 drug-resistant colonies was screened for homologous recombination at the FABP9 locus by southern blot hybridization after restriction digestion (BamHI) with an external probe [espuF: 5’ CCAGGTCTCAACATTGAGGC; espuR: 5’ CATGTCTAGCTCCAAATCCC], and one correctly targeted clone was identified. This correct targeting of the heterozygous clone was confirmed by performing additional southern blots using an alternative restriction enzyme digest (PsyI), as well as an internal neo probe. ES cells from this targeted clone were microinjected into C57BL/6 blastocysts and transferred into pseudopregnant C57BL/6 recipients. Three chimeric male mice were identified by their characteristic agouti coat color. They were mated to C57BL/6 females and the germline transmission of the targeted FABP9 allele was determined by coat color of the pups and southern blot analyses. Crossing heterozygous pairs generated mice homozygous for the targeted FABP9 allele. Mice used for phenotypic analysis were in a 129/B6 mixed background. Inbred 129Sv mice homozygous for FABP9 deletion were also derived to confirm phenotype. Mice were genotyped with primers designed to flank the recombination site to detect deletion of exons 1 and 2 of the FABP9 gene [F9: 5’AGGTGGGAAAGCCTCTGATT; F9 WT: 5’ AGGCATTCACAACCGAAAAC; F-9 KO: 5’ GCCAGAGGCCACTTGTGTAG].
Phenotypic analysis
Parameters including body weight, testis weights and seminal vesicle weights were measured in 12-week-old mice. Cauda epididymal sperm counts were estimated using a hemocytometer. Breeding trials were conducted to quantify litter sizes from FABP9−/− × FABP9−/− vs WT × WT matings. For comparing litter sizes 10 pairs each of WT and KO mice were used; the remainder of the phenotypic analyses were performed with 10–15 mice for each group. Statistical analysis was performed using JMP 8 (SAS Institute, Cary, NC). For the above traits, numeric differences between WT and FABP9−/− sperm were compared using a t test (p≤0.05 was considered significant).
Sperm abnormalities
Sperm morphology at 400× magnification was evaluated using a dark field microscope and scored as (a) apparently normal, when morphology appeared unaffected, (b) bent tail, when the flagellum was bent in a hair-pin fashion in the distal mid-piece or at the annulus, (c) cytoplasmic droplet, including both proximal and distal droplets of residual cytoplasm, and (d) abnormal head, when there was an obvious defect in sperm head morphology. These evaluations were carried out on least 100 sperm/mouse counting all the sperm in each microscopic field examined. A z-test for proportions was used to compare the mean differences of abnormal sperm populations between WT and FABP9−/− sperm (p≤0.05 was considered significant). Closer examination of sperm head abnormalities was also carried out using electron microscopy as described below.
Assessment of membrane organization
To label focal enrichments of membrane sterols in live sperm, cells were incubated with PFO-D4 (4 µg/ml) in MW medium for 20 minutes at 37°C. A stage-mounted incubation chamber (LiveCell, Neue Product Group, Westminister, MD) was used along with an objective heater (Bioptechs, Butler, PA) to maintain sperm at 37°C during imaging. Imaging was performed using a Nikon Eclipse TE 2000-U microscope (Nikon, Melville, NY) equipped with a Photometrics Coolsnap HQ CCD camera (Roper Scientific, Ottobrunn, Germany). Images were captured using Openlab v3.1 software (Improvision, Lexington, MA).
Capacitation-associated protein tyrosine phosphorylation
Incubation with different stimuli for capacitation was carried out with 2×106 sperm in 300 µl of medium with 5.5 mM glucose under one of two conditions: (a) MW base medium, (b) MW supplemented with 10 mM NaHCO3 and 3 mM 2-OHCD, for 60 minutes [shown to support both IVF (Travis et al., 2004) and capacitation-induced tyrosine phosphorylation (Travis et al., 2001a)]. After incubation, samples were processed for SDS-PAGE, transferred to a PVDF membrane and immunoblotted for phospho-tyrosine residues.
Scanning electron microscopy
Sperm collected as described above in MW medium were allowed to attach to silicon chips and fixed using 4% formaldehyde and 0.1% glutaraldehyde in 2.5 mM CaCl2 in PBS (Selvaraj et al., 2006) for 30 minutes. Samples were rinsed 5 times in distilled water for 5 minutes each and then freeze dried. The silicon surface with cells was then sputter coated with gold-palladium. Images were collected using a Zeiss LEO 1550 field emission SEM with a Gemini column (Carl Zeiss Inc., Oberkochen, Germany). A general analysis was first performed to examine the morphology of the sperm heads. A specific morphological analysis for the ventral spur was then performed counting only grossly normal sperm and classified as (a) normal, (b) small, or (c) absent for at least 50 sperm/mouse. A z-test for proportions was used to compare the mean differences of ventral spur morphologies between WT and FABP9−/− sperm (p≤0.05 was considered significant).
Transmission electron microscopy
Sperm samples were prepared by fixing sperm in suspension with 2% glutaraldehyde and 4% tannic acid in phosphate buffer (pH 7.0 – 7.5) for 4 to 6 hours at room temperature. Samples were then washed overnight in 0.1 M sodium cacodylate buffer (pH 7.4) with 7% sucrose at room temperature. They were then fixed for 2 hours with 1% OsO4 with 5% sucrose in 0.1 M sodium cacodylate at 4°C. They were treated with 0.5% uranyl acetate containing 4% sucrose for 1 hour at room temperature. Samples were then quickly dehydrated and embedded in epoxy resin. Thin sections were collected on Formvar-carbon-coated nickel grids and stained with 5% aqueous uranyl acetate followed by alkaline lead. Images were then collected using a transmission electron microscope (Philips Tecnai 12 BioTwin, FEI Company, Hillsboro, OR).
In vitro fertilization and sperm competition
TYH medium [118.8 mM NaCl, 4.78 mM KCl, 1.19 mM KH2PO4, 1.19 mM MgSO4 .7H2O, 25 mM NaHCO3, 1.71 mM CaCl2 .2H2O, 5.56 mM D-glucose, 1.01 mM Na-pyruvate, 29.2 mM Na-lactate, 4 mg/ml BSA, 0.05 mg/ml Streptomycin sulfate, 100 IU/ml Penicillin-G potassium, BSA; pH 7.4; (Toyoda et al., 1971)] pre-equilibrated in 5% CO2 at 37°C for 12 hours (pH 7.4) was used for the in vitro fertilization trials. Nineteen-day-old B6D2F1 female mice were super-ovulated by intra-peritoneal injections of PMSG (5 IU) followed after 48 hours by hCG (5 IU) (Runner and Gates, 1954). The females were euthanized 13 hours after hCG injection and oocytes were collected from the oviduct into TYH medium as a droplet under mineral oil (Sydney IVF culture oil, Cook Medical, Bloomington, IN) in a Petri dish. One hour before euthanasia of female mice, sperm from the cauda epididymides were collected from the required males in TYH medium. Sperm were allowed to capacitate at 37°C in a 5% CO2 incubator until oocyte collection. Sperm concentration for each sample was then quantified and sperm were added to the fertilization droplet to attain a final concentration of 1 million sperm/ml (final volume 100 µl). For sperm competition assays either equal numbers of sperm (1:1) from FABP9−/− and WT were introduced into the same fertilization droplet or proportions of 1:4 and 4:1 were used; the final sperm concentration was kept constant at 1 million/ml. The dish was then incubated in a chamber with an atmosphere of 5% O2, 5% CO2 and 95% N2 at 37°C. After 5 hours, eggs were rinsed briefly in fresh TYH medium and transferred to KSOM medium [95 mM NaCl, 2.5 mM KCl, 0.35 mM KH2PO4, 0.2 mM MgSO4, 1.71 mM CaCl2, 25 mM NaHCO3, 10 mM Na-lactate, 0.2 mM D-glucose, 0.2 mM Na-pyruvate, 1 mM glutamine, 0.01 mM EDTA, 1 mg/ml BSA, 1 ml MEM essential amino acids, 0.5 ml MEM non-essential amino acids, 0.05 mg/ml Streptomycin sulfate, 100 IU/ml Penicillin-G potassium, BSA; pH 7.4; (Erbach et al., 1994)] for embryo development. Embryo development to a 4-cell stage was considered as the endpoint for successful fertilization. Embryos derived from sperm competition experiments were treated with 0.5% Pronase (Roche Applied Science, Indianapolis, IN) in TYH medium for 5 minutes to remove any surface adherent sperm and subsequently genotyped to determine the performance of FABP9−/− sperm.
Total lipid extraction and thin layer chromatography
Total lipids were extracted from sperm from 3 mice using the Folch method (Folch et al., 1957). For thin layer chromatography (TLC), lipid extracts were reconstituted in 60:25:4 (chloroform:methanol:water) and spotted on a silica gel 60 plate (Merck, Damstadt, Germany) along with lipid standards and developed with the 60:25:4 solvent system (Wedgwood et al., 1974). Fluorescent bands were imaged after spraying with a primuline solution [0.005% (w/v) primuline in 80% (v/v) acetone; (Wright, 1971)] and excitation using UV-wavelength transillumination.
