Summary
The circadian clock of the cyanobacterium Synechococcus elongatus PCC 7942 is built on a three-protein central oscillator that can be reconstituted in vitro, a redox-sensitive input for synchronization with the environment, and a bacterial two-component signal transduction pathway for global transcriptional regulation. This review covers the most recent progress in our understanding of the biological and biochemical mechanism of this bacterial clock, such as the discovery of a quinone-binding activity of the oscillator protein KaiA, the molecular mechanism of circadian control of cell division, and the global control of gene expression via modulation of DNA topology.
Introduction
Most living organisms on Earth ranging from bacteria to humans experience striking changes in their environment that accompany the daily alternations of day and night, and have evolved an internal timing system named the circadian clock to cope with this predictable cycle. Synechococcus elongatus PCC 7942, a freshwater unicellular cyanobacterium, is the simplest and the most ancient form of life demonstrated to possess a circadian clock to date. The core oscillator of the cyanobacterial clock is composed of three proteins: KaiA, KaiB and KaiC [1], whose structures have been solved and biochemical activities well characterized [2-4]. KaiC is an autokinase, autophosphatase, and ATPase. Both the abundance and the phosphorylation states of KaiC cycle in vivo. Remarkably, a simple mixture of the three Kai proteins with ATP reconstitutes the circadian oscillation of KaiC phosphorylation and ATP hydrolysis in vitro, establishing the first and only cell-free circadian oscillator [5,6]. The circadian clock stays synchronized with the environment through one or more input pathways that inform the oscillator of changes in the cellular redox state, which in this photosynthetic organism correlate with the presence of light [4]. An output pathway that includes a bacterial two-component signal transduction system relays the timing information from the central oscillator to many key processes downstream. For the past two years significant progress has been made in many aspects of the clock, including redox sensing by the oscillator, the correlation between DNA topology and global transcriptional activity, and the mechanism of circadian gating of cell division in S. elongtus.
Circadian transcriptome
An early promoter trap experiment with a luciferase reporter found that all promoters tested are expressed rhythmically [7]. With the advent of a complete genome sequence and microarray technology, two groups independently examined mRNA profiles of almost all annotated genes in constant light (clock free-running) conditions, and found that transcripts from 30% to 64% of the genomic repertoire, depending on the study and the analysis method adopted, accumulate rhythmically [8,9]. This is a considerably larger fraction than was shown by similar studies conducted in eukaryotic systems [10]. Both studies found that the majority of rhythmic genes peak either at subjective dawn or subjective dusk, and that, with the exception of genes that are arranged in an operon, there is no correlation between the phase angle (relative timing of peaks) of rhythmic transcripts and the location of their genes on the chromosome.
Recent results strengthen the correlation between DNA topology and the phasing of circadian transcript peaks that was suggested previously [11-13]. Vijayan et al. surveyed both the transcriptome and DNA topology at the same time points in a circadian cycle, and found a good correlation between the global transcription profile at a given time and DNA topology as displayed by an endogenous plasmid [8]. When a gyrase inhibitor is applied at the chromosome's most supercoiled phase of the circadian cycle (subjective dusk) to quickly relax DNA superhelicity, 79% of the rhythmic ORFs change in the direction that is predicted from the established correlation; e.g., expression of ORFs that peak when DNA is most supercoiled goes down. Furthermore, the authors found a significant difference in the GC content in the promoter regions of genes that peak at different times. Collectively, these results suggest a causal relationship between DNA topology and transcription activity, and further support the oscilloid model, which states that the clock controls global gene expression by modifying chromosome topology [14].
Ito et al. surprisingly discovered at least two rhythmic transcripts in a kaiABC null background [9], which suggests the presence of kai-independent oscillations. However, given the important implications for clock mechanism in this organism, this result needs to be further validated by a different method.
