Abstract
Hypoxia is a common feature of solid tumors. Up-regulation of hypoxia-inducing factor-1 (HIF-1) occurs in the majority of primary malignant tumors and in two-thirds of metastases, while most normal tissues are negative. HIF-1 induces the glycolytic phenotype, which creates an acidic extracellular microenvironment and associated pH gradient such that drugs that are weak acids are selectively taken up and retained in acidic tumors. 7-Butyl-10-amino-camptothecin (BACPT) is a prime example of an agent that can exploit the tumor pH gradient for enhanced selectivity.
Purpose
This study profiles the antitumor activity of BACPT in vitro and its water-soluble dipeptide ester, BACPTDP, in vivo.
Methods
Antitumor activity was evaluated by proliferation assays in cancer cell lines and in murine xenograft models for human neuroblastoma (IMR-32), colon (HT29), ovarian (SK-OV-3), pancreatic (Panc-1), glioma (SF-295) and non-small-cell lung (NCI-H460) cancers.
Results
BACPT had superior antiproliferative activity compared to established drugs in monolayer cultures of human neuroblastoma and pancreatic tumor cell lines and in 3-dimensional histocultures of colon and primary ovarian cancer. Antitumor activity of BACPTDP was comparable to irinotecan in IMR-32, HT29, SF-295 and NCI-H460 xenografts, significantly greater in SK-OV-3 and in Panc-1 where complete regressions were observed. Combination of BACPT with gemcitabine produced additive to synergistic interactions in Panc-1 cells that were independent of drug ratio and optimal when gemcitabine was administered 24 h prior to BACPT.
Conclusions
BACPTDP is a water-soluble camptothecin pro-drug that spontaneously generates the lipid-soluble active agent, BACPT. This topoisomerase inhibitor exploits solid tumor physiology for improved selectivity and activity against multiple tumor types with particular promise for use in treating pediatric neuroblastoma and pancreatic carcinoma.
Keywords: Camptothecin, Glycolytic phenotype, Xenograft models, Growth inhibition, Pancreatic cancer, Drug combinations, Median effect analysis
Introduction
Despite extensive studies with camptothecins (CPTs), these and other antitumor compounds have not been adequately evaluated in models that accurately reflect human tumor physiology in vivo (reviewed in [1]). This is particularly true for solid tumors that commonly exist in hypoxic microenvironments, which promote expression of stress response genes, tumor angiogenesis, exit of tumor cells from the cell cycle and drug resistance [40, 43]. Hypoxia is now known to induce the glycolytic phenotype (the Warburg Effect), with secretion of lactic acid and consequent acidification of the extracellular microenvironment [24, 39]. This persistent acidosis can enhance tumor cell survival, lead to destruction of surrounding normal tissue and increase metastatic potential [20]. Unlike normal tissues, the glycolytic pheno-type in tumor cells creates a cellular pH gradient unique to tumor masses [21]. Drugs that are weak acids are selectively taken up and retained by acidic tumors, while those that are weak bases are excluded [22, 29]. The tumor pH gradient can be enhanced and drug efficacy increased simply by administering a single dose of glucose prior to drug treatment in murine xenografts [22]. Therefore, one approach to improve selectivity of cancer chemotherapy is to design pH-sensitive antitumor drugs that exploit the pH gradient present in human solid tumors but not in normal tissues. Camptothecins are unique in this regard in that they possess an E ring lactone that opens to form the inactive hydroxy acid at physiological pH, but remains closed and active in acidic tumor conditions. This fact could account for pH effects on activity of CPTs observed by others [19], and for most of the fourfold increase in activity for camptothecin that we observed in breast cancer cells adapted to growth in vitro at pH 6.8 [2].
Our studies in breast cancer cell models have demonstrated that other structural features are involved in this enhancement. A 7-methyl halogen substituent increases pH modulation from 10- to 40-fold, while analogs in the 10-amino CPT series are modulated 10- to 20-fold [5]. The 7-butyl-10-amino analog (BACPT) demonstrated optimal pH modulation in MCF-7/wt cells, a cyclophosphamide-resistant subline of MCF-7 (MCF-7/hc), and in hormone-insensitive MDA-MB-231 cells. The BACPT analog was chosen for further study based on the presence and impact of the pH gradient in MCF-7 cells cultured at pH 6.8 vs. 7.4. Potency was increased nearly 15-fold at acidic pH due to an increase in both drug uptake and retention [5]. Like other camptothecins, BACPT acts by inhibiting nuclear topoiso-merase I (top1) to create cytotoxic DNA double strand breaks. Molecular modeling studies based on the X-ray crystal structure for topotecan in the ternary top1/DNA complex revealed that binding of BACPT is further stabilized by a direct hydrogen bond between the 10-amino moiety and an oxygen atom of the side chain of Glu-346 [4]. The butyl substituent at the 7 position orients the drug such that it imitates a DNA base pair. The likely overall effect is to increase persistence in the top1/DNA cleavage complex, which enhances inhibitory activity. However, the increase in lipophilicity from the butyl group reduces water solubility, which has been a key impediment in the clinical development of camptothecins. To address this issue, we designed the 20(S)-β-alanine-lysine dipeptide ester BACPTDP. The dipeptide ester confers both high water solubility (160 mg/ml) and protects the E ring lactone from opening to extend serum half-life. BACPTDP hydrolyzes spontaneously to produce BACPT, releasing the natural dipeptide substituent, which avoids the diarrhea toxicity that is inherent with irinotecan.
This article reviews the antitumor activity profile for BACPT in cell culture models for human neuroblastoma, pancreatic, colon and ovarian tumor cells and for BACPTDP in murine xenograft models of human colon, ovarian, pancreatic, glioma and non-small-cell lung cancers. Given the significant activity against Panc-1 cells both in vitro and in vivo, we have also evaluated the combination of BACPT with gemcitabine, the standard of care chemotherapy in pancreatic cancer.
Methods
Cells and reagents
All cell lines were obtained from the American Type Culture Collection (ATCC, Manassas, VA). Panc-1 cells were cultured in DMEM medium containing 10% fetal bovine serum, while neuroblastoma cell lines were grown in DMEM basal medium containing 10% FBS and 1 g/l glucose at 37° C in either 5% CO2 (pH 7.4) or 17% CO2 (pH 6.8). Neuroblastoma cell lines differ in their expression of the MYCN oncogene and the norepinephrine transporter (NET). SK-N-MC and NGP express single copy, while IMR-32 and SK-N-BE(2c) express 25 and 100 copies of MYCN, respectively. SK-N-MC does not express NET, while SK-N-BE(2c) cells express high levels [8, 12, 41]. All camptothecin analogs, including BACPT and BACPTDP, were synthesized by published methods and obtained from Research Triangle Institute International, Research Triangle Park, NC. Clinically approved drugs were obtained from the Duke Pharmacy or from Clavis Pharma ASA. Clinical formulations of drugs were used. When this was not possible, drugs were formulated as concentrated stocks in dry DMSO and stored at −20° C.
Growth inhibition assay
Neuroblastoma cell lines were inoculated at 5,000 cells/well and Panc-1 cells at 3,000 cells/well in 96-well microplates. Cells were exposed to various concentrations of CPT analogs for two cell cycles; then switched to drug-free medium for one cell cycle. Surviving cells were quantified either by staining with propidium iodide [16] or by metabolic assay (ATPLite, Perkin-Elmer, Waltham, MA) as per the manufacturer’s protocol. Dose–response curves included seven dose levels assayed in triplicate with coefficients of variation around 5%. Endpoint was the concentration that produced 50% inhibition of growth compared to that of untreated controls (IC50) as derived from fitted data weighted by standard error using TableCurve software (SPSS/IBM, Chicago, IL). The inter-assay coefficient of variation in IC50 was 10–20%.
