Abstract
Bursts and oscillatory modulations in firing rate are hallmark features of abnormal neuronal activity in the parkinsonian Globus Pallidus internus (GPi). Although often implicated together in the pathophysiology of parkinsonian signs, little is known about how burst discharges and oscillatory firing (OF) relate to each other. To investigate this question, extracellular single-unit neuronal activity was recorded from 132 GPi cells in 14 Parkinson’s disease patients. We found that burst firing was equally prevalent in OF and non-oscillatory firing (NOF) cells (p>0.5). More than half of the cells were characterized by either aperiodic bursty activity or OF, but not both. OF and NOF cells had statistically-indistinguishable levels of mean burstiness (p=0.8). Even when bursting and OF co-existed in individual cells, levels of burstiness and oscillatory power were seldom correlated across time. Interestingly, however, the few OF cells with spectral peaks between 8–13-Hz (α-range) were substantially burstier than other cells (p<0.01) and showed an unique burst morphology and stronger temporal correlations between oscillatory power and burstiness. We conclude that independent mechanisms may underlie the burst discharges and OF typical of most neurons in the parkinsonian GPi.
Keywords: Parkinson’s disease, microelectrode recording, intra-operative, electrophysiology, basal ganglia, spectral analysis
INTRODUCTION
Parkinson’s disease (PD) is a chronic neurologic disorder with cardinal motor signs of muscle rigidity, tremor, slowness of movement (bradykinesia), and/or paucity of movement (akinesia). The fact that lesions placed in the globus pallidus internus (GPi) significantly ameliorate most of these signs indicates that the GPi plays an important role in the pathophysiology of parkinsonian motor signs (Coban et al., 2009; de Bie et al., 1999; Vitek et al., 2003). In animal models of parkinsonism (Filion and Tremblay, 1991; Miller and DeLong, 1987; Raz et al., 2000), and in idiopathic PD (Dogali et al., 1994; Hutchison et al., 1994; Lozano et al., 1996; Vitek et al., 1998), GPi neurons exhibit a constellation of abnormalities in spiking activity including elevated firing rates and altered firing patterns.
Although classical models of PD pathophysiology focus on altered GPi firing rates, growing evidence suggests that an increased prevalence of burst discharges (Kaneoke and Vitek, 1996) may be more important in the pathophysiology of PD (Bergman et al., 1994; Boraud et al., 1996; Boraud et al., 1998; Boraud et al., 2000; Filion and Tremblay, 1991; Hutchison et al., 1997; Starr et al., 2005; Wichmann and Soares, 2006). The mechanistic underpinnings of elevated bursting activity and its true clinical significance remain unclear, however. For example, medical and surgical therapies that reduce PD motor signs do not consistently reduce bursting activity in the BG (Chen et al., 2001; Hahn et al., 2008; Levy et al., 2001; McCairn and Turner, 2009).
In addition to increased burst discharges, oscillatory firing (OF; abnormal rhythmic modulations in firing rate) in the α- (8–13-Hz) and β- (13–30-Hz) frequency ranges is a common characteristic of BG activity in both PD patients and animal models of PD (Gatev et al., 2006; Levy et al., 2002b; Rivlin-Etzion et al., 2006a; Starr et al., 2005; Weinberger et al., 2006). Treatments that ameliorate PD signs [e.g., dopamine replacement therapy (DRT)] also reduce α- and β- frequency oscillations in spiking (Heimer et al., 2006; Levy et al., 2001) and local field potential (LFP) activity (Brown et al., 2001) in the GPi. Other anti-parkinsonian therapies, such as Deep Brain Stimulation (DBS) are also reported to decrease α- and β- frequency oscillations in the GPi (McCairn and Turner, 2009). However, not all studies support these findings (Foffani et al., 2006). Thus, the mechanisms and clinical significance of oscillatory firing also remain a topic of debate (Degos et al., 2009; Leblois et al., 2007; Mallet et al., 2008).
While oscillations and bursts are each consistent features of the parkinsonian BG, it remains unclear whether the two occur independently or are closely linked phenomena. The oscillations in neuronal firing rate associated with parkinsonism are often described as periodic bursts of neural activity, which has led to the frequent assumption that oscillations and bursts are closely linked, co-occurring phenomena (Rubin and Terman, 2004; Terman et al., 2002). For example, Galvan and Wichmann suggested that oscillations may be caused by rebound bursting within BG loops (Galvan and Wichmann, 2008). In contrast, based on theoretical considerations, Kaneoke and (1996) proposed that oscillations and bursts may represent two distinct processes. Existing evidence suggesting they may be separate phenomena includes the observation that not all OF is bursty (Wichmann and Soares, 2006), and the fact that bursts and oscillations are not affected in a similar manner by pharmacologic and surgical therapies (see above). Here, we determined the degree to which OF and bursty activity in the GPi of PD patients are related to each other and to the severity of parkinsonian signs. An improved understanding of the relationship between bursts and oscillations will facilitate the analysis of pathophysiologic relationships between types of abnormal GPi activity patterns and specific parkinsonian signs.
METHODS
Patient population
Single unit recordings in the GPi were obtained from patients with PD undergoing microelectrode-guided stereotactic surgery for the placement of GPi DBS electrodes. All patients were responsive to levodopa (3,4-dihydroxy-L-phenylalanine) and had developed levodopa-induced dyskinesias or motor fluctuations. The severity of disease was assessed prior to surgery according to 27 sections of the Unified Parkinson’s Disease Rating Scale (UPDRS). The UPDRS scores were assessed by different neurologists approximately one month prior to surgery, both off and on DRT. The responsiveness to DRT for each patient was quantified as the percentage improvement in his or her total UPDRS score following DRT. Scores were not available for 3 of the 14 patients. Anti-parkinsonian medications were withheld for at least 12-hr before the surgery and all PD patients displayed overt parkinsonian symptoms without dyskinesias during the procedure. PD patients with severe off-period dystonia were excluded from the study. All subjects gave informed consent according to a protocol approved by the University of California San Francisco Institutional Review Board. All work was carried out in accordance with the Code of Ethics of the World Medical Association.