Circadian oscillation of KaiC phosphorylation
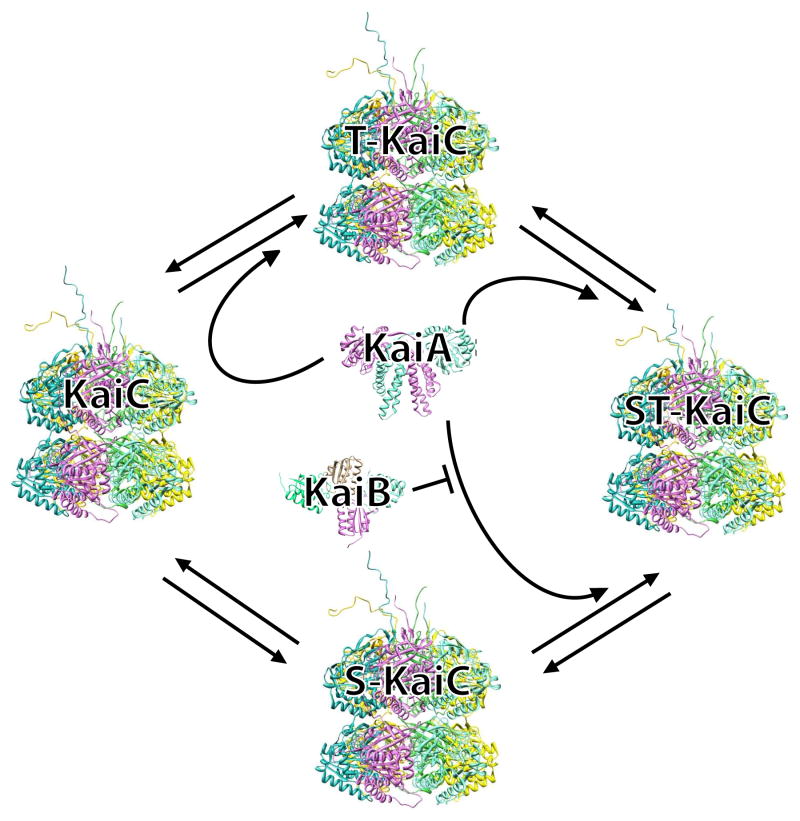
A circadian oscillation in phosphorylation of KaiC occurs in vivo as well as in vitro [6,15,16]. The mechanism of KaiC phosphorylation is comparatively well understood. Two residues in KaiC, serine at position 431 (S431) and threonine at position 432 (T432), can be phosphorylated and dephosphorylated with about a 24 hour period [17,18] (Fig. 1). T432 autophosphorylates (T-KaiC) upon stimulation of KaiC by KaiA, which binds to the C-terminal peptide of KaiC (A-Loop), and S431 subsequently autophosphorylates (ST-KaiC). ST-KaiC undergoes spontaneous dephosphorylation at T432 (S-KaiC). KaiB binds to S-KaiC, which then traps and inactivates KaiA, thus allowing further KaiC dephosphorylation. A recent review by Markson and O'Shea details the molecular events for this reaction, including time delay and synchronization, and the models that have been developed to explain the oscillation [19]. A third residue, T426, is important for KaiC function, as mutation to alanine abolishes circadian rhythm [20]; moreover, T426 can be be phosphorylated in mutants that carry substitutions at the known phosphorylation sites (S431A and S431A/T432E) [21]. The crystal structure of KaiC, which includes a mixture of phosphoforms, shows electron density around T426 suggestive of some level of phosphorylation [20]. KaiA is the primary stimulator of KaiC phosphorylation, and a mutant form of KaiA that lacks the last three residues has a stronger interaction with KaiC, resulting in changes in KaiC phosphorylation kinetics and a shorter circadian period in vivo [22].
Figure 1. Model of the circadian cycle of KaiC phosphorylation.
Two residues (S431 and T432) in KaiC undergo autophosphorylation and autodephosphorylation. KaiA shifts the equilibrium toward T-KaiC and ST-KaiC. T432 on ST-KaiC spontaneously dephosphorylates and forms S-KaiC. KaiB binds to S-KaiC and inactivates KaiA to shift the equilibrium towards unphosphorylated KaiC.
KaiC is known to dephosphorylate in the absence of KaiA in vitro [23] and in vivo [24]. Surprisingly, we found partially phosphorylated KaiC in vivo in a ΔcikAΔkaiA mutant [25], suggestive of the presence of a KaiA-independent, but CikA-suppressed activity that stimulates KaiC phosphorylation. This finding implicates another condition or component that affects KaiC phosphorylation which would need to be added to allow the in vitro reaction to fully mimic in vivo conditions.
KaiC phosphorylation at the protomer level has been well described and many aspects of the interactions among KaiA, KaiB, and KaiC are also known [17-19,22,23,26-29]. However, KaiC functions as a hexamer at all phosphorylation states and in its interactions with other clock proteins [28,29]. The stoichiometry between the KaiA dimer and KaiC hexamer that supports oscillatory phosphorylation in vitro is 1:1; thus, it is of great interest to know how phosphorylation propagates within the KaiC hexamer when only one protomer is stimulated.
What is the role of the phosphorylation cycle? Phosphorylation of the adjacent amino acids likely causes conformational change [30] in the C-terminal domain of KaiC, which moves the activity or partner interface to the next stage in the cycle. Structural evidence of a progression of conformational change is not available for wild-type KaiC, but the published structure does provide some suggestive insights. Each protomer in the hexameric crystal structure has a different conformation, although the differences are small [31]. These variations may reflect sampling of the mixed phosphorylation states for the crystal. Because KaiC is autocatalytic, it is difficult to constrain the wild-type protein to yield a homogenous population of hexamers that have the same phosphorylation state. Recently, Pattnayek et al. solved crystal structures of KaiC mutants which have controlled phosphorylation states [32]. In these structures, KaiC mutants locked in different states clearly show conformational differences.