Fatty acid mass spectrometry
Total lipids were extracted from 12 × 107 sperm using the Bligh and Dyer method (Bligh and Dyer, 1959). Fatty acid methyl esters (FAME) were prepared using sodium hydroxide followed by esterification with boron-trifluoride (BF3) in methanol, and were analyzed by gas chromatography (HP 5890; BPX-70 column, SGE, Austin, TX), using H2 carrier gas as described previously (Sarkadi-Nagy et al., 2003). FA identities were determined by covalent adduct chemical ionization tandem mass spectrometry (Lawrence and Brenna, 2006; Van Pelt and Brenna, 1999) and the quantitative profiles were calculated using methyl-17:0 as an internal standard. The results were calibrated using response factors derived from an equal weight FAME mixture. FA concentrations were expressed as percentage weight of total FA from 14 to 22 carbons. A t-test was used to compare quantitative differences of individual lipid species between WT and FABP9−/− sperm.
Results
Structural features and expression of FABP9
FABP9 is the most abundant protein of the perinuclear theca and perforatorium of murine sperm. It shares significant sequence homologies with different members of the FABP family (Fig. 1A). Comparing nucleotide substitutions, murine FABP9 is closely related to FABP8/Myelin P2. Based on its 3D homology model, we find that FABP9 shares the conserved features of all other FABPs. It has two α-helices capping an anti-parallel β-sheet that forms a barrel (Fig. 1B). The lipid-binding site is formed by the hydrophobic concavity within the β-barrel. The N-terminal tail formed by a conserved Phe5 residue forms a characteristic lid for the hydrophobic core. One unique feature of murine FABP9 compared to other FABPs was the presence of two cysteine residues (Cys28 and Cys35) in helix-2 (Fig. 1B-arrowheads in inset). These cysteine side chains are present on the surface of the protein away from the opening of the hydrophobic core giving it the potential ability to form disulfide bonds with structural elements in sperm without affecting its lipid binding property.
Primers designed for the predicted human FABP9 gave a product at the expected length (~400 bp). On sequencing, this product yielded a sequence homologous to known sequences of mouse and rat FABP9. The sequence mapped to a locus in human chromosome 8. On comparing amino acid sequences, human FABP9 showed 71% homology to mouse and 66% homology to rat FABP9 (Fig. 1C). The two cysteine residues in helix-2 in mouse and rat FABP9 were not conserved in the human FABP9 sequence. Immunoblots of various murine tissues showed the testis-specific expression of FABP9 (Fig. 1D).
Developmental expression and biochemical characterization of FABP9
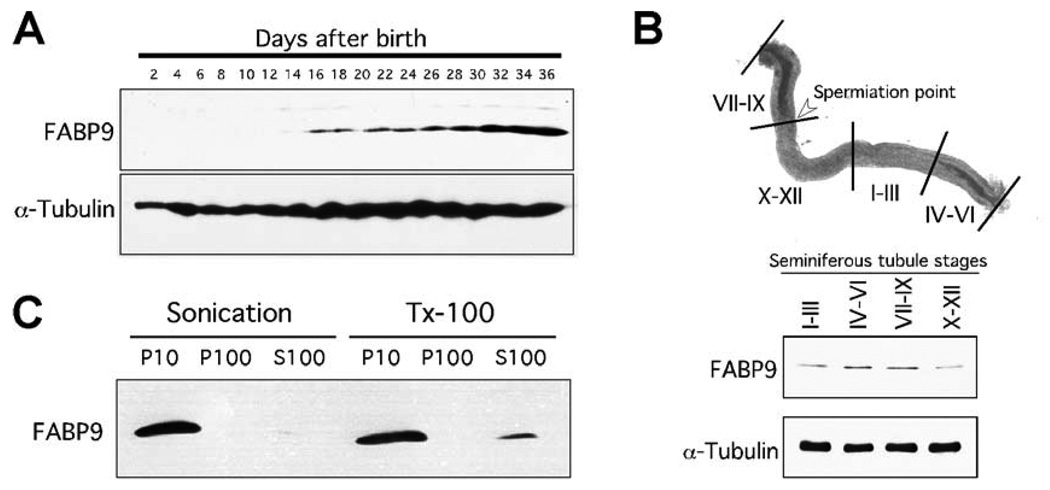
During post-natal testicular development, FABP9 expression started from day-16 consistent with the appearance of late pachytene spermatocytes (Fig. 2A) (Bellve et al., 1977). On dissected seminiferous tubules, FABP9 expression was more abundant in stages containing germ cells in advanced stages of spermiogenesis (Fig. 2B).
Fig. 2. Characterization of FABP9.
(A) FABP9 protein expression during post-natal testicular development. Immunoblot of testicular proteins collected every 2 days from mice aged day-2 to day-36 shows FABP9 protein expression starting at day-16 consistent with the appearance of pachytene spermatocytes. (B) Expression of FABP9 in different stages of germ cells in the seminiferous tubules. Highest expression was seen in tubular stages IV–VI and VII–IX which both contain germ cells in advanced stages of spermiogenesis. (C) FABP9 association with different sperm biochemical fractions separated by either physical shear (sonication) or detergent (TX-100). With sonication, FABP9 was found only in the P10 fraction that represents insoluble cytoskeletal structures and organelles. With TX-100, FABP9 was also seen predominantly in the P10 fraction although a small percentage solubilized and was found in the S100 fraction. FABP9 did not associate with the P100/membrane fraction.
Biochemical fractionation of FABP9 showed that unlike other FABPs, FABP9 was not soluble but was strongly associated with structural elements in mature sperm (Fig. 2C). This association was not disrupted by vigorous sonication, but a small fraction of FABP9 could be solubilized using TX-100. The same experiment was also performed using isolated germ cells with similar results (data not shown) suggesting that FABP9 predominantly exists in association with cytoskeletal structures even during early developmental events during spermatogenesis, consistent with its localization to the perinuclear theca and the perforatorium.
Generation and phenotypic analysis of FABP9−/− mice
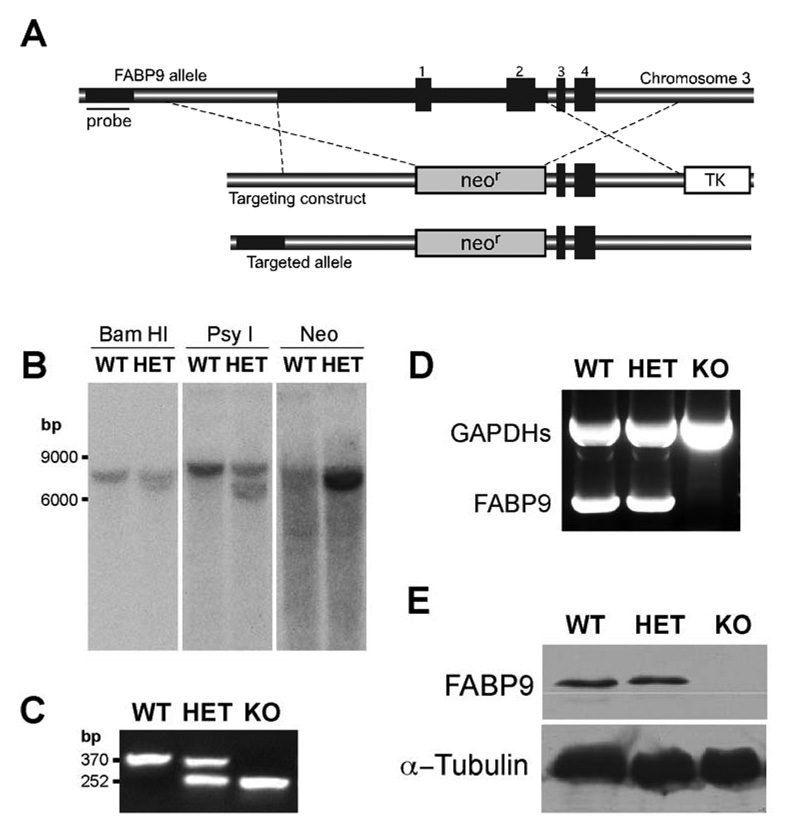
To explore potential roles of FABP9 in spermatogenesis, sperm structure and membrane organization, and fertilization, we generated mutant mice that lacked FABP9 by a targeted deletion achieved by homologous recombination in embryonic stem cells. The targeting construct was designed to replace ~2.7 kb of the FABP9 locus including exons 1 and 2 and a large part of the promoter region (Fig. 3A). In addition to preventing transcription of this locus, this would disrupt the synthesis of the entire FABP9 protein (132 amino acids). The genotypes of targeted embryonic stem cell clones were screened by Southern blot analysis of genomic DNA (Fig. 3B) and one heterozygous (FABP9+/−) clone was identified. Genomic DNA from mice generated from transmitting chimeras was also screened using Southern blot analysis and genotyping PCR (Fig. 3C). Mating between heterozygous F1 siblings was set up to derive homozygous mice (FABP9−/−). RT-PCR analysis indicated the absence of FABP9 mRNA in the FABP9−/− testis (Fig. 3D). In addition, protein extracts of FABP9−/− testis completely lacked the 15-kDa protein corresponding to FABP9 (Fig. 3E).
Fig. 3. Targeted disruption of the murine FABP9 gene.