Histoculture assay
Methods developed by Hoffman and coworkers [25] and modified by us [17] were used to evaluate agent activity against tumors growing in 3-dimensional cultures. Activity in this assay has been shown to correlate with delayed outgrowth in mouse models [17] and to clinical response [18, 38]. Tumor cell lines were grown as subcutaneous xenografts in nude mice. When tumors reached five times the inoculum volume, the animals were euthanized, the tumor aseptically excised, and placed in the respective growth medium containing antibiotics and antimicotics. Tumor tissue was washed three times with growth medium and cut in half. Necrotic areas were removed, and the tissue minced into 0.5–1.5 mm3 fragments. The fragments were then prescreened for viability by MTS metabolic assay (CellTiter 96® AQueous Non-Radioactive Cell Proliferation Assay; Promega, Madison, WI). Viable fragments (12–15 mg) were placed in a 24-well microplate, 4–6 fragments per well. Samples were incubated for 24 h in 2 ml medium/well prior to addition of test compounds. The primary ovarian specimen was obtained with the subject’s consent and approval by the Duke Health System Institutional Review Board. Tumor tissue was placed in a sterile container and submitted to the surgical pathology department at Duke University Medical Center. Excess tissue was supplied to the laboratory for analysis after being stripped of all patient identifiers.
Human tumor xenograft assay
To obtain an initial assessment of toxicity, non-tumor bearing, athymic nu/nu female mice were administered BACPTDP dissolved in saline by intraperitoneal injection on five consecutive days, rested 2 days, then dosed another five consecutive days (Q1D×5 (1, 8) or 5/2/5 schedule). Animals were checked daily for mortality and twice weekly for body weight. The endpoint was the number of survivors on day 22. BACPTDP was then evaluated in murine xenograft models for human colon (HT29), ovarian (SK-OV-3), pancreatic (Panc-1), glioma (SF-295) and non-small-cell lung (NCI-H460) cancers. BACPTDP was prepared once weekly in saline at a concentration of 0.8 mg/ml and dosed i.p. at 4.5, 6.7 and 8 mg/kg on the 5/2/5 schedule. For comparison, irinotecan (CPT-11; clinical formulation at 20 mg/ml) was run head-to-head and dosed intravenously at 40 and 60 mg/kg on a Q4D×3 schedule, where the higher dose approximates the maximum-tolerated dose (MTD). Endpoints were the time to reach either two (SK-OV-3, Panc-1), three (HT-29, NCI-H460, IMR-32) or four (SF-295) tumor volume doublings, where tumor volume is defined as: L × W2/2 = mm3 = mg, and L and W are the larger and smaller perpendicular tumor dimensions determined by caliper measurement. Median days delay in tumor outgrowth was then computed as the difference between drug-treated versus vehicle treated controls (T – C). To compare activities across tumor types with different doubling endpoints, the data were normalized and expressed as median (T – C)/C. Student’s T or the Mann–Whitney rank sum tests were used to determine significance of differences among mean growth delay times. In cases where groups contained animals whose tumors did not reach the evaluation endpoint (e.g., long-term survivors), a life-table analysis was used in which a stratified Kaplan–Meier estimation was followed by the Mantel–Haenszel log-rank test.
Drug interaction assay
Drug interaction was assessed with ATP-based growth inhibition assays by combination index and combination effect endpoints using published methods of Chou [11] and Kanzawa [26], respectively, as reported previously [6, 37].
Results
Antiproliferative activity in cell culture models
Activity in monolayer culture
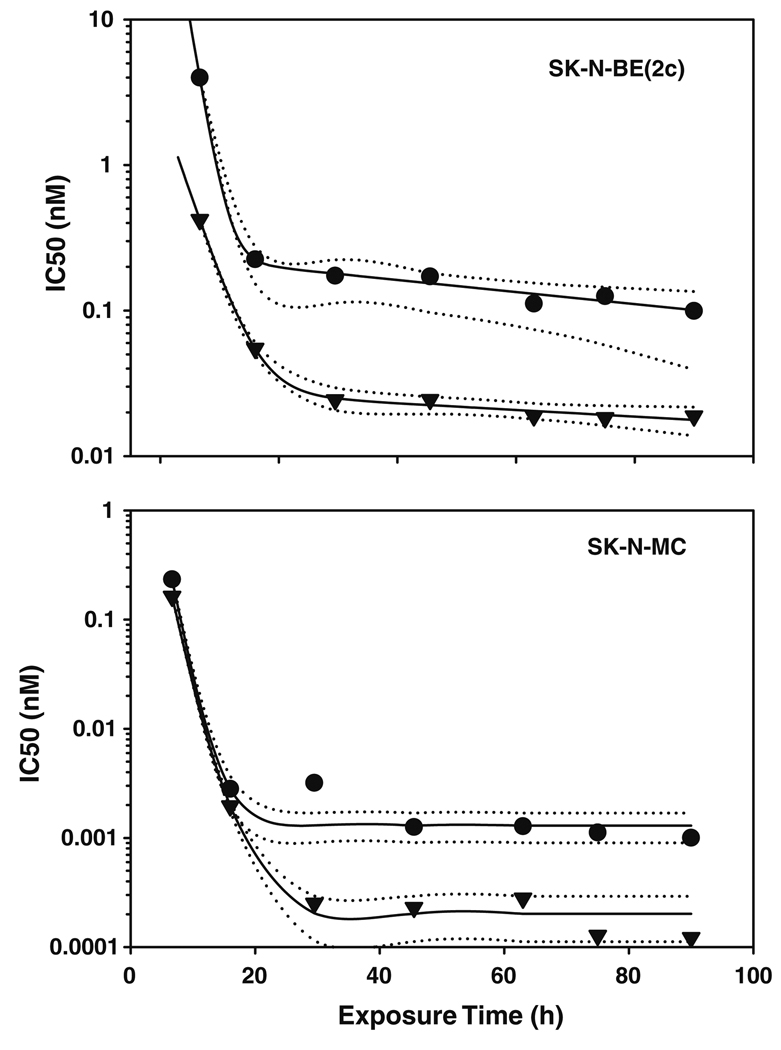
Neuroblastoma models had varied responses in vitro to BACPT at physiological pH over a six log range with SK-N-MC cells being the most sensitive and IMR-32 the least sensitive (Table 1). However, activity was enhanced at acidic pH in all four cell lines, with pH modulation ranging from 5 to nearly 50-fold. Potency of growth inhibition did not correlate with expression of the MYCN oncogene, a negative prognostic factor in neuroblastoma, or the norepinephrine transporter; the latter is commonly used to image these tumors with radiolabeled meta-iodo-benzylguanidine (MIBG). MIBG can be used to enhance tumor acidification via its ability to inhibit cellular respiration with subsequent stimulation of glycolysis. Of significance, pH modulation was independent of time of exposure to BACPT in SK-N-BE(2c) and after 20 h exposure time in SK-N-MC cells (Fig. 1). The lack of enhancement at early time points is compensated by the exquisite sensitivity of SK-N-MC cells to the drug.
Table 1.
Antiproliferative activity of BACPT in neuroblastoma cell lines
| Cell line | MYCN copy # | NET level | IC50 (nM) pH 7.4 | IC50 (nM) pH 6.8 | Fold increase at acidic pH |
|---|---|---|---|---|---|
| NGP | 1 | NDa | 1,294 | 26 | 49 |
| SK-N-MC | 1 | − | 0.0010 | 0.00012 | 8 |
| IMR-32 | 25 | + | 1,390 | 127 | 12 |
| SK-N-BE(2c) | 100 | ++++ | 0.099 | 0.019 | 5 |
Not determined
Fig. 1.
Activity of BACPT in neuroblastoma cells as a function of exposure time and extracellular pH. Neuroblastoma cell lines that do [SK-N-BE(2c)] or do not (SK-N-MC) over-express NET or MYCN were exposed to varying concentrations of BACPT for various times before being placed in drug-free medium prior to analysis of surviving cell number by ATP assay. The concentration that inhibited growth by 50% versus untreated controls (IC50) was determined as described in “Methods”. Cells were cultured at pH 7.4 (circles) or pH 6.8 (inverse triangles). Solid lines represent a curve fit of the data with dotted lines the 95% conidence intervals
Antiproliferative activity of BACPT was also assessed in Panc-1 cells, a model for advanced, aggressive pancreatic cancer. Panc-1 is poorly differentiated, expresses mutated p53 and K-ras, the c-Src oncogene and is relatively insensitive to gemcitabine and other chemotherapeutic drugs. Comparisons to BACPT were made with drugs from seven classes, including a DNA antimetabolite, antimitotic agent, topoisomerase II inhibitor, demethylating agents and a Src kinase inhibitor (Table 2). The alkylating agent temozolomide and the EGFR tyrosine kinase inhibitor erlotinib were inactive in this assay. BACPT was the most potent compound with an IC50 of 4 nM; sixfold more potent than gemcitabine, the current standard of care chemotherapy. The other drugs were two to over four logs less active. Of note, BACPT was also potent at the IC90 level of activity, a level that was not achieved by vinorelbine, 5-azacytidine or decitabine and by 5-fluorouracil only at high concentrations not clinically achievable.