Gating of cell division and KaiC ATPase activity
In a cellular context, the circadian clock must coexist with the cell cycle, which operates with a different, and varying, period. In organisms for which the relationship between these two oscillators has been examined, the circadian clock gates cell division, allowing division at some phases while inhibiting it at others [33]. Similarly, the S. elongatus circadian clock gates cell division, whereas the cell cycle has no effect on the circadian clock, regardless of the speed at which the cell cycle runs [34,35]. Recent work from our lab unveiled a linear signal transduction pathway, involving all major components of the clock, that inhibits cytokinesis (Fig. 2) [36]. Specifically, mutants with elevated KaiC ATPase are associated with cell elongation. The absence of CikA, a major player of the input pathway, causes cell elongation in a KaiC-dependent manner, suggesting that it may suppress KaiC ATPase activity, directly or indirectly. Furthermore, clock output components SasA and RpaA act downstream of KaiC in cell division control, and formation of the septation ring by FtsZ, a bacterial tubulin homolog, is targeted by this clock pathway. Yang et al. tracked circadian rhythms and cell division events simultaneously in individual cyanobacterial cells and developed a mathematical model describing the coupling of the two oscillators, which could be extended to any coupled biological oscillations [37]; this work is discussed in more depth by Pando and Van Oudenaarden in this issue. It is still unclear, however, why there is a cell division gate. Given that daughter cells inherit the circadian clock with great precision from their mother cells [38], it is possible that the gate protects the clock from perturbations that would result from cell division at a vulnerable phase.
Figure 2. Molecular Pathways of the Circadian Clock in S. elongatus.
The central oscillator of the clock is composed of KaiA, KaiB, and KaiC, which together generate circadian rhythms of KaiC phosphorylation (see Figure 1 for details) and ATPase activity that are intimately coupled. CikA, LdpA, and Pex are known components of the input pathway. Both CikA and LdpA are redox sensitive, as the addition of a quinone analog destabilizes them in vivo. CikA binds a quinone directly, and represses KaiC's phosphorylation/ATPase activity through a cryptic activity. LdpA is an iron-sulfur protein that is involved in light-dependent modulation of circadian period [43]. Its mechanism of action remains unknown. The pex gene is light suppressed, and encodes a transcription repressor of the kaiA gene [58]. Oxidized quinone binds and destabilizes KaiA, providing a direct input to the central oscillator by cellular redox. SasA is a histidine kinase that physically interacts with KaiC. RpaA is the cognate response regulator of SasA, and bears a DNA binding domain, whose target(s) has yet to be identified [59]. LabA is proposed to act negatively on RpaA [60]. Global transcription rhythms are regulated through DNA topology via an unknown mechanism. Timing of cell division is inhibited by elevated KaiC ATPase through the SasA-RpaA output pathway, and localization or polymerization of bacterial tubilin homolog FtsZ is targeted.
It is a surprise that elevated ATPase activity rather than a specific phosphorylation state of KaiC causes the closing of the gate. KaiC is an unorthodox ATPase, hydrolyzing an average of only 15 ATP molecules per KaiC monomer per day as measured in vitro [5,36]. However, this activity oscillates in a circadian manner, is correlated with circadian period, and is required for the circadian clock [5]. Given the low level of this activity, it is unlikely for KaiC to affect the overall energy balance of the cell, or do any significant physical work. It is thus important to understand the role of KaiC ATPase, although its intimate coupling with KaiC phosphorylation complicates the analysis. Terauchi et al. concluded from KaiC phosphomimetics that KaiC phosphorylation suppresses ATPase activity. However, KaiC truncation mutants that are either constitutively phosphorylated or nonphosphorylated showed the opposite correlation [36]. The discrepancy lies in the nature of the mutants that were employed, as the phosphorylatable residues are replaced in the phosphomimetics, resulting in a KaiC that is unphosphorylated, but has elevated ATPase activity. In our study, the carboxyl terminus of KaiC that controls a switch between kinase and phosphatase activities is absent, but S431 and T432 are intact. It appears that KaiC ATPase activity is influenced more by its interaction with KaiA and KaiB than by a particular phosphorylation state.
The basic timing loop
In eukaryotes transcription translation feedback loops (TTFL) are essential for the fundamental oscillation of the circadian clock [39]. The Kondo group reported that the cyanobacterial oscillator can run without a TTFL [6,16], and that a gene expression rhythm remains when KaiC is arrested at a certain phosphorylation state [40]. These data suggest that the Kai protein oscillator and a TTFL exist independently, but that both are important for a robust circadian clock. However, Johnson and colleagues found that the gene expression rhythm quickly damps when KaiC phosphorylation is clamped, and concluded that the protein oscillator is the master pacemaker, whereas TTFL is a slave. Through mathematical modeling and experiments, they showed that the integration of TTFL with the protein oscillator improves the robustness of the circadian clock, and that a TTFL can provide an entraining signal to the post-translational Kai-based pacemaker [41].