(A) Schematic showing the FABP9 locus, the targeting construct and homologous recombination. The FABP9 gene consists of 4 exons (indicated 1–4). The targeting construct was designed with upstream and downstream regions of homology on either side of a neomycin resistance cassette (neor) replacing exons 1 and 2. In this construct, the neor served for positive selection and a thymidine kinase cassette (TK) for negative selection. The correctly targeted FABP9 allele shows integration of the neor cassette in the genomic locus. The sequence upstream of the recombination site used for screening correctly targeted clones is indicated (Probe). (B) Screening for the targeted FABP9 locus using southern blots. Genomic fragments generated by digestion using BamHI and PsyI were probed for a shift in size of the targeted fragment. The correctly targeted clone was subsequently verified using a neo probe for a single and specific recombination event. (C) Gel showing genotyping PCR for verifying the targeted FABP9 locus. The WT allele produced a 370 bp band and the KO allele produced a 252 bp band. (D) Gel showing RT-PCR products for FABP9 mRNA expression in WT, HET and KO testis. FABP9 mRNA was not expressed in KO mice. A germ cell specific glyceraldehyde 3-phosphate dehydrogenase (GAPDHs) was used as a reaction control. (E) FABP9 immunoblot showing protein expression in WT, HET and KO testis extracts. There was no FABP9 expressed in KO mice. HET mice where one allele of the FABP9 gene was disrupted produced a comparable level of expression to WT mice.
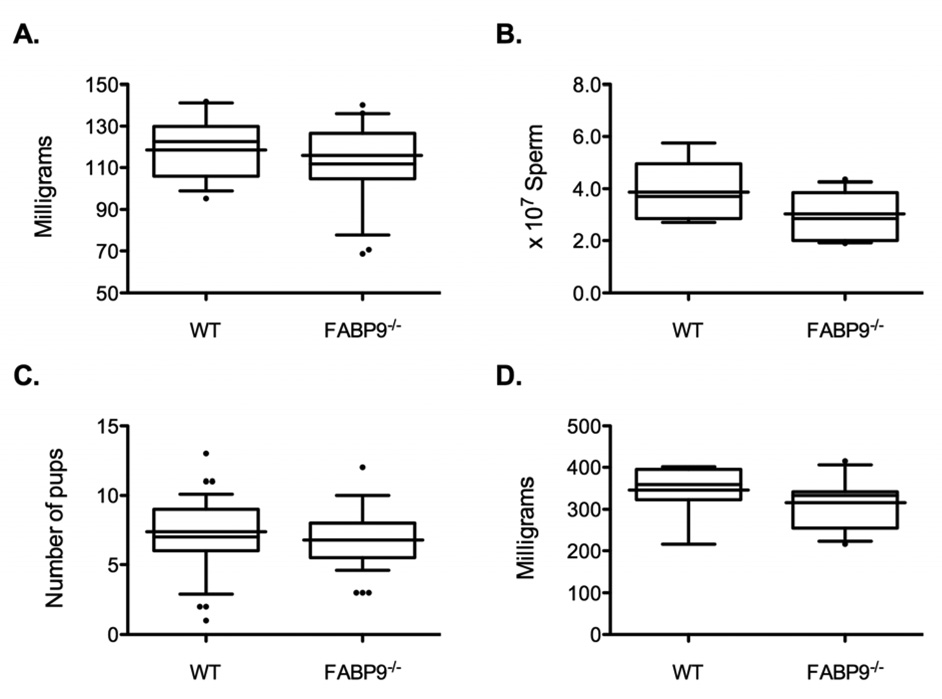
Mating of FABP9+/− male and female mice yielded the expected Mendelian frequency of FABP9−/− mice [FABP9−/−: FABP9+/−: FABP9−/− = 27 (22.0%): 61 (49.6%): 35 (28.5%) calculated with 123 offspring from 16 litters]. Both male and female FABP−/− mice appeared normal in behavior and body condition. Body weights (not shown) and testis weights (Fig 4A) of FABP9−/− mice were not significantly different from age-matched WT cohorts. Histological analysis of testis sections did not reveal any architectural differences or pathologies in the FABP9−/− mice (data not shown). Sperm production was not significantly different between FABP9−/− and WT mice (Fig. 4B). Both male and female FABP9−/− mice were fertile and produced litter sizes (6.9 ± 0.4 pups/litter) equivalent to those of WT mice (7.2 ± 0.3 pups/litter; Fig. 4C). Seminal vesicle weights were not different between FABP9−/− and WT mice (Fig. 4D).
Fig. 4. Phenotypic analysis of FABP9−/− mice.
Box plots show the spread of values for the different parameters analyzed. The lower and upper ends of the box mark the 25th and 75th quantiles; the median is represented as a horizontal line within the box, and the mean as a horizontal line through the box. Vertical whiskers extend from the ends of the box to the 10th and 90th quantiles. Comparisons for: (A) testis weights, (B) cauda epididymal sperm counts, (C) litter sizes and (D) seminal vesicle weights are shown (n=9–11/group). For these comparisons, 12-week-old FABP9−/− and WT mice were used for sperm counts and other morphometric parameters; litter sizes were obtained from breeding trials.
Spermatogenesis in FABP9−/− mice
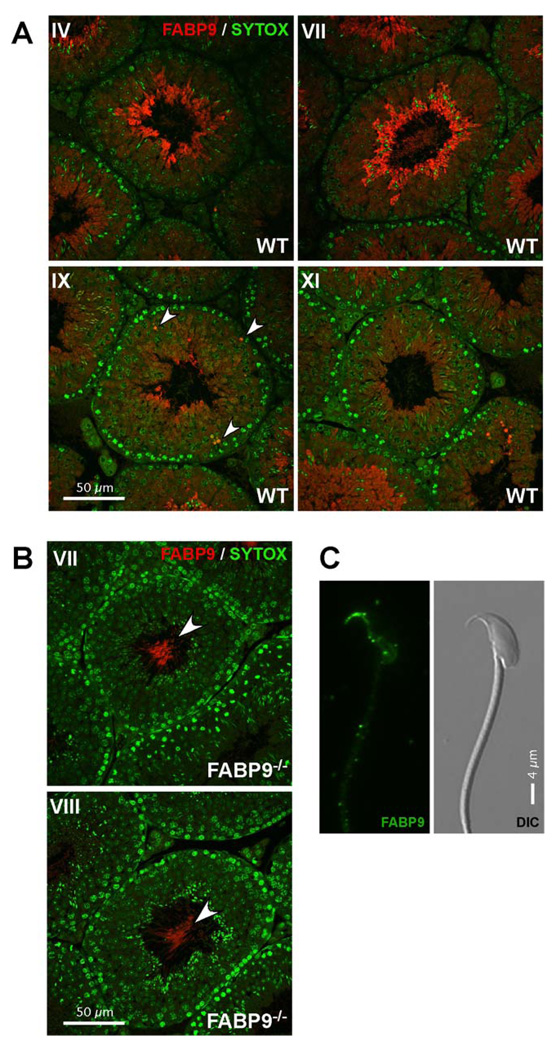
Immunofluorescence labeling of FABP9 was observed in advanced stages of germ cell development. FABP9 protein expression appeared to be particularly pronounced in elongating spermatids and more mature testicular germ cells (Fig. 5A). FABP9 was localized in part to the cytoplasm in elongating spermatids, and it was also expressed abundantly in certain rare spermatocytes (arrowheads, Fig. 5A). Germ cell development was unaffected in FABP9−/− testes and we did not detect any pathology associated with germ cells or the seminiferous epithelium. Immunofluorescence localization confirmed the absence of FABP9 in the knockout testes (Fig. 5B). However, non-specific labeling by the polyclonal antibody was seen in the principal piece of the developing sperm in tubules containing advanced stages of spermiogenesis (arrowheads; Fig. 5B). In cauda epididymal wild type sperm, the perforatorium was more intensely labeled with FABP9 with a weaker signal detected in the post-acrosomal region of the perinuclear theca (Fig. 5C). This was seen only after acetone:methanol fixation and permeabilization suggesting a deep-seated association between FABP9 and these structures. We also detected the non-specific labeling of the principal piece as seen in testicular sperm (not shown).
Fig. 5. Localization of FABP9 in developing male germ cells and sperm.
(A) FABP9 localization in testis sections. Seminiferous tubule cross-sections show FABP9 protein expression was abundant in late elongating spermatids. Earlier in male germ cell development, FABP9 localization was cytoplasmic and diffuse in spermatids. Labeling seen in the principal piece at advanced stages of spermiogenesis was deduced to be non-specific (based upon labeling observed in FABP9−/− testes). Arrowheads denote labeling of isolated, rare spermatocytes. (B) Specific labeling for FABP9 was absent in testis sections from FABP9−/− mice. Non-specific labeling was observed in the principal piece of testicular sperm (arrowheads). (C) FABP9 localization in mature sperm. In the permeabilized sperm head, FABP9 labeled the perforatorium intensely and the post-acrosomal peri-nuclear theca weakly. A corresponding DIC image is also shown.