Surgical procedures and data collection
The methods used for microelectrode-guided stereotactic implantation of DBS electrodes in the GPi were similar to those described previously (Starr, 2002). Single-unit recordings were obtained using glass-coated platinum/iridium microelectrodes with impedance 0.4–1.0-MΩ (Microprobe, Gaithersburg, MD, or FHC, Inc., Bowdoin, ME). Signals were bandpass filtered (300–5000-Hz), amplified, played on an audio monitor, displayed on an oscilloscope, and digitized (20-kHz sampling rate) using the Guideline System 3000 or 4000 (FHC, Inc.). Microelectrodes were advanced into the brain using a motorized microdrive (FHC, Inc.). In a typical surgical case, one to two microelectrode penetrations separated by 2–3-mm were made serially through the GPi on each side. The GPe and GPi were distinguished by recording a 1–2 mm interval of electrical silence corresponding to the white matter laminae between the GPe and GPi. Cells were recorded at approximately every 300–800-µm along each trajectory through the GPi. Spontaneous neuronal activity of well-isolated cells was collected for 37.7-sec on average (SD = 18-sec).
All patients were sedated with propofol for the initial surgical incision and skull opening. Propofol was stopped at least 30-min prior to neuronal recording, which is sufficient time to wash out its known effect on single unit discharge (Raz et. al, 2008). All patients were awake and alert, and were asked to remain as still as possible with eyes open during periods of neuronal recording.
Data Analysis
Digitized spike trains were imported into off-line spike sorting software (Plexon Inc., Dallas TX) for discrimination of single unit action potentials by cluster-cutting in principal components space. This software generated a record of the time of occurrence (reduced to millisecond accuracy) for each action potential waveform detected. The spike times were used to calculate discharge rate, bursting, and oscillatory activity (see following text). Analyses were performed in the Matlab computing environment (The Mathworks, Natick, Massachusetts). Neuronal data were included in this study only if action potentials could be discriminated with a high degree of certainty as indicated by the presence of a clear refractory period in the inter-spike interval (ISI) histogram (>3-msec). Neurons were excluded if the size of their action potentials varied considerably in unison with the cardiac cycle. Figure 1A illustrates the single unit isolation that was typical for the recordings used in this study.
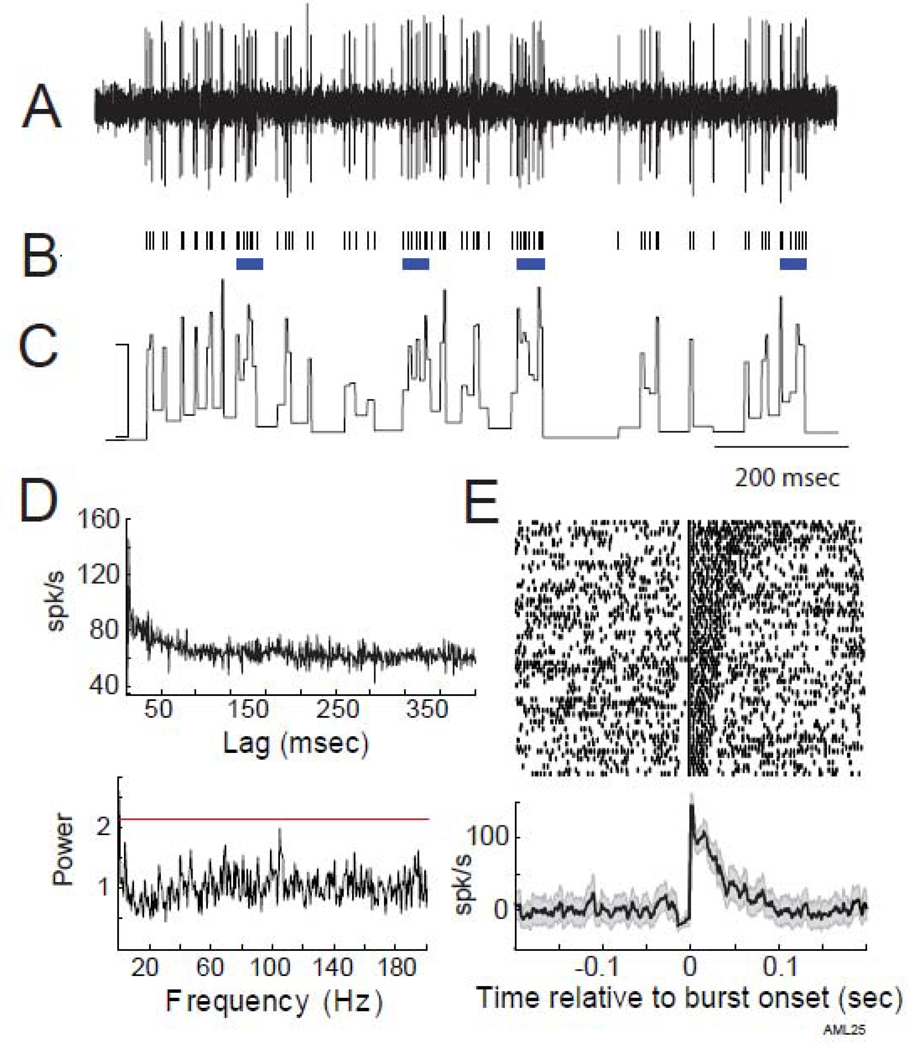
Figure 1.
Exemplar data from an NOF bursting cell. A) A short segment of raw extracellular recording illustrating the well-isolated action potentials of a single neuron. B) After spike sorting, spike times were extracted and are represented here in a rasterplot. Bursts, as detected by our detection algorithm, are identified by horizontal blue lines below the rasterplot. C) Instantaneous firing rate of this neuron during the same time segment (calibration: 200-spk/s). D) There was no evidence for oscillatory firing in either the autocorrelation (top) or normalized power spectrum (bottom) generated from all spikes from this neuron. The horizontal red line indicates the significance threshold. E) Peri-burst rasters from the same cell aligned on burst onset and sorted according to burst duration (top) with the mean peri-burst frequency of firing averaged across all bursts (bottom). Shaded gray region surrounding the peri-burst frequency of firing indicates the 95% confidence interval around the mean. Firing rates were normalized by the mean firing rate in the 200-msec preceding burst onset. Note the pre-burst “notch” in firing rate, the maximum intra-burst firing rate at burst onset, and the gradual decline in firing rate thereafter.