Table 2.
Antiproliferative activity in Panc-1 Cells
| Drug | IC50 (nM) | vs. BACPT | IC90 (nM) | vs. BACPT |
|---|---|---|---|---|
| BACPT | 4 | 1 | 86 | 1 |
| Gemcitabine | 23 | 6 | 117 | 1 |
| Vinorelbine | 103 | 25 | NAa | – |
| Etoposide | 651 | 160 | 8,750 | 102 |
| Dasatinib | 6,886 | 1,693 | 13,250 | 154 |
| 5-Flurouracil | 9,178 | 2,257 | 97,633 | 1,136 |
| 5-Azacytidine | 68,805 | 16,916 | NA | – |
| Decitabine | 68,994 | 16,963 | NA | – |
| Temozolomide | NA | – | NA | – |
| Erlotinib | NA | – | NA | – |
Not achieved at highest concentration tested
Activity in 3-dimensional histoculture
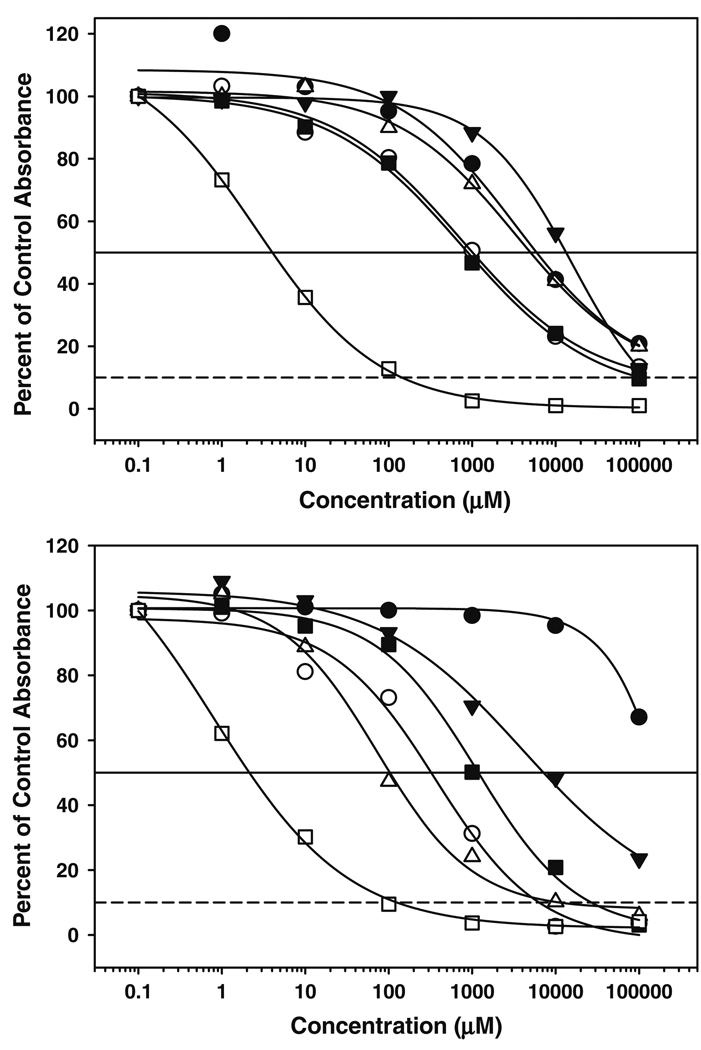
While providing high throughput, flexibility and convenience, growth inhibition analysis in monolayer cell culture models has limited relevance for solid tumors growing in vivo. Previous studies indicated that 10-amino-CPT, a precursor for BACPT, had significantly better activity than either topotecan or SN-38, the active metabolite of irinotecan, in 3-dimensional, tissue-like histocultures of human brain, neuroblastoma, breast, colon and prostate tumors and was enhanced at acidic pH [17]. Consequently, we evaluated BACPT and BACPTDP in histocultures from IMR-32 neuroblastoma and HT29 colon cell lines grown as murine xenografts. In addition, we evaluated a primary ovarian patient specimen. When IMR-32 histocultures were assessed under both hypoxic (1.5% O2) and acidic (pH 6.8) conditions, BACPT was 20-fold more potent than SN-38 (IC50 =0.2 vs. 10 µM; results not shown). BACPTDP was at least two orders of magnitude more potent than topotecan, paclitaxel, cisplatin, doxorubicin and 9-amino-CPT in HT29 histocultures at physiological pH. Similar results were observed in primary ovarian carcinoma (Fig. 2).
Fig. 2.
Antiproliferative activity of BACPTDP in 3-dimensional histoculture. Top panel HT29 human colon carcinoma cells were grown as xenograft tumors in immunocompromised mice. Tumors were excised, sliced into 1-mm3 cubes, pre-screened for metabolic activity and exposed to varying concentrations of BACPT (open square), 9-amino-CPT (filled triangle), paclitaxel (open circle), topotecan (filled circle), doxorubicin (open triangle), or cisplatin (filled inverted triangle) for 72 h as described in “Methods”. Medium was replaced and fragments incubated an additional 24 h before assay of MTS metabolic activity. Results are reported as percent of untreated control absorbance. Bottom panel primary human ovarian carcinoma tissue was acquired postoperatively from an excess surgical pathology specimen and processed as described [17]. Drug exposure and analysis were identical to that in the top panel
Antitumor activity in murine xenografts
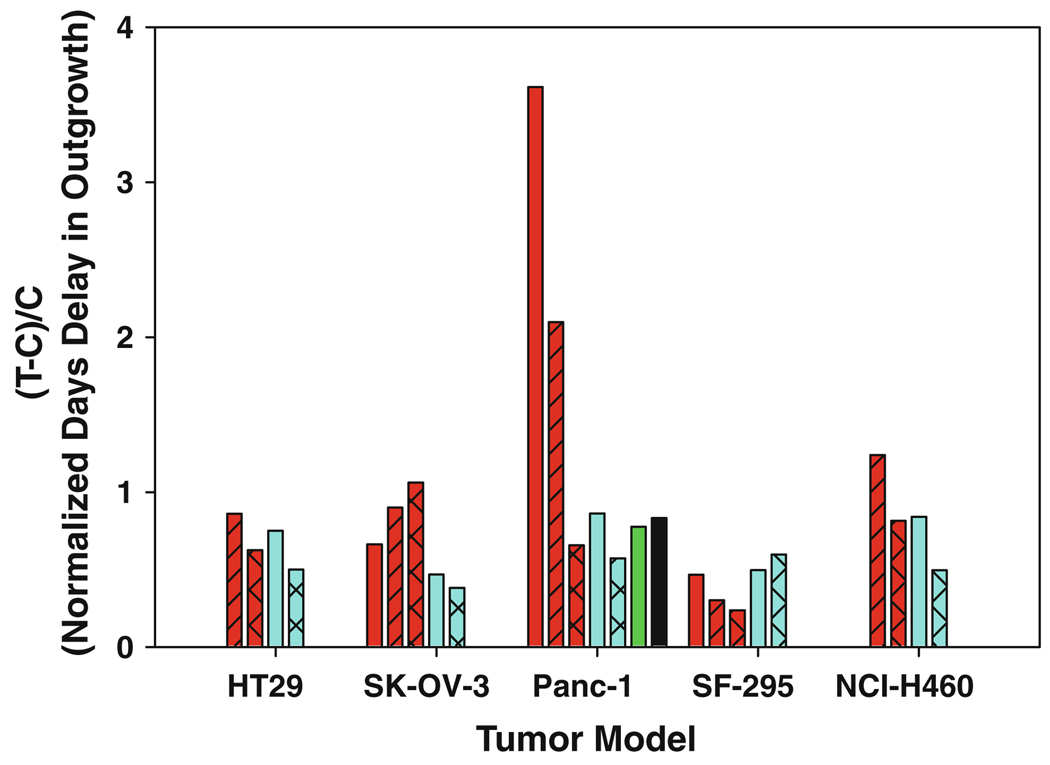
To obtain an initial assessment of toxicity, non-tumor-bearing, athymic nu/nu female mice were administered BACPTDP dissolved in saline by intraperitoneal injection on a daily × five schedule repeated after 2 days rest (5/2/5 schedule). Results shown in Table 3 indicate that BACPTDP is toxic in this model at doses of 10 and 20 mg/kg but well tolerated with only minimal body weight loss at doses of 2.5 and 5 mg/kg. Based on the toxicity data, BACPTDP was evaluated in murine xenograft models for human colon (HT29), ovarian (SK-OV-3), pancreatic (Panc-1), glioma (SF-295) and non-small-cell lung (NCI-H460) cancers. BACPTDP was dosed intraperitoneally on the 5/2/5 schedule and compared head-to-head with irinotecan (CPT-11) dosed intravenously on a commonly used Q4D×3 schedule where 60 mg/kg is at or near the MTD ([31] and W. Waud, personal communication). The results shown in Fig. 3, normalized to account for the different doubling endpoints, demonstrate that BACPTDP has antitumor activity in all xenograft models tested. Activity of BACPTDP was comparable to irinotecan in HT29, SF-295 and NCI-H460 xenografts, significantly greater in SK-OV-3 (T-C = 17 days for BACPTDP at 4.5 mg/kg vs. 6.1 days for CPT-11 at 40 mg/kg; P = 0.005). Activity of BACPTDP was superior in Panc-1 where complete regressions were observed (Table 4). The latter observation is all the more noteworthy, because the Panc-1 model is aggressive, relatively insensitive to gemcitabine, expresses the c-Src oncogene kinase activity that suppresses gemcitabine-induced, caspase-mediated apoptosis [15]. Responses to BACPTDP were dose dependent and despite significant but transient weight loss (21% maximum) at 8 mg/kg produced median survival of 67 days with 2/6 animals tumor-free at 95 days. By comparison, irinotecan at 60 mg/kg produced a median survival of 27 days and no tumor-free animals. Clearly, the latter comparison does not reflect equitoxic doses. However, at the MTD of each agent, BACPTDP (6.7 mg/kg) also exhibited significantly higher activity than CPT-11 but at onethird the total dose. BACPTDP activity was also superior to that reported for gemcitabine in independent studies by others (60 mg/kg i.p. on days 0,3,6,9, and 12 [7]), and for 9-nitro-camptothecin (rubitecan, orathecin) dosed i.v. at 2.5 mg/kg Q1D×5 (1, 8) [36]. The latter drug has clinical activity in pancreatic cancer [10, 32].