Synchronization and resetting of circadian oscillations
Light is perceived directly through photoreceptors in eukaryotic clock systems [39]. However, evidence mounts that cyanobacteria synchronize the internal circadian oscillator by sampling cellular redox state rather than sensing light directly. Two major components of the input pathway, LdpA and CikA, are both redox sensitive. LdpA is an iron-sulfur protein [42,43], whereas the pseudo-receiver domain of CikA binds a quinone [44]. The N-terminus of KaiA is also a pseudo-receiver—a domain that shares the fold of phosphorylated signal transduction receiver domains, but lacks the conserved phosphoaccepting Asp residue [45]. We showed that KaiA binds the same quinone analog that binds to CikA, and aggregates upon such binding in vitro, which negatively affects KaiC phosphorylation [46]. More specifically, only the oxidized form of quinone binds to KaiA, whereas the reduced form has no effect on KaiA structure or activity. Therefore, external light cues that change the oxidation state of electron carriers in cyanobacteria may modulate the phosphorylation states of KaiC, and thereby synchronize the internal circadian clock to the external light/dark cycle.
Recently, CikA was implicated as an output pathway component, parallel to SasA and LabA, and downstream of KaiC, through a series of genetic analyses (Fig. 2) [47]. Although the three-component classification (input, central oscillator, and output) of the circadian clock research is somewhat arbitrary, there is no doubt that CikA acts in an input pathway based on evidence both old and new. CikA is the only known protein whose absence renders the clock blind to a dark pulse, which in the wild type causes a phase shift [48]. Analysis of clock mutants that affect cell length uncovered a specific role for CikA upstream of KaiC, which functions upstream of SasA and RpaA [25]. Although the mechanism remains elusive, CikA likely suppresses the kinase and ATPase activities of KaiC indirectly. The phenotypes of period and/or amplitude of circadian rhythms in cikA mutants can be explained by its role in modifying KaiC's biochemical activity, i.e., by its role in the input pathway. Nonetheless, involvement of this multifunctional protein at different points in the clock system is quite plausible.
Rhythms in other cyanobacterial species
Bacterial circadian rhythms were first rigorously demonstrated in the cyanobacterium Synechococcus sp. RF-1 [49]. Since then, S. elongatus has emerged as the model for the prokaryotic circadian clock, largely due to the superior genetic tools available for this organism. To date, cyanobacteria remain the only prokaryotes shown to possess a true circadian clock. However, cyanobacteria are a very diverse group and differ greatly in their genome size, morphology, metabolism, and ecology; therefore, other cyanobacterial species can help us understand the evolutionary history and adaptive significance of the clock. In Crocosphaera watsonii WH 8501 staining of the chromosome, reflecting DNA topology, is rhythmic under constant light conditions [50], confirming the circadian chromosome compaction seen in S. elongatus [12]. Circadian rhythms have also been reported in Synechocystis PCC 6803 [51], Cyanothece sp. ATCC 51142 [52], and Thermosynechococcus elongatus BP-1 [53], which all have at least one set of the kaiABC genes. Of the 49 complete cyanobacterial genomes at GenBank, only Gloeobacter violaceus PCC 7421 has no apparent homolog of any of the kai genes. Gloeobacter is unique as it lacks thylakoid membranes and, phylogenetically, branches off from other cyanobacteria very early [54]. Interestingly, Prochlorococcus species have lost kaiA as a result of genome streamlining but retain kaiBC [55]. In Prochlorococcus marinus PCC 9511, rhythms of cell division and mRNA accumulation from several genes are apparent in light dark cycles, but are quickly lost when cells are released into constant light [55], failing a test of circadian clocks. Recombinant KaiC protein from Prochlorococcus sp. MED4 (ProKaiC) is phosphorylated at the neighboring Ser and Thr residues like S. elongatus KaiC, and displays a similarly weak ATPase activity. However, contrary to KaiC, ProKaiC is constitutively phosphorylated when incubated alone, and the addition of KaiA from S. elongatus or KaiB from either species has no effect on this activity [56]. Therefore, no oscillation of phosphorylation has been reconstituted under the tested conditions. Taken together, these data suggest that Prochlorococcus species may have lost the autonomous circadian clock, but retain a timing system that needs to be reset daily with environmental cues, analogous to an hourglass [57]. It remains to be seen whether the kaiBC genes are part of this timing system.
Conclusions
The seemingly simple and elegant in vitro oscillator has allowed scientists to make strides in understanding the core mechanism of the cyanobacterial clock and gain insights into how the clock synchronizes with the environment. Future studies will strive to incorporate the input and output aspects of the clock into the in vitro system. Meanwhile, the power of genetics continues to reveal how the circadian clock is integrated within the cell to coordinate physiology and behavior. Some key questions that remain are outlined in Box 1.
Box 1: Key questions.
How does the conformation of KaiC change with changes in phosphorylation state?
What is the correlation between phosphorylation state and ATPase activity?
What is the role of monomer shuffling of KaiC?
How is KaiC phosphorylation propagated and synchronized within a hexamer and among the entire population?
How does the clock control DNA topology, and vice versa?
How do CikA and LdpA relay environmental information to the central oscillator?