Burst Firing
Bursts were detected using the Poisson Surprise Method (Legendy and Salcman, 1985; Wichmann et al., 1999). Each period of increased neuronal discharge was assigned a surprise value (S) that quantified the likelihood that this period of activity was a burst (i.e., a discrete period of elevated firing rate), rather than part of the neuron’s ongoing stochastic firing pattern. The surprise value (S) can be represented as:
where P is the probability that a Poisson spike train with the same mean firing rate would generate more spikes than emitted by the burst in the same time period. Bursts were identified as a sequence of at least three inter-spike intervals (ISIs) with a Poisson surprise value >5 (P<0.00001). For each such burst, immediately-adjacent preceding and trailing spikes were added incrementally to maximize the Poisson surprise value. The onset and offset of a burst were set as the times of the first and last spikes of the burst. Figure 1B provides an example of this burst detection method in operation. The overall “burstiness” of a cell was quantified as the fraction of spikes that occurred during bursts relative to the total number of spikes in the cell’s recorded spike train. The fraction of time each cell spent in bursts was also calculated. The two measures of burstiness (fraction of spikes and fraction of time in bursts) were found to correlate very closely with each other across cells (Spearman R=0.99, p<0.0001, not shown), indicating that the two measures were redundant. All subsequent analyses used fraction of spikes in bursts as the single measure of the overall amount of burst discharges (“burstiness”) in a cell’s spike train.
We examined the magnitude and timing of changes in a neuron’s mean firing rate around the time of burst onset (i.e., burst morphology) by generating burst-triggered averages of a neuron’s instantaneous firing rate (i.e., ISI−1) for each cell in which>5 bursts were detected. To aid comparisons between cells, a cell’s mean firing rate during the 200-msec prior to burst onset was subtracted from its peri-burst average.
Oscillatory Activity
Rhythmic modulations in neuronal firing rate (i.e., “oscillatory” firing) were quantified using a spike shuffling method (Rivlin-Etzion et al., 2006b) designed to control for artifactual autocorrelations that arise from the neuronal refractory period. Neuronal spike times were represented as a delta function with a temporal resolution of 1-msec. The discrete Fourier transform was applied to non-overlapping 2048-msec segments of the spike delta function smoothed with a Hanning window of the same length. This yielded spectral density estimates for frequencies between 0.25 and 500-Hz with a resolution of 0.5-Hz. Distortions of these estimates attributable to a neuron's refractory period were compensated for by dividing the actual spectrum by a normalizing spectrum, computed from the same data but after global-shuffling of the ISIs (Rivlin-Etzion et al., 2006b). The normalizing spectrum was the mean of spectra computed from each of 1,000 random global shufflings of the same ISIs. Peaks in the normalized spectra between 0.25 and 200-Hz were tested for significance relative to the SD of the normalized spectra in the 300–500-Hz "control" range (Rivlin-Etzion et al., 2006b). Peaks in the normalized spectrum were considered significant if they exceeded a threshold designed to yield an omnibus p< 0.004 (actual p=1×10−5 after correcting for 400 comparisons). Cells with ≥1 significant peak(s) between 0.25–200-Hz were termed oscillatory firing (OF) cells. Autocorrelations were also computed to provide a qualitative assessment of a neuron’s oscillatory firing.
Temporal relationship between oscillatory and burst activity
The temporal co-variation between bursts and oscillations was determined for each cell that exhibited at least one burst and one significant spectral peak above 2-Hz. We tested whether the prevalence of bursts and the strength of oscillatory firing co-varied in time across a cell’s spike train. To obtain time-resolved estimates of the strength (power) of a cell’s oscillatory firing, the cell’s instantaneous firing frequency was first band-pass filtered using a filter custom-tuned to each cell’s significant oscillatory frequency (Parks-McClellan optimal FIR in Matlab; pass-band and stop-band cutoffs ±1-Hz and ±2-Hz, respectively, relative to a significant spectral peak). Figure 2B illustrates an example frequency of firing record before and after band-pass filtering. The band-pass range prevented analysis of oscillations <2-Hz. The resulting band-pass filtered signal was divided into 1-sec segments and root mean squared (RMS) power was computed separately for each segment. The burstiness of the cell’s spike train (% of spikes in bursts) was also computed separately for each of the same 1-sec segments. [Parallel analyses were performed using other segment lengths (range 0.1-sec to 5-sec) and the results did not differ substantively.] These time-resolved measurements of RMS oscillatory power and burstiness were entered into a Spearman correlation from which the R value represented the degree to which a cell’s oscillatory activity and burstiness co-varied in time across the recording. For cells with more than one significant spectral peak >2-Hz and at least one burst, time-resolved RMS power was computed and related to burstiness separately for each significant spectral peak. Note that at a fine temporal scale, bursts of neuronal activity always coincided with positive phases in the band-pass filtered signal such that the phase relation between bursts and oscillations was constant.
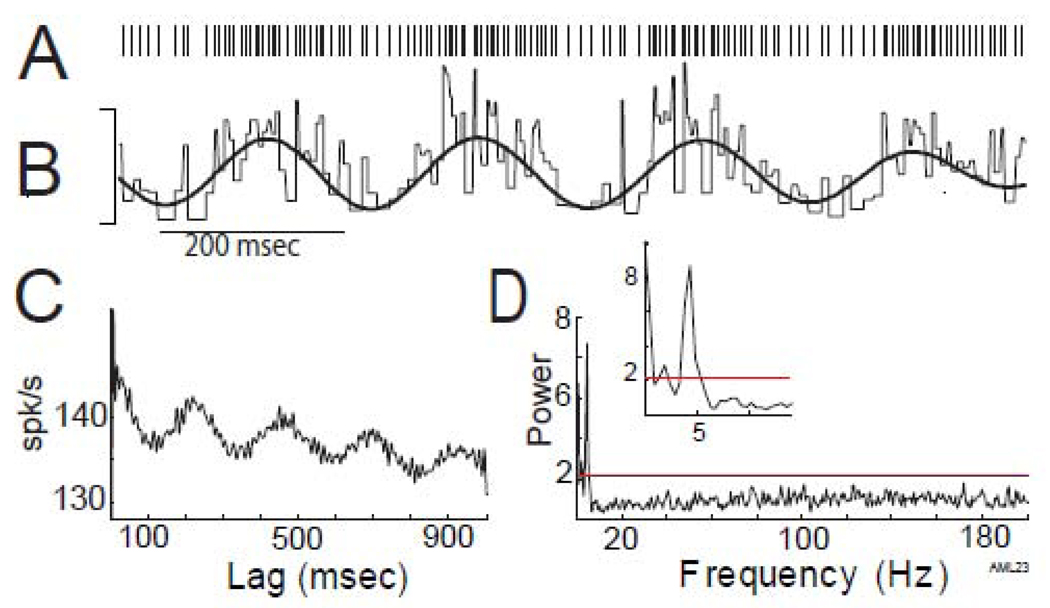
Figure 2.