Table 3.
Toxicity of BACPTDP in athymic mice
| Dose (mg/kg) | 22 Day survival | Maximum body weight loss (%) |
|---|---|---|
| 2.5 | 3/3 | 0 |
| 5 | 3/3 | 2 |
| 10 | 2/3 | 24 |
| 20 | 0/3 |
Fig. 3.
Antitumor activity of BACPTDP in human tumor xenografts. Murine xenograft models for human colon (HT29), ovarian (SK-OV-3), pancreatic (Panc-1), glioma (SF-295) and non-small-cell lung (NCI-H460) cancers were treated with BACPTDP (red bars) at 4.5 (cross-hatched), 6.7 (hatched), or 8.0 mg/kg (no hatch) on a 5/2/5 schedule and run head-to-head against irinotecan (CPT-11; blue bars) at 40 (hatched) and 60 (no hatch) mg/kg on a Q4D×3 schedule. Data for BACPTDP at 8 mg/kg are not included for the NCI-H460 xenograft due to increased toxicity in this model. Results are expressed as the overall delay in growth of the median tumor (T–C; T treated group, C control group), normalized to account for diferent tumor doubling times (two for SK-OV-3 and Panc-1; three for HT-29 and NCI-H460; four for SF-295). For comparison, published values are included for gemcitabine (black bar) and rubitecan (green bar) at their maximum tolerated doses of 60 mg/kg and at 2.5 mg/kg, respectively, in the Panc-1 model
Table 4.
Antitumor activity of BACPTDP in Panc-1 xenografts
| # | Treatment | Dose (mg/kg) |
Maximum body weight loss (%) |
Median T–C | Median survival (days) |
TFS | Comparison | P value |
|---|---|---|---|---|---|---|---|---|
| 1 | BACPTDP | 8.0 | 21 | 60.0 | 66.7 ± 7.8 | 2 | 1 vs. 4 | 0.001 |
| 2 | BACPTDP | 6.7 | 11 | 34.8 | 52.7 ± 8.3 | 0 | 2 vs. 4 | 0.009 |
| 3 | BACPTDP | 4.5 | 6 | 10.9 | 27.4 ± 2.0 | 0 | 3 vs. 5 | 0.582 |
| 4 | CPT-11 | 60 | 6 | 14.3 | 31.4 ± 1.5 | 0 | ||
| 5 | CPT-11 | 40 | 2 | 9.5 | 35.0 ± 9.3 | 0 |
Modulation of extracellular pH enhances BACPTDP activity in vivo
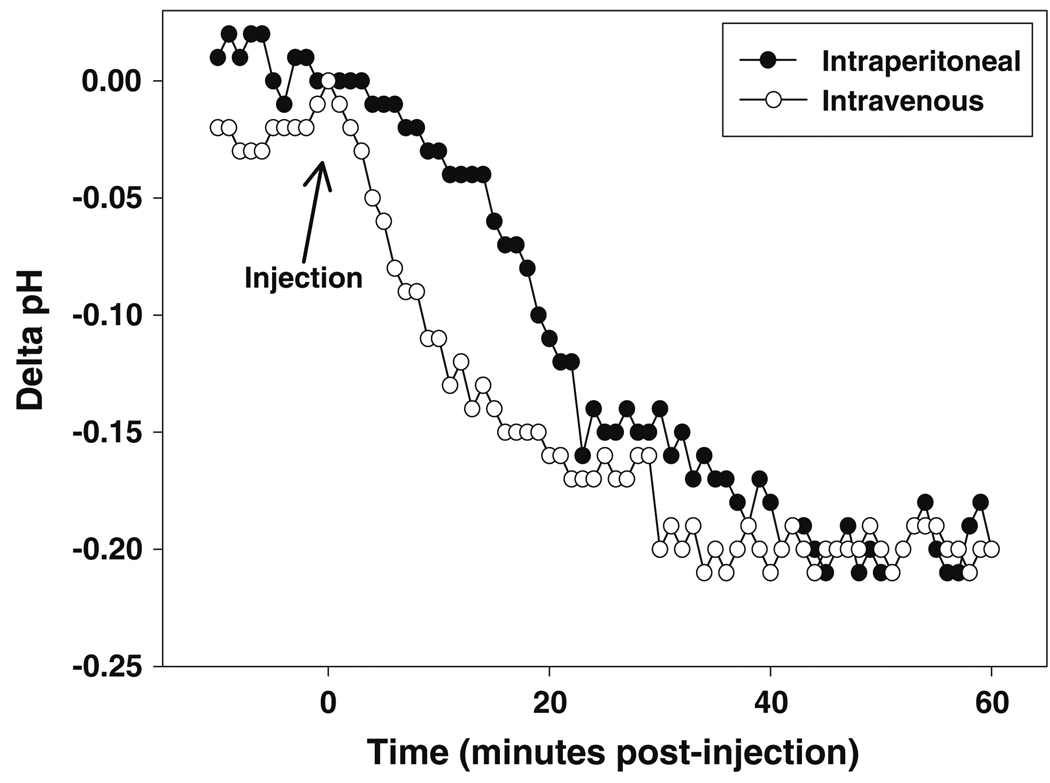
The extracellular pH of IMR-32 neuroblastoma xenografts can be reduced in a selective manner in vivo by pre-treatment with glucose in combination with MIBG to increase glycolysis while simultaneously inhibiting cellular respiration. Either intravenous or intraperitoneal pretreatment with MIBG/glucose acidified tumor extracellular pH by 0.2 pH unit by 30 min, an effect that was maintained for at least 60 min (Fig. 4). Antitumor activities of irinotecan and BACPTDP were minimal in this model, which employed a single 5 day cycle of treatment (Table 5). In contrast, pH modulation by MIBG plus glucose significantly delayed tumor growth for both of the CPT analogs compared to untreated controls, with BACPTDP showing the most effect (Table 5).