Abnormalities in FABP9−/− sperm
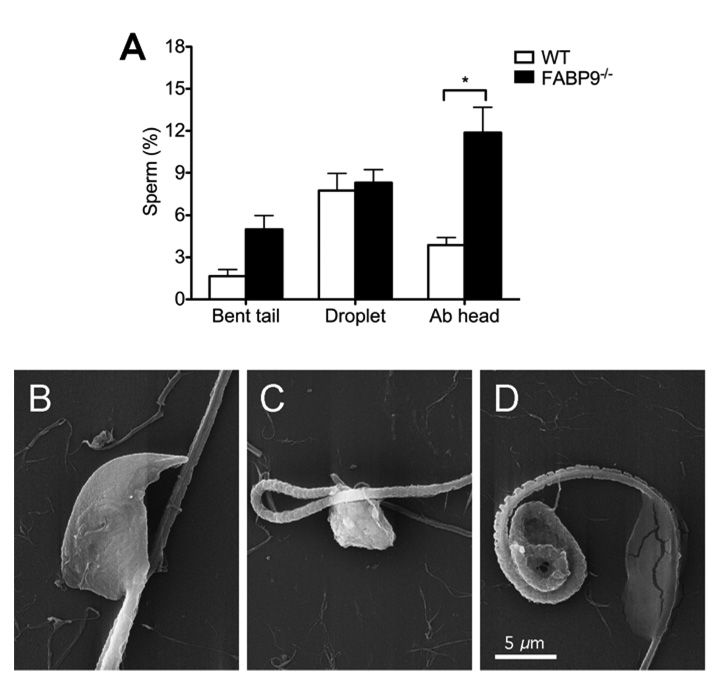
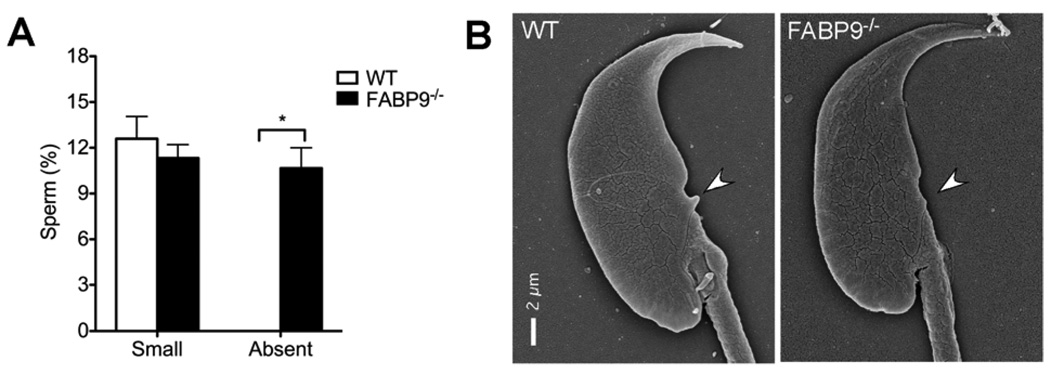
Comparison of sperm morphology between FABP9−/− and WT mice revealed significant but modest differences in numbers of abnormal heads in FABP9−/− sperm (3.88 ± 0.54 % in WT vs 11.88 ± 1.81 % in FABP9−/− sperm; Fig. 6A; p=0.05). Sperm head abnormalities included misshaped apical parts of the sperm head and more severe shape distortions involving both apical and distal parts of the head (Fig 6B, C and D). At the ultrastructural level, significantly higher numbers of FABP9−/− sperm were also found to completely lack a morphological feature of the sperm head called the ventral spur (0% in WT vs 10.7 ± 1.3 % in FABP9−/− sperm; Fig. 7A; p=0.004). Representative SEMs of a WT sperm head showing normal ventral spur morphology and a FABP9−/− sperm head that is lacking a ventral spur are shown (Fig. 6B).
Fig. 6. Sperm abnormalities in FABP9−/− mice.
(A) Abnormalities in sperm from WT and FABP9−/− mice were evaluated at 400× magnification using dark-field microscopy. Sperm were counted and categorized as follows: tails bent at the connecting piece, midpiece or principal piece (Bent tail), carrying either a proximal droplet or distal cytoplasmic droplet (Droplet), or with abnormalities of the sperm head (Ab head) [n=7 mice/group]. Differences between WT and FABP9−/− sperm regarding head abnormalities were statistically significant (* P=0.05). (B) SEM showing a morphological abnormality of head shape in FABP9−/− sperm. (C) SEM showing a severe abnormality of the sperm head and a bent tail in FABP9−/− sperm. (D) SEM showing severe abnormality of a coiled midpiece and a distal cytoplasmic droplet in FABP9−/− sperm.
Fig. 7. Ultrastructural morphology of the ventral spur in FABP9−/− sperm.
Ventral spur morphology was evaluated in otherwise morphologically normal WT and FABP9−/− sperm heads. Deviations from normal ventral spur sizes were qualitatively classified as “Small” or “Absent” and counted. FABP9−/− mice had significantly higher numbers of sperm where ventral spurs were extremely rudimentary or absent (P<0.01) [n=3 mice/group]. (C) Panels show SEM of an apparently normal WT sperm head and a FABP9−/− sperm head showing underdevelopment of the ventral spur.
Sperm membrane organization and sperm capacitation in FABP9−/− mice
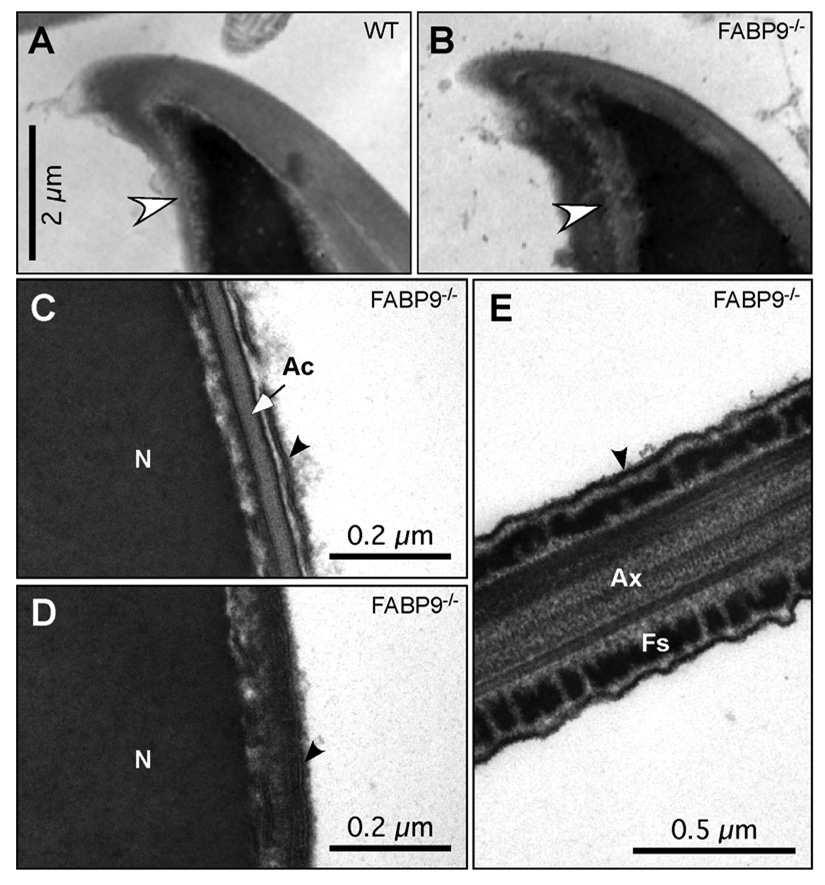
To investigate whether FABP9 was responsible for tethering membrane components to the perinuclear theca, we examined the ultrastructural organization of the perforatorium, perinuclear theca and associated membranes in WT and FABP9−/− sperm. From the sections obtained, although we could not examine the dense mass of perforatorial material and the rods that form the perforatorium, we did not find any difference in structural contours between FABP9−/− compared to WT sperm heads (Fig. 8A and B). Transmission electron micrographs (TEMs) also showed normal membrane ultrastructure, with the plasma membrane positioned in close apposition to the underlying acrosome and perinuclear theca of the sperm head and fibrous sheath of the sperm tail in FABP9−/− sperm (Fig. 8C, D and E).
Fig. 8. Membrane tethering, and perforatorium and perinuclear theca morphology in FABP9−/− mice.