Oscillatory modulation in firing rate in the absence of burst firing. A) Rasterplot of a short segment of a spike train containing rhythmic modulations in firing rate but no burst firing. B) The raw instantaneous firing rate of this neuron (thin line) during the same time segment (calibration: 200-spk/s) overlaid by a band-pass filtered (4.4-Hz) version of the same instantaneous firing frequency (thick line). This spike train exhibited prominent oscillatory modulations in firing rate at 4.4-Hz, demonstrated by multiple peaks at regular intervals in the autocorrelation (C) and a significant peak centered on 4.4-Hz in the normalized power spectrum (D, exploded in inset).
RESULTS
Cell database and general discharge characteristics
One-hundred and thirty-two GPi cells were studied in 14 PD patients. Recording durations of individual cells were 37.7±18-sec (mean +/− SD). Fifty-five cells were classified as oscillatory firing (OF) cells. The remaining 77 cells with no significant spectral peaks were classified as non-oscillatory firing (NOF) cells. Results from the oscillation analysis were summarized by grouping OF cells by standard frequency bands [0.25–3 (delta), 3–8 (theta), 8–13 (alpha), 13–30 (beta), 30–60 (low gamma),>60-Hz (high gamma)] (Table 1). A chi-squared analysis revealed that oscillatory firing occurred most often at frequencies below 8-Hz (χ2=34.4, p<0.0001, 5 df). One-hundred and eight cells (81.8%) had at least 1 burst, while sixty-four cells (48.5%) had >5 bursts.
Table 1. Firing properties of OF cells by frequency band.
The number of cells (and percentage relative to all OF cells) with significant spectral peaks is indicated for each frequency band. Means ± SEM of the burstiness (% spikes in bursts) and average firing rates (Avg FR) are also provided for each frequency band.
| Frequency band | Num. (%) cells | Burstiness (%) | Avg FR (spk/s) |
|---|---|---|---|
| All frequencies | 55 (100) | 4.5 ± 0.9 | 101 ± 3.6 |
| < 3 Hz (delta) | 24 (44)* | 5.6 ± 1.4 | 94.7 ± 4.4 |
| 3–8 Hz (theta) | 25 (45)* | 4.6 ± 1.4 | 110.1 ± 5.4 |
| 8–13 Hz (alpha) | 5 (9) | 15.7 ± 4.5† | 86.8 ± 9.2 |
| 13–30 Hz (beta) | 6 (11) | 5.5 ± 2.7 | 116.6 ± 15.2 |
| 30–60 Hz (gamma low) | 3 (5) | 1.6 ± 1.1 | 87.3± 8.9 |
| > 60 Hz (gamma high) | 10 (18) | 2.9 ± 1.6 | 98.9 ± 9.3 |
OF was more common at frequencies <8-Hz (chi-square test, p<0.01).
burstiness was elevated in the five cells with alpha-range oscillatory firing when compared to all other OF cells combined (Mann-Whitney test, p<0.01). The number of cells listed in different frequency bands is greater than the total number of OF cells because 13 cells had more than one spectral peak.
Bursts and oscillations as independent phenomena
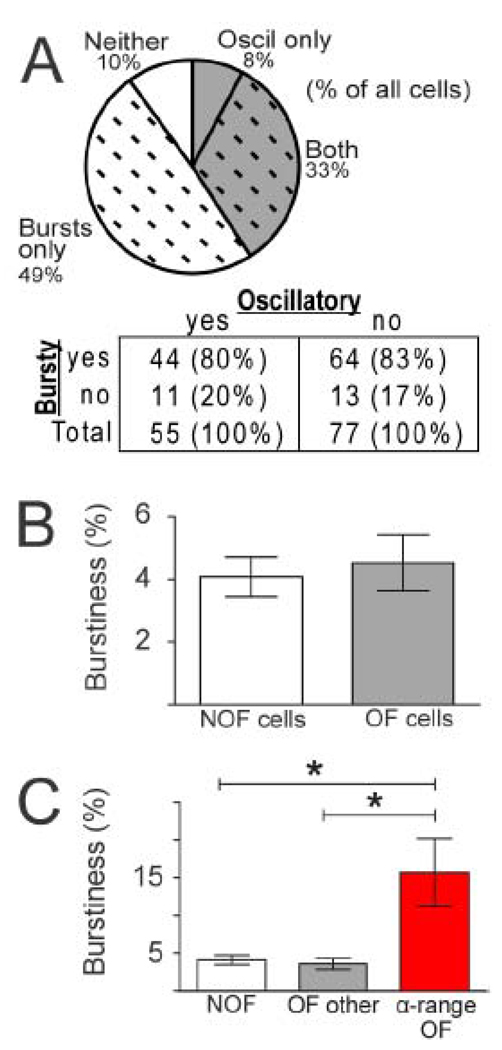
Bursts and oscillations were found to be independent properties of spiking activity in the parkinsonian GPi. The fraction of cells with bursty firing (i.e., at least one burst) was very similar for OF neurons (44 of 55 cells, 80%) and NOF neurons (64 of 77 cells, 83%;χ2=0.2, p>0.5; Fig. 3A inset). Furthermore, most cells exhibited either bursts or oscillations, but not both.
Figure 3.
A) Bursty firing (dashed hatching, Bursty "yes" in table inset) was found in equally large fractions of cells that had oscillatory firing (gray shading, Oscillatory “yes” in table inset) and cells that did not have oscillatory firing (Oscillatory “no” in table inset). B) The mean burstiness (% spikes in bursts) of OF cells was not significantly different from that of NOF cells (Mann-Whitney test, p=0.8). C) The mean burstiness of cells with spectral peaks in the α-range was significantly greater than that of NOF cells and all other OF cells (Kruskal-Wallis test, p<0.01). Columns represent mean +/− SEM. *denotes p<0.01 in Dunn’s post tests.
Figure 1 illustrates the firing characteristics of a typical GPi cell that exhibited bursts without oscillations. Bursts (as detected by our algorithm) are identified by blue horizontal lines below the rasterplot in Figure 1B and are sorted according to burst duration in the burst onset-aligned rasterplot in Figure 1E. Even though the activity of the exemplar cell in Figure 1 was quite bursty (28% of this cell’s spikes occurred in bursts), its autocorrelation and power spectrum were notably flat (Fig. 1D, top and bottom, respectively), providing no evidence for oscillatory modulations in firing.