Fig. 4.
pH modulation by MIBG plus glucose in a rapidly growing human neuroblastoma tumor xenograft. Mice were anesthetized with ketamine and xylazine prior to insertion of a pH electrode into the tumor. Following equilibration, MIBG (32 mg/kg) and glucose (1.5 g/kg) were administered either intraperitoneally (open circle) or intravenously (filled circle) and the intratumoral pH monitored for 1 h
Table 5.
Impact of pH modulation on antitumor activity in IMR-32 neuroblastoma xenografts
| Treatment | Median survival (days) |
95% Lower confidence limit |
95% Upper confidence limit |
Log rank statistic |
P value |
|---|---|---|---|---|---|
| Control | 9.0 ± 0.7 | 7.6 | 10.4 | ||
| MIBG/Glu | 10.0 ± 0.8 | 8.4 | 11.6 | 1.727 | 0.189 |
| CPT-11 | 8.0 ± 2.4 | 3.3 | 12.7 | 0.313 | 0.576 |
| BACPTDP | 11.0 ± 0.4 | 10.2 | 11.8 | 3.346 | 0.067 |
| CPT-11 + MIBG/Glu | 11.0 ± 1.5 | 8.1 | 13.9 | 5.023 | 0.025 |
| BACPTDP + MIBG/Glu | 14.0 ± 4.0 | 6.1 | 21.9 | 9.743 | 0.002 |
Groups of 10 Balb/C nude mice bearing 150 mm3 subcutaneous xenografts of IMR32 neuroblastoma tumor were treated intraperitoneally on days 1–5 with BACPTDP (5 mg/kg) or irinotecan (40 mg/kg), or pretreated with MIBG (32 mg/kg) plus glucose (1.5 g/kg) 30 min prior to administration of BACPTDP or irinotecan. Survival was measured as time to reach three tumor volume doublings (computed as L × W2/2), followed by Kaplan–Meier survival analysis with log rank test for significance
Interaction of BACPT in combination with gemcitabine in pancreatic cancer cells
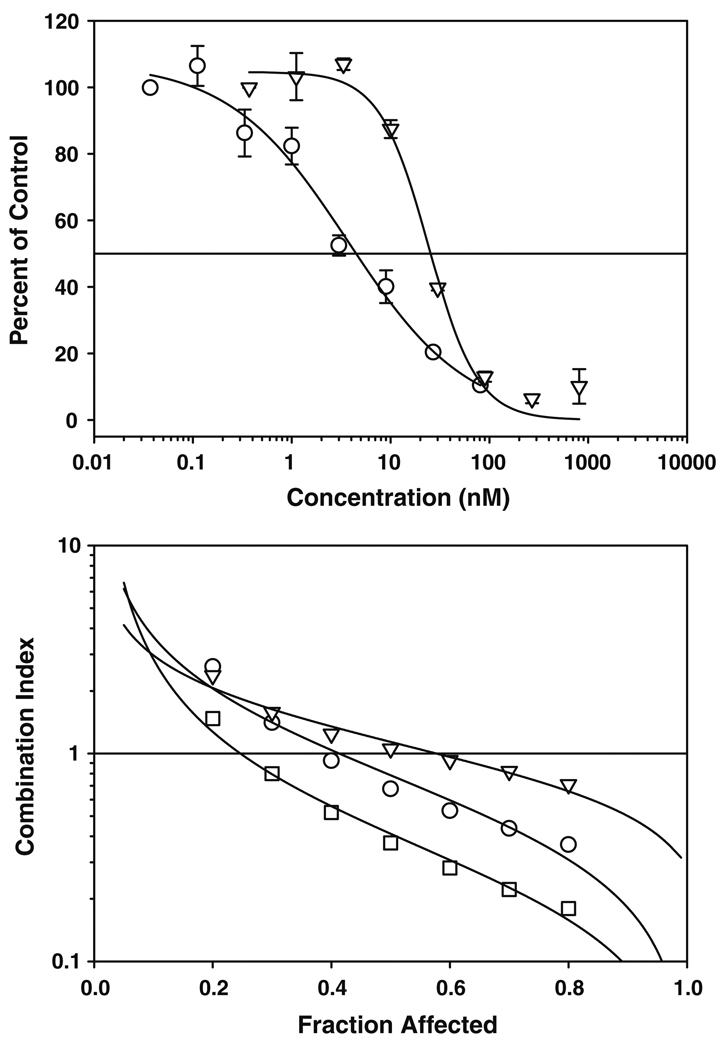
Gemcitabine is considered by most to be the standard-of-care for pancreatic cancer. Thus, interaction of BACPT with gemcitabine was evaluated by median effect analysis with the combination index (CI) endpoint. The results in Fig. 5 revealed that BACPT has additive (CI = 1) to synergistic (CI < 1) antiproliferative activity in combination with gemcitabine, particularly at higher levels of effect (fraction affected >0.5 = the IC50). Significant synergy was observed when gemcitabine was administered 24 h prior to BACPT, indicating sequence dependence for the interaction. This results in a significant dose reduction index (Table 6), such that at the IC50 the concentration of gemcitabine can be reduced threefold and that of BACPT over 20-fold and still obtain the activity of each agent used alone. Of note, significant drug antagonism was not seen at meaningful levels of activity (Fa ≥ 0.5), which is important for clinical planning. The impact of drug ratio was also evaluated and the additive/synergistic interaction proved to be ratio independent (Fig. 6), which is also advantageous for clinical use, since binary drug pharmacokinetics are difficult to control in vivo. In summary, BACPT was the most potent of ten compounds from seven drug classes tested in the chemorefractory Panc-1 model and demonstrated strong, sequence-dependent synergistic activity when used in binary combination with gemcitabine.
Fig. 5.
Impact of drug sequence on interaction of BACPT and gemcitabine in Panc-1 cells. Top panel dose–response curves for Panc-1 cells treated with BACPT (open circle) or gemcitabine (open inverted triangle) for 3 days followed by 2 days in drug-free medium. Surviving cells were quantified by ATP assay. Bottom panel Panc-1 cells were exposed simultaneously to BACPT and gemcitabine (open circle), to BACPT followed 24 h by gemcitabine (open inverted triangle), or gemcitabine followed by BACPT (open square) at a molar ratio of 100:1 (gemcitabine:BACPT) for 3 days followed by 2 days in drug-free medium. Surviving cells were quantified by ATP assay and the combination index computed as a function of the fraction of cells affected (fa), where fa = 0.5 is the IC50
Table 6.
Dose reduction index for gemcitabine followed by BACPT
| Fraction affected |
Dose reduction index | |
|---|---|---|
| Gemcitabine | BACPT | |
| 0.2 | 1.0 | 2.0 |
| 0.3 | 1.6 | 5.3 |
| 0.4 | 2.3 | 11.0 |
| 0.5 | 3.1 | 21.0 |
| 0.6 | 3.9 | 38.0 |
| 0.7 | 4.8 | 69.1 |
| 0.8 | 5.8 | 133.8 |
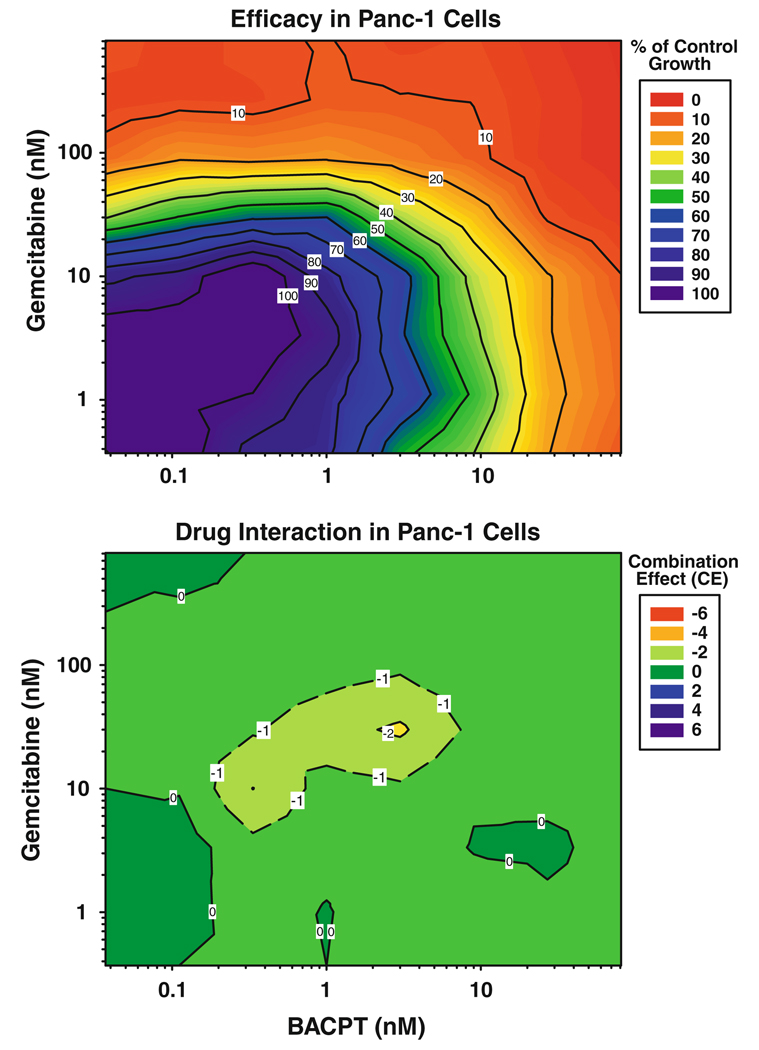
Fig. 6.