Ultrastructural examination of membrane attachment to the underlying structures revealed no abnormalities in FABP9−/− sperm (panels B–E). (A) The apical hook region of a WT sperm head. (B) The same region of a FABP9−/− sperm head is shown. Note that the two views are slightly oblique to one another, but both show the apical acrosome extending up the convex curve, both show the extension of the nucleus into the hook, and both show normal tethering of the acrosome to the underlying structure and normal tethering of the plasma membrane overlying the acrosome. Sperm ultrastructure and membrane tethering were also normal in null sperm in the equatorial region (C), the post-acrosomal region (D), as well as the principal piece (E). Black arrows point to the bilayer structure of the plasma membrane. [N: nucleus; Ac: acrosome; Ax: axoneme; Fs: fibrous sheath]
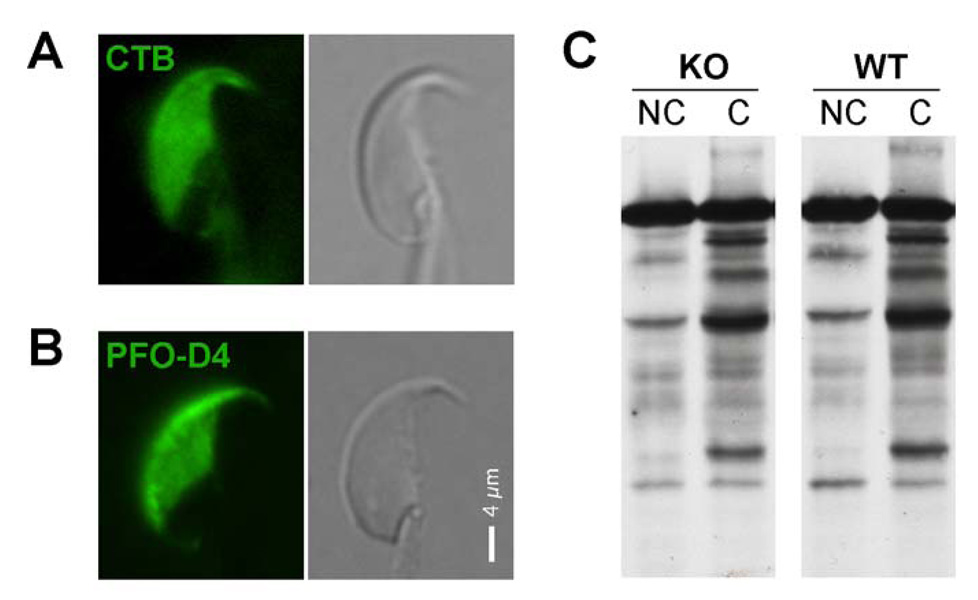
To examine lipid distribution in addition to membrane tethering, we localized GM1 and focal enrichments of membrane sterols to evaluate the known micron-scale lipid segregation in the plasma membrane of the sperm head. Sperm from FABP9−/− mice did not show any defects in the segregation of GM1 and enrichments of sterols in the plasma membrane overlying the acrosome (Fig. 9A and Fig. 9B respectively). We also investigated the capability of FABP9−/− sperm to respond to stimuli for capacitation by evaluating the capacitation-associated protein tyrosine phosphorylation profile. Sperm from FABP9−/− mice showed a normal response to bicarbonate ions and 2-OHCD in the medium and displayed hyperactivated motility (data not shown) and a normal profile of tyrosine phosphorylated proteins (Fig. 9C) as seen in capacitated sperm.
Fig. 9. Membrane organization and signaling in FABP9−/− mice.
Localization in FABP9−/− sperm of (A) glycosphingolipid GM1 and (B) focal enrichments of membrane sterols. EGFP-conjugated D4 of perfringolysin O (PFO-D4) was used to localize focal enrichments of sterols and CTB was used to localize GM1. These segregations to the plasma membrane overlying the acrosome were consistent both with previous reports (Selvaraj et al., 2006; Selvaraj et al., 2009) and with WT cohorts (not shown). (C) Tyrosine phosphorylation of FABP9−/− and WT sperm proteins after exposure to stimuli to induce capacitation. Capacitation-associated phosphorylation profile of sperm proteins was not affected in FABP9−/− sperm compared to WT. [NC: non-capacitated; C: capacitated]
In vitro fertilization performance of FABP9−/− sperm
Our breeding data showed no differences between WT and FABP9−/− males in fertility or litter size. However, in natural matings, ejaculated sperm are exposed to significant lipid modifications as a result of exposure to seminal plasma and the uterine and oviductal fluids. These modifications might compensate for or rescue specific lipid deficits and thereby mask possible functional phenotypes in FABP9−/− sperm. Therefore, we examined the functional ability of epididymal FABP9−/− sperm to fertilize oocytes in vitro. In vitro fertilization (IVF) trials were carried out using FABP9−/− and WT sperm either separately or in competition with each other. Our results showed no significant differences in overall fertilization rates when FABP9−/− and WT sperm were used separately (Table 1). When sperm were used in competition, the numbers of WT and FABP+/− embryos obtained were 9:8 for equal sperm proportions (1WT:1KO; n=2), 6:23 when used at 1WT:4KO (n=2) and 19:1 when used at 4WT:1KO (n=2). These data confirmed that FABP−/− sperm did not exhibit any significant deficits in fertilizing mature oocytes in vitro.
Table 1.
In vitro fertilization performance of FABP9−/− sperm
| Trial # | Genotype | Fertilization rate† | % Fertilized† |
|---|---|---|---|
| 1 | WT | 10/11 | 90.9 |
| KO | 39/46 | 84.7 | |
| 2 | WT | 7/8 | 87.5 |
| KO | 12/12 | 100 | |
| 3 | WT | 41/49 | 83.6 |
| KO | 33/40 | 82.5 | |
Determined based on the number of 4-cell embryos in culture.
Lipid profiles of FABP9−/− sperm
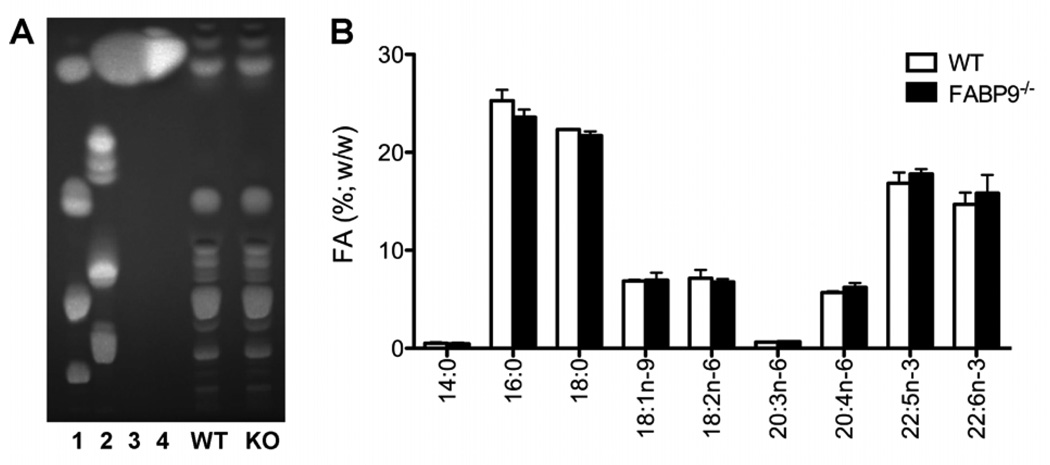
To investigate whether the deficiency of FABP9 led to changes in sperm lipids, we analyzed total lipids and performed a detailed analysis of fatty acids in WT and FABP9−/− sperm. Based on phospholipid, sphingolipid and fatty acid standards, several classes of lipids were observed in the analysis of total lipids using TLC. Comparison of relative profiles of total lipids did not show any detectable differences between WT and FABP9−/− sperm (Fig. 10A). Specific analysis for fatty acids using covalent adduct chemical ionization tandem mass spectrometry detected 14:0, 16:0, 18:0, 18:1n-9, 18:2n-6, 20:3n-6, 20:4n-6, 22:5n-3 and 22:6n-3 fatty acids in both WT and FABP9−/− sperm. Comparison of the quantitative fatty acid profile of WT and FABP9−/− sperm showed no significant changes in different fatty acid concentrations (Fig. 10B).
Fig. 10. Lipid profiles of FABP9−/− and WT sperm.
(A) Total lipids were extracted from sperm and developed on a silica gel TLC plate using 60:25:4 (chloroform:methanol:water) as the solvent system. Lipid standards indicated are as follows. (1) Polar lipid mix: cholesterol, phosphatidyl ethanolamine, lecithin, lyso-lecithin; (2) Sphingolipid mix: cerebrosides, sulfatides, sphingomyelin; (3) Fatty acid mix: methyl palmitate, methyl stearate, methyl oleate-9, methyl linoleate-9,12, methyl linoleate-9,12,15; (4) Poly-unsaturated fatty acid (PUFA) mix: C14, C16, C16:1n-7, C18:1n-7, C18:1n-9, C18:2n-6, C20:1n-9, C18:4n-3, C22:1n-11, C20:5n-3, C22:1n-9, C22:5n-3, C22:6n-3. Fluorescent bands were imaged after spraying with a primuline solution. (B) Quantitative profiles of sperm fatty acids were determined by covalent adduct chemical ionization tandem mass spectrometry and calculated using methyl-17:0 as an internal standard. Detectable fatty acids were 14:0, 16:0, 18:0, 18:1n-9, 18:2n-6, 20:3n-6, 20:4n-6, 22:5n-3 and 22:6n-3. There were no significant differences in fatty acid levels between WT and FABP9−/− sperm.
Discussion
In both mouse and rat sperm, previous studies have shown that FABP9 is the major protein of the perforatorium and perinuclear theca (Korley et al., 1997; Oko and Morales, 1994). Therefore, FABP9 has been suggested to be a critical building block in the organization of the perinuclear theca and attachment of sperm plasma membrane to those underlying structures. However, both the assembly and functions of sperm ultrastructural elements such as the perinuclear theca and perforatorium are inadequately understood. The facts that FABP9 is a testis-specific member of this family of proteins and that it is phosphorylated during capacitation further suggested to us the likely importance of FABP9 in spermatogenesis and/or the function of mature sperm.