Other GPi cells (e.g., Fig. 2) had no detectable bursts, but exhibited prominent oscillatory activity, as evidenced by periodicity in the auto-correlogram (Fig. 2C) and a significant peak in the power spectrum (Fig. 2D). Like the examples shown in Figures 1 and 2, most cells exhibited bursts without oscillations (64 cells), or oscillations without bursts (11 cells) (Fig. 3A). These observations are inconsistent with the idea that oscillatory and bursty activity are closely-related phenomena in the parkinsonian GPi.
The independence of bursting and oscillatory activity was substantiated further by comparing the mean burstiness of all OF cells with that of NOF cells. The mean fraction of spikes occurring in bursts for all OF cells (4.5% +/− 0.9; mean +/− SE) was very similar to that for NOF cells (4.1% +/− 0.6; Mann-Whitney test, p=0.8; Fig. 3B). Therefore, burst firing occurred at statistically-indistinguishable levels in cells that displayed oscillatory activity and in cells that did not. Furthermore, across all OF cells, there was no general relationship between the power of a cell’s significant spectral peaks and the cell’s burstiness (Spearman R=0.09, p=0.4; not shown).
Cells with α-range oscillatory activity were burstier than other cells
Oscillatory activity within specific frequency bands is thought to be more “pathologic” than oscillatory activity in other frequency bands (Gatev et al., 2006; Levy et al., 2002a; Rivlin-Etzion et al., 2006a; Starr et al., 2005; Weinberger et al., 2006). Therefore, we explored the possibility that burstiness was more closely associated with oscillatory activity within specific frequencies. The mean burstiness values of OF neurons (% spikes in bursts) were compared across different frequency bands. For cells with oscillations in multiple frequency bands (n=13), the cell’s burstiness value was included in each of those frequency bands. This analysis revealed substantially higher burstiness in cells that had ≥1 significant spectral peak in the α-range (15.7 +/− 5.4% spikes in bursts; mean +/− SEM, Fig. 3C) compared with other OF cells (3.6% +/− 0.7) and NOF cells (4% +/− 0.6; Kruskal-Wallis test, p<0.01; Dunn’s post-tests p<0.01 Fig. 3C). Thus, OF cells with spectral peaks in the α-range were substantially burstier than all other cells. While only 4% of all cells (5 cells total) exhibited oscillatory activity in the α-range, these five cells were recorded from five different patients.
Unique burst morphology of α-range OF cells
The unusually high burstiness of cells with α-range oscillatory activity prompted further analyses. Figure 4 depicts the firing characteristics of GPi cells with α-range oscillatory firing. Comparison with Figure 1 draws attention to the unique features of bursting exhibited by α-range cells. Bursts in pallidal cells typically appear as an abrupt increase in firing rate preceded by a small reduction in firing (i.e., a longer than normal ISI; Fig. 1E) (Wichmann and Soares, 2006). Additionally, firing rates within bursts usually peak at the time of burst onset and trail off smoothly to the baseline firing rate after that (Fig. 1E bottom).
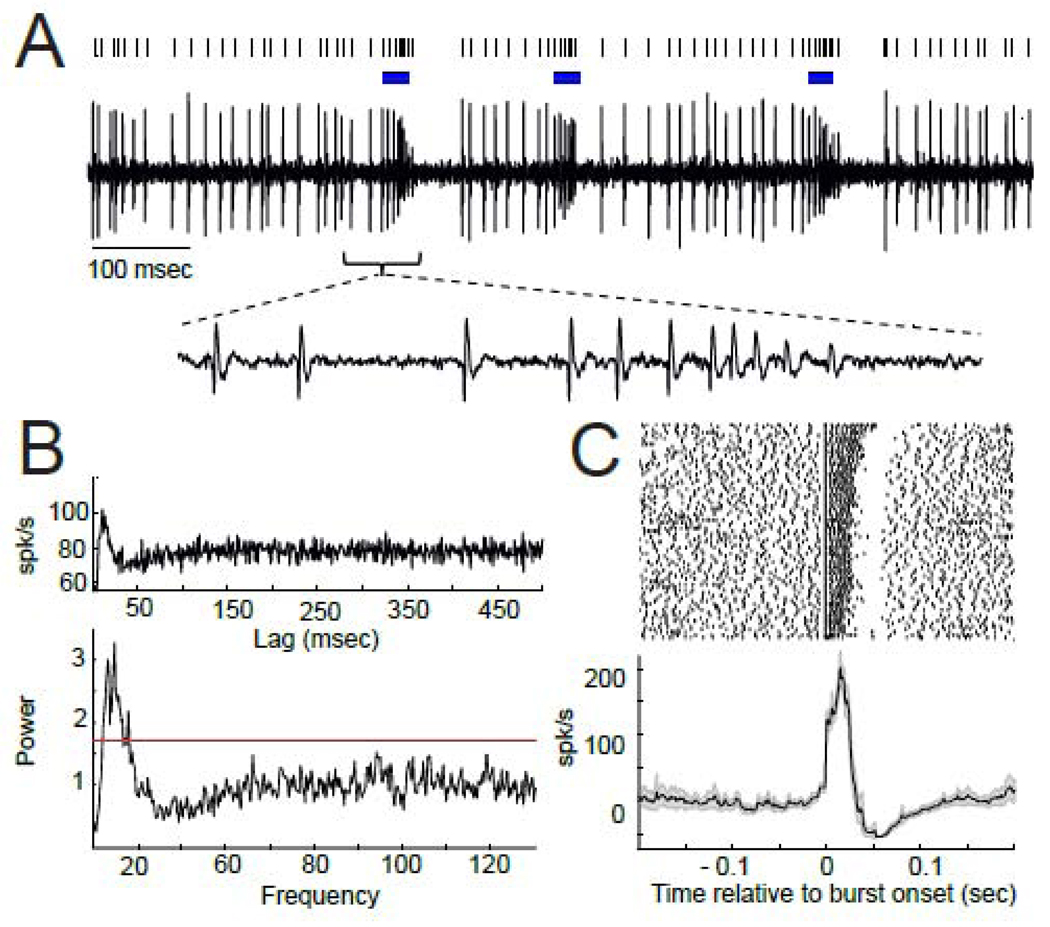
Figure 4.