Impact of drug ratio on the interaction of gemcitabine followed by BACPT in Panc-1 cells. 3-dimensional drug interaction analysis was performed for gemcitabine followed 24 h later with BACPT in Panc-1 cells as described in “Methods”. Top panel percent inhibition of tumor cell growth compared to untreated controls, color-coded to indicate the range of response from minimum (blue) to maximum (red) inhibition. Bottom panel the corresponding combination effect (CE) surface is presented as a color-coded contour map where green represents additivity (CE = 0), red antagonism (CE < 0) and blue synergy (CE > 0)
Discussion
Other investigators have studied 7-alkyl-substituted 10-amino camptothecins. The 7-ethyl-10-amino CPT analog was first synthesized and evaluated by Yaegashi et al., in 1994, and reported to have potent activity in human KB and murine L1210 in vitro tumor models [44]. Guiotto et al. [23] reported that this analog also had antitumor activity in vivo against murine Meth A fibrosarcoma, notably as the sodium salt, which exists predominantly in the carboxylate form. A PEGylated formulation also had activity against murine P388 and Adriamycin-resistant P388 leukemia comparable to irinotecan. Recently, Kruszewski replaced the 7-ethyl group with the more lipophilic 7-trimethysilylethyl substituent to improve stability in blood by increasing membrane binding while reducing binding to human serum albumin; antitumor activities were not reported [28]. Meanwhile, Burke et al. reported nanomolar antiproliferative activity for BACPT in two human breast cancer models [9], activity that was maintained upon conjugation to antibodies directed against breast cancer surface antigens.
Our interest in BACPT initially stemmed from the analog’s enhanced activity in breast cancer cell lines when cultured at acidic pH [4, 5], and thus it’s potential to exploit the tumor pH gradient. Uptake and efflux data in MCF-7 cells [5] support the concept that BACPT behaves as a weak acid. This is certainly true for the carboxylate or open E ring form of the compound, which contains seven predicted protonization states (as modeled by ADME Suite software, ACD/Labs, Toronto, ON). At physiological pH, this in silico approach predicts that 67% of the drug species are negatively (−1) charged, while 33% are uncharged, making it a weak acid. Acidic pH (6.8) reduces the predicted net negative charge by nearly half to 34%, thus enhancing cell uptake of the uncharged species. The carboxylate form of CPT is generally considered inactive as an inhibitor of the molecular drug target, nuclear topoisomerase I. In contrast, the presumed active form of BACPT is the lactone, which is predicted to be 95% neutral at pH 7.4. Under acidic pH (6.8) conditions, the compound is predicted to become more basic (17% positively [+1] charged), which would decrease cellular uptake. Clearly, the majority of BACPT lactone species remains uncharged and therefore cell permeable. However, the precise mechanism that underlies the apparent ability of BACPT to exploit the tumor pH gradient is unclear and likely complex, since it involves not only the lactone versus carboxylate equilibrium and charge dynamics, but also the impact of membrane versus protein binding on this equilibrium. Human serum albumin, in particular, has a high affinity for the carboxylate form and is thought to govern blood stability. Another intriguing possibility has recently emerged with the discovery by Pommier and co-workers of mitochondrial topoisomerase I [45]. The enzyme functions at the alkaline pH characteristic of mitochondria, an organelle that is critical for apoptosis as well as for respiration. Mitochondrial topoisomerase I is inhibited by camptothecin upon incubation at pH 8.0, conditions that would favor formation of the carboxylate. Hence, mitochondrial topoisomerase I could be a cytotoxic target for the carboxylate form of the camptothecins. At pH 6.8, a significant amount of BACPT carboxylate is predicted to be uncharged and thus cell-permeable. Intracellular pH is physiological, which will generate negatively charged BACPT carboxylate species. Such weak acid species will accumulate in alkaline compartments like the mitochondria where they can potentially target mitochondrial top1. This hypothesis would be consistent with the punctuate cytoplasmic localization rather than nuclear localization observed for BACPT (D. Adams, unpublished observation), and reported for topotecan and gimatecan [14].
BACPT has unique physical–chemical properties in addition to its charge dynamics. For example, the analog exhibits high membrane permeability as reflected in its calculated Log D (2.8) at physiological or acidic pH and its solubility in dichloromethane (145 mg/ml), an unexpected result given the corresponding solubility of CPT (0.6 mg/ml), 10-amino-CPT (0.2 mg/ml) or 7-butyl-CPT (20 mg/ml). The 20S dipeptide formulation converts the lipophilic drug into a pro-drug that is much more water soluble (160 mg/ml) than irinotecan, yet does not require hepatic carboxyesterase activation and does not induce diarrhea. Moreover, the intrinsic fluorescence of BACPT is 40-fold greater than that of CPT, topotecan or irinotecan/SN-38, which permits tissue localization and therapeutic drug monitoring [3].
We have extended and confirmed our findings in breast cancer with evaluations of BACPT in neuroblastoma and pancreatic cancer cell culture models, both of which over-express the top 1 genotoxic target [42]. In addition, neuroblastoma retains the norepinephrine transporter and, therefore, selectively takes up the norepinephrine analog, meta-iodo-benzyl-guanidine (MIBG), a standard radioimaging agent for this pediatric tumor. Since MIBG acts to inhibit cellular respiration, it can also be used to modulate tumor extracellular pH by forcing glucose metabolism into the glycolytic pathway. Accordingly, when MIBG/glucose modulation was combined with BACPTDP treatment, there was a significant increase in survival of mice bearing IMR-32 xenografts. Since MIBG has been used to treat as well as image pediatric neuroblastoma, this cancer represents a clinical population that could potentially benefit from BACPTDP alone and in combination with MIBG.
Likewise, BACPTDP could also benefit patients with pancreatic cancer. Pancreatic carcinoma is characterized by avascular morphology and is generally hypoxic [27]. Hypoxia contributes to the aggressive nature of the disease via elevated expression of hypoxia inducible factor-1 (HIF-1) that in turn up-regulates expression of growth and proangiogenic factors to promote tumor invasion and metastasis. In addition, HIF-1 up-regulates expression of numerous glycolytic enzymes to activate the glycolytic phenotype [39]. BACPTDP was designed to exploit these conditions with enhanced tumor uptake, retention and antitumor activity via the classical topoisomerase I genotoxic mechanism that results in DNA damage and subsequent apoptosis. However, it is now possible that BACPTDP could also act by a second, non-genotoxic mechanism: direct inhibition of HIF-1α. Rapisarda and colleagues used a luciferase reporter construct driven by a hypoxia-responsive element to screen ~2,000 compounds from the National Cancer Institute “Diversity Set” for small molecule inhibitors of HIF-1[34]. Three of the four initial hits from this assay were camptothecin analogs, including topotecan. Recently, SN-38, the active metabolite of irinotecan, was also shown to inhibit HIF-1α and to synergize with rapamycin at low concentrations to induce extensive cell death in HT29 colon carcinoma cells under hypoxic but not normoxic conditions [33]. Since inhibition of HIF-1 requires catalytically active topoisomerase I but not DNA replication, this mechanism is distinct from that which induces genotoxic lesions [35], could be effective in non-cycling tumor cells and could explain the anti-angiogenic activity observed with both topotecan and irinotecan [13, 30]. Hence, the tumor biology of pancreatic cancer is particularly suited to treatment with BACPTDP as documented by the activity observed in Panc-1 xenograft tumors. Moreover, in vitro drug interaction studies indicated that BACPTDP could be a useful partner for combination with gemcitabine, particularly when given after treatment with this standard-of-care agent. Dose reduction data suggested that the synergistic interaction will permit a significant reduction in BACPTDP dose that could reduce toxicity while still maintaining efficacy.