Phylogenetic analysis indicates that FABP9 potentially evolved from a gene duplication event along with FABP4, FABP5 and FABP8 (Karanth et al., 2008). Despite the high level of homology shared by FABPs, sequence analysis of FABP9 showed unique elements not present in other FABPs. Two cysteine residues (Cys28 and Cys35) were found to be unique in mouse and rat FABP9. Formation of disulfide bonds by these conserved residues could mark an important functional transition in this FABP from being a soluble transport protein to an anchored lipid tethering protein. It is also well known that disulfide bonds play critical roles in the organization of the sperm head perinuclear theca (Calvin and Bedford, 1971), suggesting that it might anchor sperm membranes to this underlying support. Our biochemical characterization of murine FABP9 supported this theory by showing that FABP9 was not soluble but instead was tethered to cytoskeletal elements in sperm, such as the perinuclear theca and rods of the perforatorium. Of interest, we found that FABP9 was transcribed in human testis although human sperm heads are spatulate in shape. Sequence for the human FABP9 transcript did not contain the two cysteine residues as seen in the mouse suggesting that there could be inter-species differences in functional characteristics of this protein. However, protein expression and localization of FABP9 in either human sperm or other testicular cells remains to be determined.
In the mouse, FABP9 protein was detected as early as day-16 of testicular development but prominent expression was visualized only in late elongating spermatids. This expression pattern of FABP9 late during spermiogenesis strongly suggested its involvement in a terminal differentiation process after the acrosome and subacrosomal layer have already capped the nucleus. We also observed rare and sporadic FABP9 expression in some spermatocytes that could indicate apoptotic cells (Kido and Namiki, 2000). Unexpectedly, the lack of FABP9 did not have a significant effect on the apoptotic regulation of germ cell numbers during spermatogenesis as had been suggested (Kido et al., 2005).
In addition to a lack of observable change in spermatogenesis, sperm from FABP9−/− mice did not show any functional deficits compared to WT sperm at a population level. Membrane lipid organization and attachment were not affected by the loss of FABP9 suggesting that FABP9 is not essential for the compartmentalization of membrane domains in sperm. Moreover, structural features of the sperm head including the perforatorium were also not outwardly affected in FABP9−/− sperm at the level of SEM as had been predicted by previous studies (Korley et al., 1997; Oko and Morales, 1994). Although this is an unexpected observation as FABP9 has been reported to be the major protein in these structures, it can be explained by previous studies that identify other cytoskeletal elements in the sperm head also associated with both the perinuclear theca and perforatorium (Aul and Oko, 2002; Longo et al., 1987; Oko and Morales, 1994). However, we note that we were not able to make a fine comparison at the TEM level between WT and FABP9−/− sperm regarding the internal organization of the perforatorium, that might not be reflected outwardly at the SEM level.
Functionally, FABP9−/− sperm performed on par with WT sperm in assays for in vivo fertility, in vitro capacitation and IVF suggesting that the Ser3 phosphorylation of FABP9 that occurs during capacitation (Platt et al., 2009), is also not essential.
Finally, the loss of FABP9 did not generate differences in total lipid or fatty acid profiles suggesting that membrane lipid and fatty acid production, transport and metabolism were not affected in FABP9−/− germ cells during spermatogenesis. One possible explanation for this lack of difference is compensation by a functionally redundant protein. Recent studies have discovered another member of the FABP family of proteins that are expressed in germ cells. This newly identified FABP12 does not share Cys28 but contains the Cys35 residue. However, a compensatory role for this protein is speculative because FABP12 transcripts have a distinct developmental expression pattern and only a 55% sequence homology to FABP9 (Liu et al., 2008). Alternatively, transfer of proteins or lipids from Sertoli cells to germ cells during spermatogenesis could compensate for the lack of FABP9.
Although reproductive functions in FABP9−/− mice were unaffected, the null males did have a higher rate (~8% increase compared to WT sperm) of sperm head abnormalities. Moreover, ultrastructural examination of FABP9−/− sperm showed that the ventral spur was absent in a significant but modest proportion of sperm (~10% of FABP9−/− sperm compared to none in WT sperm). The ventral spur is formed by an extension of the sperm head cytoskeleton surrounded by the post-acrosomal dense lamina (Oko and Clermont, 1988). In sperm from Australian hydromyine rodents, which have a very pronounced ventral spur that is easy to isolate, it has been shown that FABP9 is the dominant protein in this structure (Breed et al., 2000). Therefore, underdevelopment of this structure can reasonably be correlated with the loss of FABP9. From an evolutionary perspective, the tremendous variations observed in sperm head morphology among species of rodent are highly noteworthy. For example, several species of wild murid rodents from southern Asia and Australia have spatulate or globular heads (Breed, 1993, 1997). Given this morphological diversity among fertile rodent species, it is perhaps not unreasonable to speculate that a structural deficit such as absence of the ventral spur in FABP9−/− sperm would not affect fertility; however, it must be acknowledged that the fertilizing ability of “spur-less” sperm was not directly evaluated in our studies. It is possible that morphologically normal sperm that formed the major population overshadowed the deficits, if any, of the “spurless” sperm in our functional assays.
In summary, the present study tests several hypotheses related to FABP9 function that have existed since the first description of this protein some decades ago. We report surprising findings that several of the prominent roles suggested for FABP9 were minimally affected in FABP9−/− sperm. Although there were higher rates (~8% compared to WT sperm) of structural defects of the FABP9−/− sperm head, and a proportion (~10%) of FABP9−/− sperm specifically lacked the ventral spur, many FABP9−/− sperm remained morphologically normal suggesting that FABP9 only played a minor role in the development and function of sperm. These findings highlight our lack of knowledge of how sperm are formed and reinforce the concept of functional redundancy at multiple levels in spermatogenesis and mature sperm.
Acknowledgements
This work was supported by National Institutes of Health grant R01-HD-045664 (A.J.T). We thank Neal Copeland, Institute for Molecular and Cellular Biology, Singapore for providing the plasmids and hosts used for recombineering the FABP9 construct, and Gary Hunnicutt of the Population Council at Rockefeller University, New York for providing the human testis cDNA library. We also thank Ke-Yu Deng, Transgenic Mouse Core Facility at Cornell University and Robert Munroe, Department of Biomedical Sciences at Cornell University for assisting with blastocyst microinjections and embryo transfers for generating chimeric mice. We acknowledge assistance from Kathy Mott and Jackie Wright with mouse colony management and breeding. Scanning electron microscopy was performed at the Keck Integrated Microscopy Facility (NSF-MRSEC program; DMR 0520404) at Cornell University.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aponte GW. PYY-mediated fatty acid induced intestinal differentiation. Peptides. 2002;23:367–376. doi: 10.1016/s0196-9781(01)00613-1. [DOI] [PubMed] [Google Scholar]
- Asano A, Selvaraj V, Buttke DE, Nelson JL, Green KM, Evans JE, Travis AJ. Biochemical characterization of membrane fractions in murine sperm: identification of three distinct sub-types of membrane rafts. J Cell Physiol. 2009;218:537–548. doi: 10.1002/jcp.21623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aul RB, Oko RJ. The major subacrosomal occupant of bull spermatozoa is a novel histone H2B. Dev Biol. 2002;242:376–387. [PubMed] [Google Scholar]
- Austin CR. Observations on the penetration of the sperm in the mammalian egg. Aust J Sci Res B. 1951;4:581–596. doi: 10.1071/bi9510581. [DOI] [PubMed] [Google Scholar]