How are clock components distributed in the cell?
What is the function of the circadian cell division gate?
Acknowledgments
Our research is funded by grants from the National Institutes of Health (R01 GM62419, P01 NS39546) and the American Recovery and Reinvestment Act.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Ishiura M, Kutsuna S, Aoki S, Iwasaki H, Andersson CR, Tanabe A, Golden SS, Johnson CH, Kondo T. Expression of a gene cluster kaiABC as a circadian feedback process in cyanobacteria. Science. 1998;281:1519–1523. doi: 10.1126/science.281.5382.1519. [DOI] [PubMed] [Google Scholar]
- 2.Johnson CH, Egli M, Stewart PL. Structural insights into a circadian oscillator. Science. 2008;322:697–701. doi: 10.1126/science.1150451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Johnson CH, Mori T, Xu Y. A cyanobacterial circadian clockwork. Curr Biol. 2008;18:R816–825. doi: 10.1016/j.cub.2008.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dong G, Golden SS. How a cyanobacterium tells time. Curr Opin Microbiol. 2008;11:541–546. doi: 10.1016/j.mib.2008.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Terauchi K, Kitayama Y, Nishiwaki T, Miwa K, Murayama Y, Oyama T, Kondo T. ATPase activity of KaiC determines the basic timing for circadian clock of cyanobacteria. Proc Natl Acad Sci U S A. 2007;104:16377–16381. doi: 10.1073/pnas.0706292104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nakajima M, Imai K, Ito H, Nishiwaki T, Murayama Y, Iwasaki H, Oyama T, Kondo T. Reconstitution of circadian oscillation of cyanobacterial KaiC phosphorylation in vitro. Science. 2005;308:414–415. doi: 10.1126/science.1108451. [DOI] [PubMed] [Google Scholar]
- 7.Liu Y, Tsinoremas NF, Johnson CH, Lebedeva NV, Golden SS, Ishiura M, Kondo T. Circadian orchestration of gene expression in cyanobacteria. Genes Dev. 1995;9:1469–1478. doi: 10.1101/gad.9.12.1469. [DOI] [PubMed] [Google Scholar]
- **8.Vijayan V, Zuzow R, O'Shea EK. Oscillations in supercoiling drive circadian gene expression in cyanobacteria. Proc Natl Acad Sci USA. 2009;106:22564–22568. doi: 10.1073/pnas.0912673106. [DOI] [PMC free article] [PubMed] [Google Scholar]; The authors confirmed previous findings that DNA superhelicity cycles under constant conditions, and established a direct correlation between genome-wide transcription pattern and DNA topology.
- 9.Ito H, Mutsuda M, Murayama Y, Tomita J, Hosokawa N, Terauchi K, Sugita C, Sugita M, Kondo T, Iwasaki H. Cyanobacterial daily life with Kai-based circadian and diurnal genome-wide transcriptional control in Synechococcus elongatus. Proc Natl Acad Sci USA. 2009;106:14168–14173. doi: 10.1073/pnas.0902587106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vitalini MW, de Paula RM, Park WD, Bell-Pedersen D. The rhythms of life: circadian output pathways in Neurospora. J Biol Rhythms. 2006;21:432–444. doi: 10.1177/0748730406294396. [DOI] [PubMed] [Google Scholar]
- 11.Min H, Liu Y, Johnson CH, Golden SS. Phase determination of circadian gene expression in Synechococcus elongatus PCC 7942. J Biol Rhythms. 2004;19:103–112. doi: 10.1177/0748730403262056. [DOI] [PubMed] [Google Scholar]
- 12.Smith RM, Williams SB. Circadian rhythms in gene transcription imparted by chromosome compaction in the cyanobacterium Synechococcus elongatus. Proc Natl Acad Sci U S A. 2006;103:8564–8569. doi: 10.1073/pnas.0508696103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Woelfle MA, Xu Y, Qin X, Johnson CH. Circadian rhythms of superhelical status of DNA in cyanobacteria. Proc Natl Acad Sci U S A. 2007;104:18819–18824. doi: 10.1073/pnas.0706069104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Woelfle MA, Johnson CH. No promoter left behind: global circadian gene expression in cyanobacteria. J Biol Rhythms. 2006;21:419–431. doi: 10.1177/0748730406294418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nishiwaki T, Satomi Y, Nakajima M, Lee C, Kiyohara R, Kageyama H, Kitayama Y, Temamoto M, Yamaguchi A, Hijikata A, et al. Role of KaiC phosphorylation in the circadian clock system of Synechococcus elongatus PCC 7942. Proc Natl Acad Sci U S A. 2004;101:13927–13932. doi: 10.1073/pnas.0403906101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tomita J, Nakajima M, Kondo T, Iwasaki H. No transcription-translation feedback in circadian rhythm of KaiC phosphorylation. Science. 2005;307:251–254. doi: 10.1126/science.1102540. [DOI] [PubMed] [Google Scholar]