In summary, BACPTDP is a water-soluble camptothecin pro-drug that spontaneously generates the lipid-soluble active agent, BACPT. The drug has multiple molecular mechanisms and can exploit solid tumor physiology for improved selectivity and activity against numerous tumor types with particular promise for pediatric neuroblastoma and pancreatic carcinoma.
Acknowledgments
This work was supported in part by Small Business Innovation Research grant CA125871 from the National Cancer Institute.
Abbreviations
- CPT
Camptothecin
- BACPTDP
7-butyl-10-amino-camptothecin-(20S) β-alanine-lysine
Contributor Information
David J. Adams, Email: adams041@mc.duke.edu, Duke University Medical Center, Durham, NC, USA.
William R. Waud, Southern Research Institute, Birmingham, AL, USA
Mansukh C. Wani, Research Triangle Institute International, Research Triangle Park, NC, USA
Govindarajan Manikumar, Research Triangle Institute International, Research Triangle Park, NC, USA.
James L. Flowers, Eno Research and Consulting, LLC, Hillsborough, NC, USA
Timothy A. Driscoll, Duke University Medical Center, Durham, NC, USA
Lee Roy Morgan, DEKK-TEC, Inc., New Orleans, LA, USA.
References
- 1.Adams DJ. The impact of tumor physiology on camptothecin-based drug development. Curr Pharma Med Chem Anticancer Agents. 2005;5:1–13. doi: 10.2174/1568011053352596. [DOI] [PubMed] [Google Scholar]
- 2.Adams DJ, Dewhirst MW, Flowers JL, Gamcsik MP, Colvin OM, Manikumar G, Wani MC, Wall ME. Camptothecin analogues with enhanced antitumor activity at acidic pH. Cancer Chemother Pharmacol. 2000;46:263–271. doi: 10.1007/s002800000157. [DOI] [PubMed] [Google Scholar]
- 3.Adams DJ, Black RD, Bolick NG, Richardson RA, Spasojevic I, Manikumar G, Wani MC, Dewhirst MW, Colvin OM. Utilization of a fiber optic biosensor to assess uptake of a fluorescent camptothecin in human tumor xenografts. AACR Meet Abstracts 981-a-.2005. [Google Scholar]
- 4.Adams DJ, da Silva MW, Flowers JL, Kohlhagen G, Pommier Y, Colvin OM, Manikumar G, Wani MC. Camptothecin analogs with enhanced activity against human breast cancer cells. I. Correlation of potency with lipophilicity and persistence in the cleavage complex. Cancer Chemother Pharmacol. 2006;57:135–144. doi: 10.1007/s00280-005-0007-6. [DOI] [PubMed] [Google Scholar]
- 5.Adams DJ, Wahl ML, Flowers JL, Sen B, Colvin M, Dewhirst MW, Manikumar G, Wani MC. Camptothecin analogs with enhanced activity against human breast cancer cells. II. Impact of the tumor pH gradient. Cancer Chemother Pharmacol. 2006;57:145–154. doi: 10.1007/s00280-005-0008-5. [DOI] [PubMed] [Google Scholar]
- 6.Adams DJ, Sandvold ML, Myhren F, Jacobsen TF, Giles F, Rizzieri DA. Anti proliferative activity of ELACYT (TM) (CP-4055) in combination with cloretazine ( VNP40101 M), idarubicin, gemcitabine, irinotecan and topotecan in human leukemia and lymphoma cells. Leuk Lymphoma. 2008;49:786–797. doi: 10.1080/10428190801935752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Adams D, Brueim S, Maelandsmo G, Fodstad O, Myhren F, Sandvold M. Drug combinations with gemcitabine 5’-elaidic acid ester (CP-4126) that exhibit synergistic activity in pancreatic cancer. Annual Meeting of the American Association for Cancer Research, AACR; Denver, CO. 2009. [Google Scholar]
- 8.Biedler J, Helson L, Spengler B. Morphology and growth, tumorigenicity, and cytogenetics of human neuroblastoma cells in continuous culture. Cancer Res. 1973;33:2643–2652. [PubMed] [Google Scholar]
- 9.Burke PJ, Senter PD, Meyer DW, Miyamoto JB, Anderson M, Toki BE, Manikumar G, Wani MC, Kroll DJ, Jeffrey SC. Design, synthesis, and biological evaluation of antibody-drug conjugates comprised of potent camptothecin analogues. Bioconj Chem. 2009;20:1242–1250. doi: 10.1021/bc9001097. [DOI] [PubMed] [Google Scholar]
- 10.Burris HA, Rivkin S, Reynolds R, Harris J, Wax A, Gerstein H, Mettinger KL, Staddon A. Phase II trial of oral rubitecan in previously treated pancreatic cancer patients. Oncologist. 2005;10:183–190. doi: 10.1634/theoncologist.10-3-183. [DOI] [PubMed] [Google Scholar]
- 11.Chou TC. Theoretical basis, experimental design, and computerized simulation of synergism and antagonism in drug combination studies. Pharmacol Rev. 2006;58:621–681. doi: 10.1124/pr.58.3.10. [DOI] [PubMed] [Google Scholar]
- 12.Ciccarone V, Spengler B, Meyers M, Biedler J, Ross R. Phenotypic diversification in human neuroblastoma cells: expression of distinct neural crest lineages. Cancer Res. 1989;49:219–225. [PubMed] [Google Scholar]
- 13.Clements MK, Jones CB, Cumming M, Daoud SS. Antiangiogenic potential of camptothecin and topotecan. Cancer Chemother Pharmacol. 1999;44:411–416. doi: 10.1007/s002800050997. [DOI] [PubMed] [Google Scholar]
- 14.Croce AC, Bottiroli G, Supino R, Favini E, Zuco V, Zunino F. Subcellular localization of the camptothecin analogues, topotecan and gimatecan. Biochem Pharmacol. 2004;67:1035–1045. doi: 10.1016/j.bcp.2003.10.034. [DOI] [PubMed] [Google Scholar]
- 15.Duxbury MS, Ito H, Zinner MJ, Ashley SW, Whang EE. siRNA directed against c-Src enhances pancreatic adenocarcinoma cell gemcitabine chemosensitivity. J Am Coll Surg. 2004;198:953–959. doi: 10.1016/j.jamcollsurg.2004.01.037. [DOI] [PubMed] [Google Scholar]
- 16.Flowers JL, Ludeman SM, Gamcsik MP, Colvin OM, Shao KL, Boal JH, Springer JB, Adams DJ. Evidence for a role of chloroethylaziridine in the cytotoxicity of cyclophosphamide. Cancer Chemother Pharmacol. 2000;45:335–344. doi: 10.1007/s002800050049. [DOI] [PubMed] [Google Scholar]
- 17.Flowers JL, Hoffman RM, Driscoll TA, Wall ME, Wani MC, Manikumar G, Friedman HS, Dewhirst M, Colvin OM, Adams DJ. The activity of camptothecin analogues is enhanced in histocultures of human tumors and human tumor xenografts by modulation of extracellular pH. Cancer Chemother Pharmacol. 2003;52:253–261. doi: 10.1007/s00280-003-0635-7. [DOI] [PubMed] [Google Scholar]
- 18.Furukawa T, Kubota T, Hoffman RM. Clinical-applications of the histoculture drug response assay. Clin Cancer Res. 1995;1:305–311. [PubMed] [Google Scholar]
- 19.Gabr A, Kuin A, Aalders M, ElGawly H, Smets LA. Cellular pharmacokinetics and cytotoxicity of camptothecin and topotecan at normal and acidic pH. Cancer Res. 1997;57:4811–4816. [PubMed] [Google Scholar]
- 20.Gatenby RA, Gawlinski ET. The glycolytic phenotype in carcinogenesis and tumor invasion: Insights through mathematical models. Cancer Res. 2003;63:3847–3854. [PubMed] [Google Scholar]
- 21.Gerweck LE, Seetharaman K. Cellular pH gradient in tumor versus normal tissue: potential exploitation for the treatment of cancer. Cancer Res. 1996;56:1194–1198. [PubMed] [Google Scholar]