- Austin CR. The capacitation of the mammalian sperm. Nature. 1952;170:326. doi: 10.1038/170326a0. [DOI] [PubMed] [Google Scholar]
- Bellve AR, Cavicchia JC, Millette CF, O'Brien DA, Bhatnagar YM, Dym M. Spermatogenic cells of the prepuberal mouse. Isolation and morphological characterization. J Cell Biol. 1977;74:68–85. doi: 10.1083/jcb.74.1.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernlohr DA, Coe NR, Simpson MA, Hertzel AV. Regulation of gene expression in adipose cells by polyunsaturated fatty acids. Adv Exp Med Biol. 1997;422:145–156. doi: 10.1007/978-1-4757-2670-1_12. [DOI] [PubMed] [Google Scholar]
- Bligh EG, Dyer WJ. A rapid method of total lipid extraction and purification. Can J Biochem Physiol. 1959;37:911–917. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- Borchers T, Hohoff C, Buhlmann C, Spener F. Heart-type fatty acid binding protein - involvement in growth inhibition and differentiation. Prostaglandins Leukot Essent Fatty Acids. 1997;57:77–84. doi: 10.1016/s0952-3278(97)90496-8. [DOI] [PubMed] [Google Scholar]
- Breed WG. Novel organization of the spermatozoon in two species of murid rodents from southern Asia. J Reprod Fertil. 1993;99:149–158. doi: 10.1530/jrf.0.0990149. [DOI] [PubMed] [Google Scholar]
- Breed WG. Evolution of the spermatozoon in Australasian rodents. Aust J Zool. 1997;45:459–478. [Google Scholar]
- Breed WG, Idriss D, Oko RJ. Protein composition of the ventral processes on the sperm head of Australian hydromyine rodents. Biol Reprod. 2000;63:629–634. doi: 10.1095/biolreprod63.2.629. [DOI] [PubMed] [Google Scholar]
- Burton PB, Hogben CE, Joannou CL, Clark AG, Hsuan JJ, Totty NF, Sorensen C, Evans RW, Tynan MJ. Heart fatty acid binding protein is a novel regulator of cardiac myocyte hypertrophy. Biochem Biophys Res Commun. 1994;205:1822–1828. doi: 10.1006/bbrc.1994.2882. [DOI] [PubMed] [Google Scholar]
- Calvin HI, Bedford JM. Formation of disulphide bonds in the nucleus and accessory structures of mammalian spermatozoa during maturation in the epididymis. J Reprod Fertil Suppl. 1971;13 Suppl 13:65–75. [PubMed] [Google Scholar]
- Chang MC. Fertilizing capacity of spermatozoa deposited into the fallopian tubes. Nature. 1951;168:697–698. doi: 10.1038/168697b0. [DOI] [PubMed] [Google Scholar]
- Chmurzynska A. The multigene family of fatty acid-binding proteins (FABPs): function, structure and polymorphism. J Appl Genet. 2006;47:39–48. doi: 10.1007/BF03194597. [DOI] [PubMed] [Google Scholar]
- Cowan SW, Newcomer ME, Jones TA. Crystallographic studies on a family of cellular lipophilic transport proteins. Refinement of P2 myelin protein and the structure determination and refinement of cellular retinol-binding protein in complex with all-trans-retinol. J Mol Biol. 1993;230:1225–1246. doi: 10.1006/jmbi.1993.1238. [DOI] [PubMed] [Google Scholar]
- Davis BK. Inhibitory effect of synthetic phospholipid vesicles containing cholesterol on the fertilizing ability of rabbit spermatozoa. Proc Soc Exp Biol Med. 1976;152:257–261. doi: 10.3181/00379727-152-39374. [DOI] [PubMed] [Google Scholar]
- Deng C, Wynshaw-Boris A, Zhou F, Kuo A, Leder P. Fibroblast growth factor receptor 3 is a negative regulator of bone growth. Cell. 1996;84:911–921. doi: 10.1016/s0092-8674(00)81069-7. [DOI] [PubMed] [Google Scholar]
- Erbach GT, Lawitts JA, Papaioannou VE, Biggers JD. Differential growth of the mouse preimplantation embryo in chemically defined media. Biol Reprod. 1994;50:1027–1033. doi: 10.1095/biolreprod50.5.1027. [DOI] [PubMed] [Google Scholar]
- Eswar N, John B, Mirkovic N, Fiser A, Ilyin VA, Pieper U, Stuart AC, Marti-Renom MA, Madhusudhan MS, Yerkovich B, Sali A. Tools for comparative protein structure modeling and analysis. Nucleic Acids Res. 2003;31:3375–3380. doi: 10.1093/nar/gkg543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eylar EH, Szymanska I, Ishaque A, Ramwani J, Dubiski S. Localization of the P2 protein in peripheral nerve myelin. J Immunol. 1980;124:1086–1092. [PubMed] [Google Scholar]
- Falomir-Lockhart LJ, Laborde L, Kahn PC, Storch J, Corsico B. Protein-membrane interaction and fatty acid transfer from intestinal fatty acid-binding protein to membranes. Support for a multistep process. J Biol Chem. 2006;281:13979–13989. doi: 10.1074/jbc.M511943200. [DOI] [PubMed] [Google Scholar]
- Fawcett DW. A comparative view of sperm ultrastructure. Biol Reprod. 1970;2 Suppl 2:90–127. [PubMed] [Google Scholar]
- Fiser A, Do RK, Sali A. Modeling of loops in protein structures. Protein Sci. 2000;9:1753–1773. doi: 10.1110/ps.9.9.1753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folch J, Lees M, Sloane Stanley GH. A simple method for the isolation and purification of total lipides from animal tissues. J Biol Chem. 1957;226:497–509. [PubMed] [Google Scholar]
- Fouquet JP, Valentin A, Kann ML. Perinuclear cytoskeleton of acrosome-less spermatids in the blind sterile mutant mouse. Tissue Cell. 1992;24:655–665. doi: 10.1016/0040-8166(92)90036-7. [DOI] [PubMed] [Google Scholar]
- Graber R, Sumida C, Nunez EA. Fatty acids and cell signal transduction. J Lipid Mediat Cell Signal. 1994;9:91–116. [PubMed] [Google Scholar]
- Hotamisligil GS, Johnson RS, Distel RJ, Ellis R, Papaioannou VE, Spiegelman BM. Uncoupling of obesity from insulin resistance through a targeted mutation in aP2, the adipocyte fatty acid binding protein. Science. 1996;274:1377–1379. doi: 10.1126/science.274.5291.1377. [DOI] [PubMed] [Google Scholar]
- Hunter DJ, Macmaster R, Roszak AW, Riboldi-Tunnicliffe A, Griffiths IR, Freer AA. Structure of myelin P2 protein from equine spinal cord. Acta Crystallogr D Biol Crystallogr. 2005;61:1067–1071. doi: 10.1107/S0907444905014162. [DOI] [PubMed] [Google Scholar]
- Karanth S, Denovan-Wright EM, Thisse C, Thisse B, Wright JM. The evolutionary relationship between the duplicated copies of the zebrafish fabp11 gene and the tetrapod FABP4, FABP5, FABP8 and FABP9 genes. FEBS J. 2008;275:3031–3040. doi: 10.1111/j.1742-4658.2008.06455.x. [DOI] [PubMed] [Google Scholar]
- Kido T, Arata S, Suzuki R, Hosono T, Nakanishi Y, Miyazaki J, Saito I, Kuroki T, Shioda S. The testicular fatty acid binding protein PERF15 regulates the fate of germ cells in PERF15 transgenic mice. Dev Growth Differ. 2005;47:15–24. doi: 10.1111/j.1440-169x.2004.00775.x. [DOI] [PubMed] [Google Scholar]
- Kido T, Namiki H. Expression of testicular fatty acid-binding protein PERF 15 during germ cell apoptosis. Dev Growth Differ. 2000;42:359–366. doi: 10.1046/j.1440-169x.2000.00520.x. [DOI] [PubMed] [Google Scholar]
- Korley R, Pouresmaeili F, Oko R. Analysis of the protein composition of the mouse sperm perinuclear theca and characterization of its major protein constituent. Biol Reprod. 1997;57:1426–1432. doi: 10.1095/biolreprod57.6.1426. [DOI] [PubMed] [Google Scholar]
- Kotaja N, De Cesare D, Macho B, Monaco L, Brancorsini S, Goossens E, Tournaye H, Gansmuller A, Sassone-Corsi P. Abnormal sperm in mice with targeted deletion of the act (activator of cAMP-responsive element modulator in testis) gene. Proc Natl Acad Sci U S A. 2004;101:10620–10625. doi: 10.1073/pnas.0401947101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kusumi A, Sako Y. Cell surface organization by the membrane skeleton. Curr Opin Cell Biol. 1996;8:566–574. doi: 10.1016/s0955-0674(96)80036-6. [DOI] [PubMed] [Google Scholar]
- Lalli M, Clermont Y. Structural changes of the head components of the rat spermatid during late spermiogenesis. Am J Anat. 1981;160:419–434. doi: 10.1002/aja.1001600406. [DOI] [PubMed] [Google Scholar]
- Lawrence P, Brenna JT. Acetonitrile covalent adduct chemical ionization mass spectrometry for double bond localization in non-methylene-interrupted polyene fatty acid methyl esters. Anal Chem. 2006;78:1312–1317. doi: 10.1021/ac0516584. [DOI] [PubMed] [Google Scholar]
- Liu P, Jenkins NA, Copeland NG. A highly efficient recombineering-based method for generating conditional knockout mutations. Genome Res. 2003;13:476–484. doi: 10.1101/gr.749203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu RZ, Li X, Godbout R. A novel fatty acid-binding protein (FABP) gene resulting from tandem gene duplication in mammals: transcription in rat retina and testis. Genomics. 2008;92:436–445. doi: 10.1016/j.ygeno.2008.08.003. [DOI] [PubMed] [Google Scholar]
- Longo FJ, Krohne G, Franke WW. Basic proteins of the perinuclear theca of mammalian spermatozoa and spermatids: a novel class of cytoskeletal elements. J Cell Biol. 1987;105:1105–1120. doi: 10.1083/jcb.105.3.1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marti-Renom MA, Stuart AC, Fiser A, Sanchez R, Melo F, Sali A. Comparative protein structure modeling of genes and genomes. Annu Rev Biophys Biomol Struct. 2000;29:291–325. doi: 10.1146/annurev.biophys.29.1.291. [DOI] [PubMed] [Google Scholar]