- 17.Rust MJ, Markson JS, Lane WS, Fisher DS, O'Shea EK. Ordered phosphorylation governs oscillation of a three-protein circadian clock. Science. 2007;318:809–812. doi: 10.1126/science.1148596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nishiwaki T, Satomi Y, Kitayama Y, Terauchi K, Kiyohara R, Takao T, Kondo T. A sequential program of dual phosphorylation of KaiC as a basis for circadian rhythm in cyanobacteria. Embo J. 2007;26:4029–4037. doi: 10.1038/sj.emboj.7601832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Markson JS, O'Shea EK. The molecular clockwork of a protein-based circadian oscillator. FEBS Lett. 2009;583:3938–3947. doi: 10.1016/j.febslet.2009.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Xu Y, Mori T, Pattanayek R, Pattanayek S, Egli M, Johnson CH. Identification of key phosphorylation sites in the circadian clock protein KaiC by crystallographic and mutagenetic analyses. Proc Natl Acad Sci U S A. 2004;101:13933–13938. doi: 10.1073/pnas.0404768101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Xu Y, Mori T, Qin X, Yan H, Egli M, Johnson CH. Intramolecular regulation of phosphorylation status of the circadian clock protein KaiC. PLoS ONE. 2009;4:e7509. doi: 10.1371/journal.pone.0007509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chen Y, Kim YI, Mackey SR, Holtman CK, Liwang A, Golden SS. A novel allele of kaiA shortens the circadian period and strengthens interaction of oscillator components in the cyanobacterium Synechococcus elongatus PCC 7942. Journal of Bacteriology. 2009;191:4392–4400. doi: 10.1128/JB.00334-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *23.Kim YI, Dong G, Carruthers CW, Jr, Golden SS, LiWang A. The day/night switch in KaiC, a central oscillator component of the circadian clock of cyanobacteria. Proc Natl Acad Sci U S A. 2008:12825–12830. doi: 10.1073/pnas.0800526105. [DOI] [PMC free article] [PubMed] [Google Scholar]; The authors show that binding of the KaiA C-terminal domain to the KaiC C-terminal peptide causes KaiC phosphorylation. This is the first detailed molecular mechanism of KaiC phosphorylation activated by KaiA.
- 24.Kitayama Y, Iwasaki H, Nishiwaki T, Kondo T. KaiB functions as an attenuator of KaiC phosphorylation in the cyanobacterial circadian clock system. Embo J. 2003;22:2127–2134. doi: 10.1093/emboj/cdg212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- **25.Dong G, Yang Q, Wang Q, Kim YI, Wood TL, Osteryoung KW, van Oudenaarden A, Golden SS. Elevated ATPase activity of KaiC applies a circadian checkpoint on cell division in Synechococcus elongatus. Cell. 2010;140:529–539. doi: 10.1016/j.cell.2009.12.042. [DOI] [PMC free article] [PubMed] [Google Scholar]; We observed gating of cell division at the single-cell level, and uncovered a pathway involving all major components of the clock as well as the specific state of KaiC responsible for this phenomenon.
- 26.Mutoh R, Mino H, Murakami R, Uzumaki T, Takabayashi A, Ishii K, Ishiura M. Direct interaction between KaiA and KaiB revealed by a site-directed spin labeling electron spin resonance analysis. Genes Cells. 2010 doi: 10.1111/j.1365-2443.2009.01377.x. [DOI] [PubMed] [Google Scholar]
- 27.Nakajima M, Ito H, Kondo T. In vitro regulation of circadian phosphorylation rhythm of cyanobacterial clock protein KaiC by KaiA and KaiB. FEBS Lett. 2010;584:898–902. doi: 10.1016/j.febslet.2010.01.016. [DOI] [PubMed] [Google Scholar]
- *28.Pattanayek R, Williams DR, Pattanayek S, Mori T, Johnson CH, Stewart PL, Egli M. Structural model of the circadian clock KaiB-KaiC complex and mechanism for modulation of KaiC phosphorylation. Embo J. 2008;27:1767–1778. doi: 10.1038/emboj.2008.104. [DOI] [PMC free article] [PubMed] [Google Scholar]; The authors solve the KaiC-KaiB complex structure which gives insight into how KaiB blocks KaiA function and shifts the equilibrium toward unphosphorylated KaiC.