Cells with α-range OF exhibited elevated burst firing and a unique form of burstiness. A) A segment of raw data from an α-range OF cell with raster plot above indicating times of burst firing (blue lines). A sub-panel below shows a time-expanded view of raw spike data from an exemplar burst to illustrate the accelerating firing rate during the burst accompanied by progressively decrementing action potentials. B) Marked modulations in the cell’s autocorrelation (top) and significant spectral peaks in the α and β frequency ranges of the power spectrum (bottom) are consistent with strong OF. C) Peri-burst rasters from the same cell aligned to the onset of the burst (top) and the mean peri-burst frequency of firing across all bursts (bottom). Otherwise, the figure follows the conventions outlined for Fig. 1. Note the absence of a pre-burst notch in firing rate, the accelerating firing rate during the burst, and abrupt cessation of firing with burst offset.
In contrast, the firing rates of α-range cells showed no evidence of a deceleration or pause preceding burst onset (Fig. 4). Within bursts, these cells' firing rates accelerated progressively until the burst was terminated abruptly by a long-lasting cessation in firing (Fig. 4A, C). This within-burst acceleration in firing rate was accompanied by a progressive diminution in action potential magnitude (Fig. 4A inset), likely due to progressive failure of the cell’s voltage-gated channels to reactivate fully.
The differences in burst morphology were substantiated by a comparison of population averages of peri-burst firing rates across the different cell categories (Fig. 5). Bursts in NOF cells and other (non-α) OF cells showed a clear notch in firing rate immediately before burst onset, their peak intra-burst firing rate occurred at the time of burst onset, and they exhibited a gradual decline in firing rate thereafter. In contrast, bursts in α-range OF cells showed no sign of a pre-burst notch, their firing rates accelerated during bursts, and they were followed by a marked post-burst decrease in firing rate. The bursts of α-range OF cells shared a strikingly similar morphology, as illustrated by the narrow 95% confidence intervals in Figure 5.
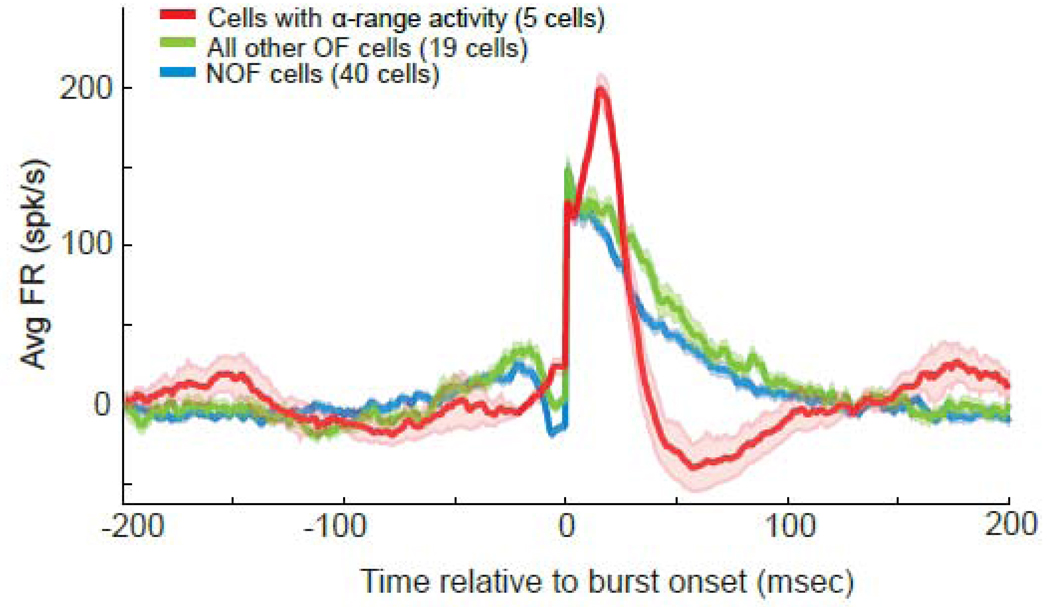
Figure 5.
Population averages of mean peri-burst frequency of firing for α-range OF cells, NOF cells, and all other OF cells with > 5 bursts. Firing rates were normalized individually by each cell’s mean firing rate in the 200-msec preceding burst onset prior to averaging across cells. Shaded areas denote 95% confidence intervals for each population mean. Note the shorter burst duration and greater maximum intra-burst firing rate that occurred later in the bursts in α-range OF cells in comparison with all other OF and NOF cells.
Quantitative analyses of burst metrics supported the differences in burst morphology described above. When compared with bursts in other cell populations, the bursts of α-range OF cells: (a) had a shorter duration, and a (b) greater maximum intra-burst firing rate (c) that peaked later in bursts (Kruskal-Wallis tests, all p values < 0.05; Table 2). The distinctive burst properties of α-range OF cells could not be attributed to differences in the strength of oscillatory activity. The mean power of significant spectral peaks in the α-range was no different from that of significant peaks in any other frequency band (Dunn’s post-test comparisons, p>0.05). It is also unlikely that this type of bursting activity was due to some form of injury discharge. The average recording duration for the five α-range cells was 51 +/− 7.5-sec. Visual inspection of the raw neuronal recording data confirmed that the similar unique bursting activity was present throughout the duration of each recording.
Table 2. Comparing peri-burst morphology of different cell populations.
Means ± SEM of burst duration, maximum intra-burst firing rate, and latency of maximum firing rate are provided for each category.
| Burst duration | Max Intra-burst FR | Latency of Max FR | |
|---|---|---|---|
| NOF cells | 46 +/− 2 ms* | 163 +/− 7 spk/s* | 8.7 +/− 1.6 ms * |
| α-range cells | 32 +/− 3 ms | 205 +/− 9 spk/s | 17.6 +/− .7 ms |
| All other OF cells | 53 +/− 3 ms* | 162 +/− 5 spk/s* | 8 +/− 2 ms * |
The cells with α-range activity had a shorter burst duration, and a longer maximum intra-burst firing rate that occurred later in the burst than other cells (Kruskal-Wallis test, p<0.05, Dunn's post test in comparison with α-range cells, p<0.05).