- Oko R. Comparative analysis of proteins from the fibrous sheath and outer dense fibers of rat spermatozoa. Biol Reprod. 1988;39:169–182. doi: 10.1095/biolreprod39.1.169. [DOI] [PubMed] [Google Scholar]
- Oko R, Clermont Y. Isolation, structure and protein composition of the perforatorium of rat spermatozoa. Biol Reprod. 1988;39:673–687. doi: 10.1095/biolreprod39.3.673. [DOI] [PubMed] [Google Scholar]
- Oko R, Maravei D. Protein composition of the perinuclear theca of bull spermatozoa. Biol Reprod. 1994;50:1000–1014. doi: 10.1095/biolreprod50.5.1000. [DOI] [PubMed] [Google Scholar]
- Oko R, Morales CR. A novel testicular protein, with sequence similarities to a family of lipid binding proteins, is a major component of the rat sperm perinuclear theca. Dev Biol. 1994;166:235–245. doi: 10.1006/dbio.1994.1310. [DOI] [PubMed] [Google Scholar]
- Oko R, Moussakova L, Clermont Y. Regional differences in composition of the perforatorium and outer periacrosomal layer of the rat spermatozoon as revealed by immunocytochemistry. Am J Anat. 1990;188:64–73. doi: 10.1002/aja.1001880108. [DOI] [PubMed] [Google Scholar]
- Platt MD, Salicioni AM, Hunt DF, Visconti PE. Use of differential isotopic labeling and mass spectrometry to analyze capacitation-associated changes in the phosphorylation status of mouse sperm proteins. J Proteome Res. 2009;8:1431–1440. doi: 10.1021/pr800796j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Runner MN, Gates A. Conception in prepuberal mice following artificially induced ovulation and mating. Nature. 1954;174:222–223. doi: 10.1038/174222b0. [DOI] [PubMed] [Google Scholar]
- Sali A, Blundell TL. Comparative protein modelling by satisfaction of spatial restraints. J Mol Biol. 1993;234:779–815. doi: 10.1006/jmbi.1993.1626. [DOI] [PubMed] [Google Scholar]
- Sarkadi-Nagy E, Wijendran V, Diau GY, Chao AC, Hsieh AT, Turpeinen A, Nathanielsz PW, Brenna JT. The influence of prematurity and long chain polyunsaturate supplementation in 4-week adjusted age baboon neonate brain and related tissues. Pediatr Res. 2003;54:244–252. doi: 10.1203/01.PDR.0000072795.38990.F2. [DOI] [PubMed] [Google Scholar]
- Schmitt MC, Jamison RS, Orgebin-Crist MC, Ong DE. A novel, testis-specific member of the cellular lipophilic transport protein superfamily, deduced from a complimentary deoxyribonucleic acid clone. Biol Reprod. 1994;51:239–245. doi: 10.1095/biolreprod51.2.239. [DOI] [PubMed] [Google Scholar]
- Schurer NY, Stremmel W, Grundmann JU, Schliep V, Kleinert H, Bass NM, Williams ML. Evidence for a novel keratinocyte fatty acid uptake mechanism with preference for linoleic acid: comparison of oleic and linoleic acid uptake by cultured human keratinocytes, fibroblasts and a human hepatoma cell line. Biochim Biophys Acta. 1994;1211:51–60. doi: 10.1016/0005-2760(94)90138-4. [DOI] [PubMed] [Google Scholar]
- Selvaraj V, Asano A, Buttke DE, McElwee JL, Nelson JL, Wolff CA, Merdiushev T, Fornes MW, Cohen AW, Lisanti MP, Rothblat GH, Kopf GS, Travis AJ. Segregation of micron-scale membrane sub-domains in live murine sperm. J Cell Physiol. 2006;206:636–646. doi: 10.1002/jcp.20504. [DOI] [PubMed] [Google Scholar]
- Selvaraj V, Asano A, Buttke DE, Sengupta P, Weiss RS, Travis AJ. Mechanisms underlying the micron-scale segregation of sterols and GM1 in live mammalian sperm. J Cell Physiol. 2009;218:522–536. doi: 10.1002/jcp.21624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaughnessy S, Smith ER, Kodukula S, Storch J, Fried SK. Adipocyte metabolism in adipocyte fatty acid binding protein knockout mice (aP2−/−) after short-term high-fat feeding: functional compensation by the keratinocyte [correction of keritinocyte] fatty acid binding protein. Diabetes. 2000;49:904–911. doi: 10.2337/diabetes.49.6.904. [DOI] [PubMed] [Google Scholar]
- Storch J, Thumser AE. The fatty acid transport function of fatty acid-binding proteins. Biochim Biophys Acta. 2000;1486:28–44. doi: 10.1016/s1388-1981(00)00046-9. [DOI] [PubMed] [Google Scholar]
- Stremmel W, Strohmeyer G, Borchard F, Kochwa S, Berk PD. Isolation and partial characterization of a fatty acid binding protein in rat liver plasma membranes. Proc Natl Acad Sci U S A. 1985;82:4–8. doi: 10.1073/pnas.82.1.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toyoda Y, Yokoyama M, Hoshi T. [Studies on the fertilization of mouse eggs in vitro. I. In vitro fertilization of eggs by fresh epididymal spermatozoa] Jpn J Reprod Dev. 1971;16:147–151. [Google Scholar]
- Trapp BD, Dubois-Dalcq M, Quarles RH. Ultrastructural localization of P2 protein in actively myelinating rat Schwann cells. J Neurochem. 1984;43:944–948. doi: 10.1111/j.1471-4159.1984.tb12828.x. [DOI] [PubMed] [Google Scholar]
- Travis AJ, Jorgez CJ, Merdiushev T, Jones BH, Dess DM, Diaz-Cueto L, Storey BT, Kopf GS, Moss SB. Functional relationships between capacitation-dependent cell signaling and compartmentalized metabolic pathways in murine spermatozoa. J Biol Chem. 2001a;276:7630–7636. doi: 10.1074/jbc.M006217200. [DOI] [PubMed] [Google Scholar]
- Travis AJ, Kopf GS. In: The spermatozoon as a machine: compartmentalized pathways bridge cellular structure and function. In: Assisted reproductive technology: Accomplishments and new horizons. DeJonge CJ, Barratt CL, editors. Cambridge University Press; 2002. pp. 26–39. [Google Scholar]
- Travis AJ, Merdiushev T, Vargas LA, Jones BH, Purdon MA, Nipper RW, Galatioto J, Moss SB, Hunnicutt GR, Kopf GS. Expression and localization of caveolin-1, and the presence of membrane rafts, in mouse and Guinea pig spermatozoa. Dev Biol. 2001b;240:599–610. doi: 10.1006/dbio.2001.0475. [DOI] [PubMed] [Google Scholar]
- Travis AJ, Tutuncu L, Jorgez CJ, Ord TS, Jones BH, Kopf GS, Williams CJ. Requirements for glucose beyond sperm capacitation during in vitro fertilization in the mouse. Biol Reprod. 2004;71:139–145. doi: 10.1095/biolreprod.103.025809. [DOI] [PubMed] [Google Scholar]
- Trotter PJ, Ho SY, Storch J. Fatty acid uptake by Caco-2 human intestinal cells. J Lipid Res. 1996;37:336–346. [PubMed] [Google Scholar]
- Van Pelt CK, Brenna JT. Acetonitrile chemical ionization tandem mass spectrometry to locate double bonds in polyunsaturated fatty acid methyl esters. Anal Chem. 1999;71:1981–1989. doi: 10.1021/ac981387f. [DOI] [PubMed] [Google Scholar]
- Visconti PE, Galantino-Homer H, Ning X, Moore GD, Valenzuela JP, Jorgez CJ, Alvarez JG, Kopf GS. Cholesterol efflux-mediated signal transduction in mammalian sperm. beta-cyclodextrins initiate transmembrane signaling leading to an increase in protein tyrosine phosphorylation and capacitation. J Biol Chem. 1999;274:3235–3242. doi: 10.1074/jbc.274.5.3235. [DOI] [PubMed] [Google Scholar]
- Wedgwood JF, Strominger JL, Warren CD. Transfer of sugars from nucleoside diphosphosugar compounds to endogenous and synthetic dolichyl phosphate in human lymphocytes. J Biol Chem. 1974;249:6316–6324. [PubMed] [Google Scholar]
- Weiss RS, Enoch T, Leder P. Inactivation of mouse Hus1 results in genomic instability and impaired responses to genotoxic stress. Genes Dev. 2000;14:1886–1898. [PMC free article] [PubMed] [Google Scholar]
- Widstrom RL, Norris AW, Spector AA. Binding of cytochrome P450 monooxygenase and lipoxygenase pathway products by heart fatty acid-binding protein. Biochemistry. 2001;40:1070–1076. doi: 10.1021/bi001602y. [DOI] [PubMed] [Google Scholar]
- Wolfrum C, Borrmann CM, Borchers T, Spener F. Fatty acids and hypolipidemic drugs regulate peroxisome proliferator-activated receptors alpha - and gamma-mediated gene expression via liver fatty acid binding protein: a signaling path to the nucleus. Proc Natl Acad Sci U S A. 2001;98:2323–2328. doi: 10.1073/pnas.051619898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright RS. A reagent for the non-destructive location of steroids and some other lipophilic materials on silica gel thin-layer chromatograms. J Chromatogr. 1971;59:220–221. doi: 10.1016/s0021-9673(01)80033-9. [DOI] [PubMed] [Google Scholar]
- Yang Y, Spitzer E, Kenney N, Zschiesche W, Li M, Kromminga A, Muller T, Spener F, Lezius A, Veerkamp JH, Smith GH, Salomon DS, Grosse R. Members of the fatty acid binding protein family are differentiation factors for the mammary gland. J Cell Biol. 1994;127:1097–1109. doi: 10.1083/jcb.127.4.1097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmerman AW, Veerkamp JH. New insights into the structure and function of fatty acid-binding proteins. Cell Mol Life Sci. 2002;59:1096–1116. doi: 10.1007/s00018-002-8490-y. [DOI] [PMC free article] [PubMed] [Google Scholar]