- 29.Pattanayek R, Williams DR, Pattanayek S, Xu Y, Mori T, Johnson CH, Stewart PL, Egli M. Analysis of KaiA-KaiC protein interactions in the cyano-bacterial circadian clock using hybrid structural methods. EMBO J. 2006;25:2017–2028. doi: 10.1038/sj.emboj.7601086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhang J, Corden JL. Identification of phosphorylation sites in the repetitive carboxyl-terminal domain of the mouse RNA polymerase II largest subunit. J Biol Chem. 1991;266:2290–2296. [PubMed] [Google Scholar]
- 31.Pattanayek R, Wang J, Mori T, Xu Y, Johnson CH, Egli M. Visualizing a circadian clock protein: crystal structure of KaiC and functional insights. Mol Cell. 2004;15:375–388. doi: 10.1016/j.molcel.2004.07.013. [DOI] [PubMed] [Google Scholar]
- **32.Pattanayek R, Mori T, Xu Y, Pattanayek S, Johnson CH, Egli M. Structures of KaiC circadian clock mutant proteins: a new phosphorylation site at T426 and mechanisms of kinase, ATPase and phosphatase. PLoS ONE. 2009;4:e7529. doi: 10.1371/journal.pone.0007529. [DOI] [PMC free article] [PubMed] [Google Scholar]; The crystal structures of mutant KaiC variants show an additional possible phosphorylation site (T426) and conformational differences from wild type. Those mutants and wild-type KaiC structures give insight into how KaiC conformation changes by phosphorylation.
- 33.Mori T. Cell division cycles and circadian rhythms. Bacterial Circadian Programs. 2009 [Google Scholar]
- 34.Mori T, Johnson CH. Independence of circadian timing from cell division in cyanobacteria. J Bacteriol. 2001;183:2439–2444. doi: 10.1128/JB.183.8.2439-2444.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mori T, Binder B, Johnson CH. Circadian gating of cell division in cyanobacteria growing with average doubling times of less than 24 hours. Proc Natl Acad Sci U S A. 1996;93:10183–10188. doi: 10.1073/pnas.93.19.10183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Baca I, Sprockett D, Dvornyk V. Circadian input kinases and their homologs in cyanobacteria: evolutionary constraints versus architectural diversification. J Mol Evol. 2010;70:453–465. doi: 10.1007/s00239-010-9344-0. [DOI] [PubMed] [Google Scholar]
- *37.Yang Q, Pando B, Dong G, Golden S, van Oudenaarden A. Circadian gating of the cell cycle revealed in single cyanobacterial cells. Science. 2010;327:1522. doi: 10.1126/science.1181759. [DOI] [PMC free article] [PubMed] [Google Scholar]; The authors combined experimental and theoretical approaches to model broadly how two cycles (the circadian cycle and the cell cycle) within the same cell are coupled with each other.
- 38.Mihalcescu I, Hsing W, Leibler S. Resilient circadian oscillator revealed in individual cyanobacteria. Nature. 2004;430:81–85. doi: 10.1038/nature02533. [DOI] [PubMed] [Google Scholar]
- 39.Bell-Pedersen D, Cassone VM, Earnest DJ, Golden SS, Hardin PE, Thomas TL, Zoran MJ. Circadian rhythms from multiple oscillators: lessons from diverse organisms. Nat Rev Genet. 2005;6:544–556. doi: 10.1038/nrg1633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kitayama Y, Nishiwaki T, Terauchi K, Kondo T. Dual KaiC-based oscillations constitute the circadian system of cyanobacteria. Genes Dev. 2008;22:1513–1521. doi: 10.1101/gad.1661808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *41.Qin X, Byrne M, Xu Y, Mori T, Johnson CH. Coupling of a core post-translational pacemaker to a slave transcription/translation feedback loop in a circadian system. PLoS Biol. 2010;8:e1000394. doi: 10.1371/journal.pbio.1000394. [DOI] [PMC free article] [PubMed] [Google Scholar]; The authors suggest that a previously observed transcription cycle that is independent of KaiC phosphorylation cycle is likely unstable, and concluded that the post-translational oscillator and the transcription-translation oscillator are not parallel oscillators, but rather have a definitive hierarchical relationship.
- 42.Ivleva NB, Bramlett MR, Lindahl PA, Golden SS. LdpA: a component of the circadian clock senses redox state of the cell. Embo J. 2005;24:1202–1210. doi: 10.1038/sj.emboj.7600606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Katayama M, Kondo T, Xiong J, Golden SS. ldpA encodes an iron-sulfur protein involved in light-dependent modulation of the circadian period in the cyanobacterium Synechococcus elongatus PCC 7942. J Bacteriol. 2003;185:1415–1422. doi: 10.1128/JB.185.4.1415-1422.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ivleva NB, Gao T, LiWang AC, Golden SS. Quinone sensing by the circadian input kinase of the cyanobacterial circadian clock. Proc Natl Acad Sci U S A. 2006;103:17468–17473. doi: 10.1073/pnas.0606639103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ye S, Vakonakis I, Ioerger TR, LiWang AC, Sacchettini JC. Crystal structure of circadian clock protein KaiA from Synechococcus elongatus. J Biol Chem. 2004;279:20511–20518. doi: 10.1074/jbc.M400077200. [DOI] [PubMed] [Google Scholar]
- **46.Wood TL, Bridwell-Rabb J, Kim YI, Gao T, Chang YG, Liwang A, Barondeau DP, Golden SS. The KaiA protein of the cyanobacterial circadian oscillator is modulated by a redox-active cofactor. Proc Natl Acad Sci U S A. 2010;107:5804–5809. doi: 10.1073/pnas.0910141107. [DOI] [PMC free article] [PubMed] [Google Scholar]; The authors demonstrate that oxidized quinone bind to the pseudo-receiver domain of KaiA cause aggregation and affect the phosphorylation states of KaiC. This study gives insight how cyanobacteria sense external light and synchronize oscillator by day/night cycle.