Burstiness, OF, and Firing Rate
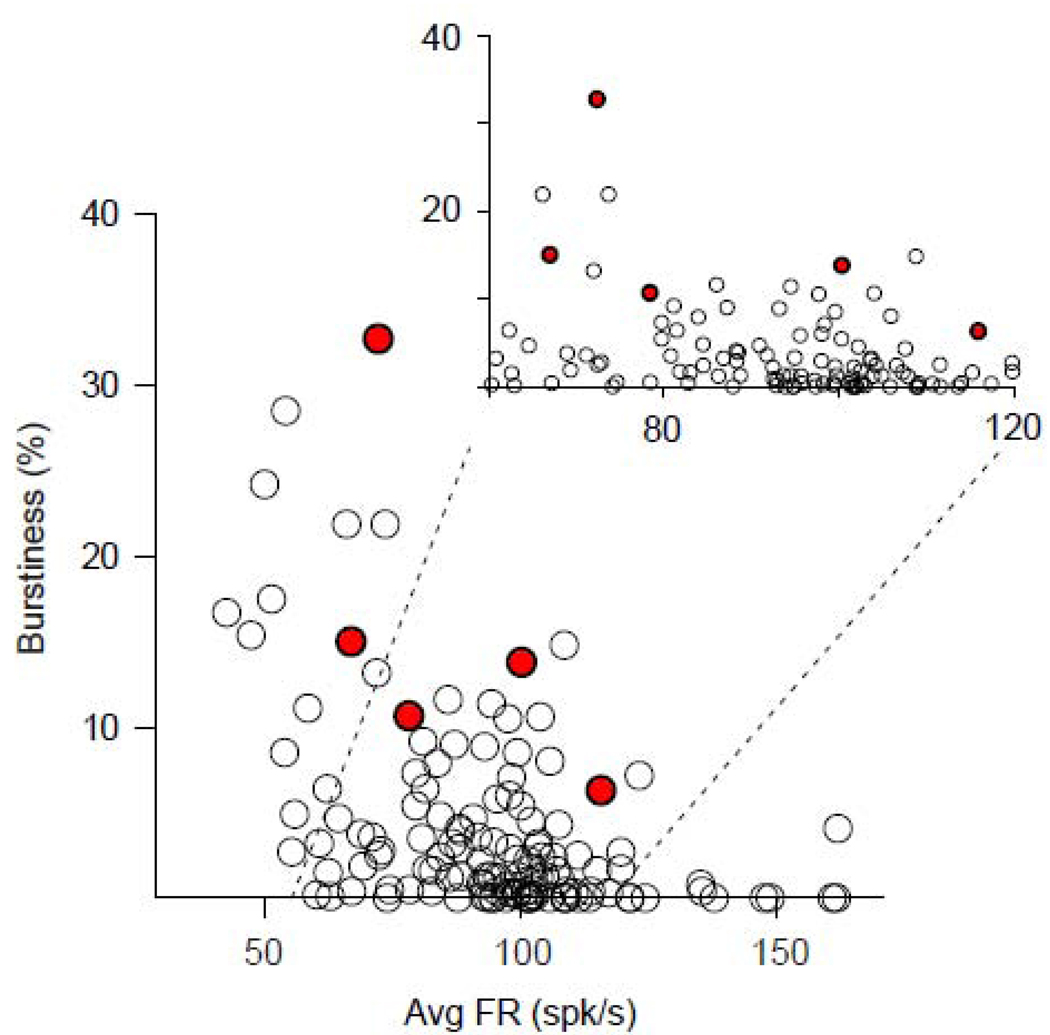
Burstiness varied as an inverse function of a cell’s mean firing rate within the general population of GPi neurons. There was a significant (p<0.0001; Spearman R=−0.48) negative correlation between a neuron’s firing rate and its burstiness (Fig. 6). The increased burstiness of α-range OF cells followed that general relationship. Notably, cells with α-range activity tended to be burstier (Fig. 6, red dots) than other cells with similar firing rates (Fig. 6, open symbols).
Figure 6.
Burstiness was correlated inversely with mean firing rate (Spearman R=−0.48, p<0.0001). Each point in the graph represents one burst-firing cell. The values for α-range OF cells (red circles) fall within the general anti-correlated relationship identified for all bursty neurons.
Unlike burstiness, OF was associated with higher firing rates. Specifically, the mean firing rate of OF cells (101±3.6-spk/s; mean +/− SEM) was greater than that of NOF cells (89.7±2.1-spk/s; Mann-Whitney test, p<0.01). Interestingly, the mean firing rate of α-range OF cells (86.8±9.2 spk/s) was lower than that of other OF cells (102.4±3.8 spk/s) and even of NOF cells (89.7±2.1 spk/s) although those differences were not significant due to the small number of α-range OF cells (Dunn’s post test p>0.05).