- 22.Gerweck LE, Vijayappa S, Kozin S. Tumor pH controls the in vivo efficacy of weak acid and base chemotherapeutics. Mol Cancer Ther. 2006;5:1275–1279. doi: 10.1158/1535-7163.MCT-06-0024. [DOI] [PubMed] [Google Scholar]
- 23.Guiotto A, Canevari M, Orsolini P, Lavanchy O, Deuschel C, Kaneda N, Kurita A, Matsuzaki T, Yaegashi T, Sawada S, Veronese FM. Synthesis, characterization, and preliminary in vivo tests of new poly(ethylene glycol) conjugates of the antitumor agent 10-amino-7-ethylcamptothecin. J Med Chem. 2004;47:1280–1289. doi: 10.1021/jm031072e. [DOI] [PubMed] [Google Scholar]
- 24.Heiden MGV, Cantley LC, Thompson CB. Understanding the Warburg effect: the metabolic requirements of cell proliferation. Science. 2009;324:1029–1033. doi: 10.1126/science.1160809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hoffman RM. 3-Dimensional histoculture—origins and applications in cancer-research. Cancer Cells Mon Rev. 1991;3:86–92. [PubMed] [Google Scholar]
- 26.Kanzawa F, Nishio K, Fukuoka K, Fukuda M, Kunimoto T, Saijo N. Evaluation of synergism by a novel three-dimensional model for the combined action of cisplatin and etoposide on the growth of a human small-cell lung-cancer cell line, SBC-3. Int J Cancer. 1997;71:311–319. doi: 10.1002/(sici)1097-0215(19970502)71:3<311::aid-ijc1>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 27.Koong AC, Mehta VK, Le QT, Fisher GA, Terris DJ, Brown JM, Bastidas AJ, Vierra M. Pancreatic tumors show high levels of hypoxia. Int J Radiat Oncol Biol Phys. 2000;48:919–922. doi: 10.1016/s0360-3016(00)00803-8. [DOI] [PubMed] [Google Scholar]
- 28.Kruszewski S, Kruszewska DM. Affinity of new anticancer agent, 7-trimethylsilyl-ethyl-10-amino-camptothecin, to membranes and HSA determined by fluorescence spectroscopy methods. Optica Applicata. 2008;38:625–633. [Google Scholar]
- 29.Mahoney BP, Raghunand N, Baggett B, Gillies RJ. Tumor acidity, ion trapping and chemotherapeutics I. Acid pH affects the distribution of chemotherapeutic agents in vitro. Biochem Pharmacol. 2003;66:1207–1218. doi: 10.1016/s0006-2952(03)00467-2. [DOI] [PubMed] [Google Scholar]
- 30.O’Leary JJ, Shapiro RL, Ren CJ, Chuang N, Cohen HW, Potmesil M. Antiangiogenic effects of camptothecin analogues 9- amino-20(S)camptothecin, topotecan, and CPT-11 studied in the mouse cornea model. Clin Cancer Res. 1999;5:181–187. [PubMed] [Google Scholar]
- 31.Onda T, Nakamura I, Seno C, Matsumoto S, Kitagawa M, Okamoto K, Nishikawa K, Suzuki M. Superior antitumor activity of NK012, 7-ethyl-10-hydroxycamptothecin-incorporating micellar nanoparticle, to irinotecan. AACR Meet Abstracts 720-b-.2006. [Google Scholar]
- 32.Papish SW, Ramanathan RK, Pincus J, Hirmand M, Burris HA. Patients rescued by crossover to rubitecan in phase III study of rubitecan capsules versus 5-FU in pancreatic cancer. J Clin Oncol. 2005;23:349S. [Google Scholar]
- 33.Pencreach E, Guerin E, Nicolet C, Lelong-Rebel I, Voegeli AC, Oudet P, Larsen AK, Gaub MP, Guenot D. Marked activity of irinotecan and rapamycin combination toward colon cancer cells in vivo and in vitro is mediated through cooperative modulation of the mammalian target of rapamycin/hypoxia-inducible factor-1 alpha axis. Clin Cancer Res. 2009;15:1297–1307. doi: 10.1158/1078-0432.CCR-08-0889. [DOI] [PubMed] [Google Scholar]
- 34.Rapisarda A, Uranchimeg B, Scudiero DA, Selby M, Sausville EA, Shoemaker RH, Melillo G. Identification of small molecule inhibitors of hypoxia-inducible factor 1 transcriptional activation pathway. Cancer Res. 2002;62:4316–4324. [PubMed] [Google Scholar]
- 35.Rapisarda A, Uranchimeg B, Sordet O, Pommier Y, Shoemaker RH, Melillo G. Topoisomerase I-mediated inhibition of hypoxia-inducible factor 1: mechanism and therapeutic implications. Cancer Res. 2004;64:1475–1482. doi: 10.1158/0008-5472.can-03-3139. [DOI] [PubMed] [Google Scholar]
- 36.Sands H, Mishra A, Stoeckler JD, Hollister B, Chen SF. Preclinical activity of an i.v. formulation of rubitecan in IDD-P (TM) against human solid tumor xenografts. Anti-Cancer Drugs. 2002;13:965–975. doi: 10.1097/00001813-200210000-00009. [DOI] [PubMed] [Google Scholar]
- 37.Shanks RH, Rizzieri DA, Flowers JL, Colvin OM, Adams DJ. Preclinical evaluation of gemcitabine combination regimens for application in acute myeloid leukemia. Clin Cancer Res. 2005;11:4225–4233. doi: 10.1158/1078-0432.CCR-04-2106. [DOI] [PubMed] [Google Scholar]
- 38.Singh B, Li RG, Xu L, Poluri A, Patel S, Shaha AR, Pfister D, Sherman E, Goberdhan A, Hoffman RM, Shah J. Prediction of survival in patients with head and neck cancer using the histoculture drug response assay. Head Neck J Sci Spec Head Neck. 2002;24:437–442. doi: 10.1002/hed.10066. [DOI] [PubMed] [Google Scholar]
- 39.Stubbs M, Bashford CL, Griffiths JR. Understanding the tumor metabolic phenotype in the genomic era. Curr Mol Med. 2003;3:49–59. doi: 10.2174/1566524033361645. [DOI] [PubMed] [Google Scholar]
- 40.Tomida A, Tsuruo T. Drug resistance mediated by cellular stress response to the microenvironment of solid tumors. Anti Cancer Drug Design. 1999;14:169–177. [PubMed] [Google Scholar]
- 41.Tumilowicz J, Nichols W, Cholon J, Greene A. Definition of a continuous human cell line derived from neuroblastoma. Cancer Res. 1970;30:2110–2118. [PubMed] [Google Scholar]
- 42.Vassal G, Pondarre C, Cappelli C, Terrier-Lacombe MJ, Boland I, Morizet J, Benard J, Venuat AM, Ardouin P, Hartmann O, Gouyette A. DNA-topoisomerase I, a new target for the treatment of neuroblastoma. Eur J Cancer. 1997;33:2011–2015. doi: 10.1016/s0959-8049(97)00296-7. [DOI] [PubMed] [Google Scholar]
- 43.Wachsberger PR, Landry J, Storck C, Davis K, O’Hara MD, Owen CS, Leeper DB, Coss RA. Mammalian cells adapted to growth at pH 6.7 have elevated HSP27 and are resistant to cisplatin. Int J Hypertherm. 1997;13:251–255. doi: 10.3109/02656739709023533. (discussion) [DOI] [PubMed] [Google Scholar]
- 44.Yaegashi T, Sawada S, Nagata H, Furuta T, Yokokura T, Miyasaka T. Synthesis and antitumor-activity of 20(s)-camptothecin derivatives—a-ring-substituted 7-ethylcamptothecins and their e-ring-modified water-soluble derivatives. Chem Pharm Bull. 1994;42:2518–2525. doi: 10.1248/cpb.42.2518. [DOI] [PubMed] [Google Scholar]
- 45.Zhang HL, Barcelo JM, Lee B, Kohlhagen G, Zimonjic DB, Popescu NC, Pommier Y. Human mitochondrial topoisomerase I. Proc Natl Acad Sci USA. 2001;98:10608–10613. doi: 10.1073/pnas.191321998. [DOI] [PMC free article] [PubMed] [Google Scholar]