- 47.Taniguchi Y, Takai N, Katayama M, Kondo T, Oyama T. Three major output pathways from the KaiABC-based oscillator cooperate to generate robust circadian kaiBC expression in cyanobacteria. Proc Natl Acad Sci U S A. 2010;107:3263–3268. doi: 10.1073/pnas.0909924107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Schmitz O, Katayama M, Williams SB, Kondo T, Golden SS. CikA, a bacteriophytochrome that resets the cyanobacterial circadian clock. Science. 2000;289:765–768. doi: 10.1126/science.289.5480.765. [DOI] [PubMed] [Google Scholar]
- 49.Huang TC, Tu J, Chow TJ, Chen TH. Circadian rhythm of the prokaryote Synechococcus sp RF-1. Plant Physiol. 1990;92:531–533. doi: 10.1104/pp.92.2.531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *50.Pennebaker K, Mackey KR, Smith RM, Williams SB, Zehr JP. Diel cycling of DNA staining and nifH gene regulation in the unicellular cyanobacterium Crocosphaera watsonii strain WH 8501 (Cyanophyta) Environ Microbiol. 2010;12:1001–1010. doi: 10.1111/j.1462-2920.2010.02144.x. [DOI] [PMC free article] [PubMed] [Google Scholar]; The authors observed rhythmic staining of chromosomal DNA in Crocosphaera, confirming a similar result in S. elongatus and suggesting that transcriptional control through modulation of DNA topology may be a common circadian mechanism in cyanobacteria.
- 51.Aoki S, Kondo T, Ishiura M. Circadian expression of the dnaK gene in the cyanobacterium Synechocystis sp strain PCC 6803. J Bacteriol. 1995;177:5606–5611. doi: 10.1128/jb.177.19.5606-5611.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Elvitigala T, Stockel J, Ghosh BK, Pakrasi HB. Effect of continuous light on diurnal rhythms in Cyanothece sp ATCC 51142. BMC Genomics. 2009;10:226. doi: 10.1186/1471-2164-10-226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Onai K, Morishita M, Itoh S, Okamoto K, Ishiura M. Circadian rhythms in the thermophilic cyanobacterium Thermosynechococcus elongatus: compensation of period length over a wide temperature range. J Bacteriol. 2004;186:4972–4977. doi: 10.1128/JB.186.15.4972-4977.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Nakamura Y, Kaneko T, Sato S, Mimuro M, Miyashita H, Tsuchiya T, Sasamoto S, Watanabe A, Kawashima K, Kishida Y, et al. Complete genome structure of Gloeobacter violaceus PCC 7421, a cyanobacterium that lacks thylakoids. DNA Res. 2003;10:137–145. doi: 10.1093/dnares/10.4.137. [DOI] [PubMed] [Google Scholar]
- 55.Holtzendorff J, Partensky F, Mella D, Lennon JF, Hess WR, Garczarek L. Genome streamlining results in loss of robustness of the circadian clock in the marine cyanobacterium Prochlorococcus marinus PCC 9511. J Biol Rhythms. 2008;23:187–199. doi: 10.1177/0748730408316040. [DOI] [PubMed] [Google Scholar]
- 56.Axmann IM, Dühring U, Seeliger L, Arnold A, Vanselow JT, Kramer A, Wilde A. Biochemical evidence for a timing mechanism in Prochlorococcus. Journal of Bacteriology. 2009;191:5342–5347. doi: 10.1128/JB.00419-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Mullineaux CW, Stanewsky R. The rolex and the hourglass: a simplified circadian clock in prochlorococcus? J Bacteriol. 2009;191:5333–5335. doi: 10.1128/JB.00719-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kutsuna S, Kondo T, Aoki S, Ishiura M. A period-extender gene, pex, that extends the period of the circadian clock in the cyanobacterium Synechococcus sp strain PCC 7942. J Bacteriol. 1998;180:2167–2174. doi: 10.1128/jb.180.8.2167-2174.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Takai N, Nakajima M, Oyama T, Kito R, Sugita C, Sugita M, Kondo T, Iwasaki H. A KaiC-associating SasA-RpaA two-component regulatory system as a major circadian timing mediator in cyanobacteria. Proc Natl Acad Sci U S A. 2006;103:12109–12114. doi: 10.1073/pnas.0602955103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Taniguchi Y, Katayama M, Ito R, Takai N, Kondo T, Oyama T. labA: a novel gene required for negative feedback regulation of the cyanobacterial circadian clock protein KaiC. Genes Dev. 2007;21:60–70. doi: 10.1101/gad.1488107. [DOI] [PMC free article] [PubMed] [Google Scholar]