Weak temporal co-variation between bursts and oscillations in individual cells
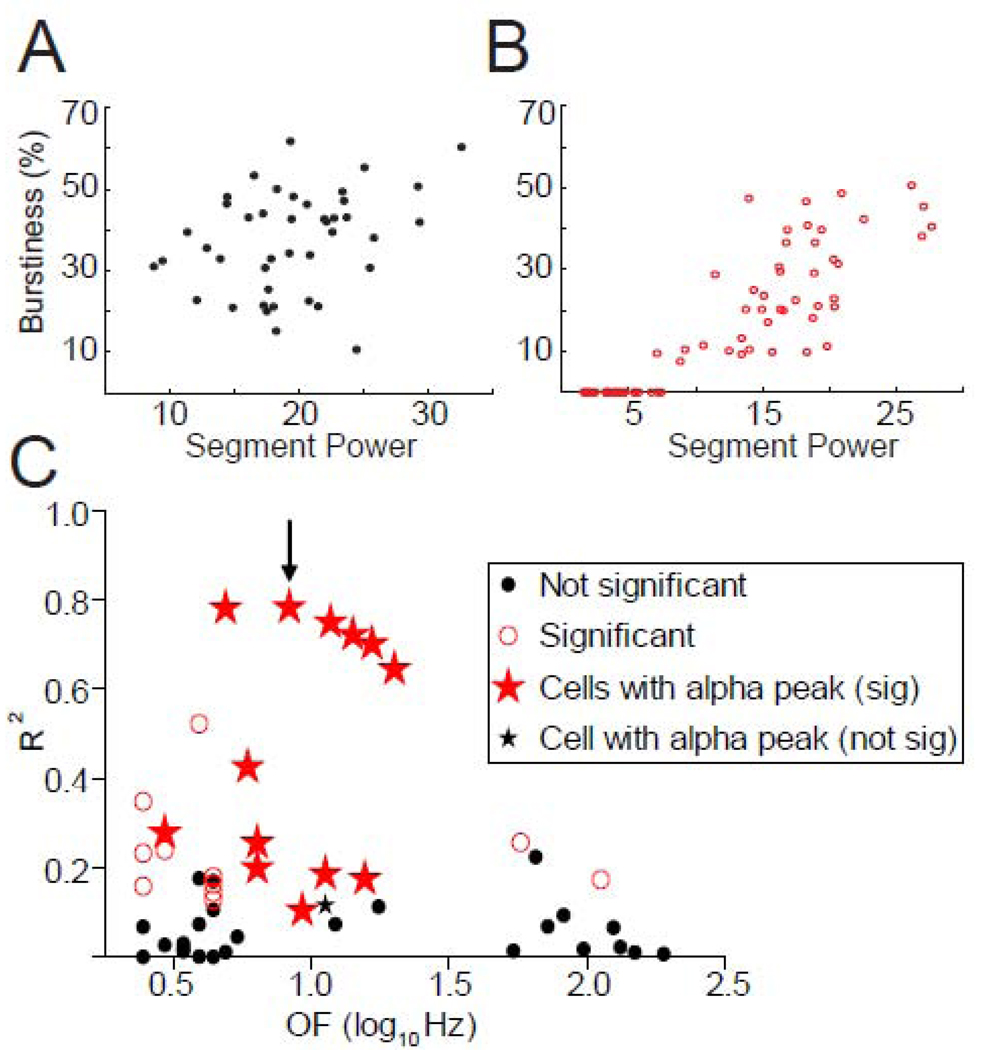
To test the independence of bursts and oscillations further, we quantified relationships between the strength of oscillatory activity and burstiness across time in individual neurons. The degree to which burstiness and oscillatory power co-varied across time was quantified by correlation analysis for each cell with bursts and oscillations (>2–Hz). A minority of cells (34/132) displayed both burst firing (≥1 bursts) and oscillatory activity >2-Hz. Second-by-second burstiness values ranged between 0–50% in 85% of these cells, suggesting that burstiness varied adequately across time to allow detection of temporal correlations if they were present. Time-resolved oscillatory power and burstiness were not correlated in 20 of those 34 cells (Spearman correlations, p>0.05), as demonstrated by an example cell in Fig. 7A (Spearman R2=7%, p>0.05). Among the 14 cells that did show significant correlations with one or more of their oscillatory peaks (p<0.05), the correlations were typically positive, but weak, with variations in oscillatory power accounting for ≤40% of the variance in burstiness in most cells (Spearman correlations, Fig. 7C). Interestingly, α-range OF cells exhibited some of the strongest correlations. Figure 7B illustrates the strongest temporal relationship identified between burstiness and oscillatory activity (8.3-Hz in this case; arrow, Fig. 7C).
Figure 7.
A) Correlation analysis revealed a lack of any relationship between RMS power and burstiness in most cells, such as the example cell shown here (Spearman correlation, R2=7%, p>0.05). B) Some cells exhibited a positive correlation between RMS power and burstiness. Burstiness (% spikes in bursts) is plotted versus mean RMS power (8.3-Hz in this case), depicting the relationship for the highest R2 value detected from any correlation (red, B; arrow, C; Spearman correlation, R2=78%, p<0.01). Note that this cell happens to be an α-range OF cell. C) Identical correlation analyses were performed for all cells with bursts and oscillations >2-Hz. This figure depicts relationships between oscillatory activity and burstiness across different frequency ranges. Each point represents an R2 value for each significant oscillatory peak detected. Therefore, if a cell exhibited multiple oscillatory peaks, multiple points represent this cell. Note that the oscillations in all α-range OF cells (red stars) but one (black star) had significant and positive correlations and some of the highest R2-values.
Clinical Relationships
All patients were tremulous with one exception. Bradykinesia and rigidity were also present in every patient. Total UPDRS scores ranged from 31–66 (43.3 +/− 3.1; mean +/− SEM). Responsiveness to DA therapy ranged from 30% to 86% (54 +/− 6%).
We found no significant relationship between the power of oscillatory firing, burstiness, or mean firing rate and either UPDRS scores or responsiveness to DRT (% change in UPDRS; Spearman correlations, all p values>0.5). UPDRS scores and responsiveness to DRT were similar in patients with α-range OF cells and those in whom none were found (Mann-Whitney tests, all p values >0.5).
DISCUSSION
Bursts and Oscillations as Independent Properties
Neuronal activity in the parkinsonian BG is characterized by an increased prevalence of oscillatory firing and bursting activity, leading many to assume that the two are closely linked phenomena. To the best of our knowledge, that assumption has not been tested empirically. Here we found little support for the idea. Burst firing (≥1 burst) was found in equally large fractions of cells regardless of whether they were OF or NOF cells. Furthermore, cells with oscillatory activity were characterized by a level of burstiness (mean % of spikes in bursts) that was statistically-indistinguishable from that of NOF cells. Even within the subset of cells that exhibited both bursts and oscillations, second-to-second changes in burstiness and oscillatory power were seldom strong predictors of each other. Together, these results are consistent with the idea that bursts and oscillations are, for the most part, independent pathophysiologic features of the parkinsonian GPi. These observations cast doubt on earlier hypotheses that oscillatory activity in the BG is generated by rebound bursts in the subthalamic nucleus (STN) (Plenz and Kitai, 1999) that trigger additional bursts in other BG regions, creating self-sustaining oscillatory activity throughout the BG (Galvan and Wichmann, 2008). Instead, we found that the activity of many cells was characterized exclusively by bursts or oscillations, and that NOF cells were just as bursty as OF cells.
The presence in some neurons of correlated modulations in burstiness and oscillatory power (Fig. 7) indicates that the two phenomena may wax and wane together in a subset of cells. Most temporal correlations were relatively weak, however, and were significant only in a minority of the neurons (14/34). In general, burstiness and oscillatory power varied independently across time within individual neurons, even on a 1-sec time scale.
These findings, however, are subject to potential confounds. The activity of some cells may have been characterized by brief epochs of oscillatory activity without meeting the standards set here to identify significant oscillatory activity. In such situations, temporal relationships between bursts and oscillatory activity would not have been detected, causing any relationship between bursty and oscillatory activity to be underestimated. Oscillatory firing and bursts might be more closely linked in a subset of GPi neurons that were not encountered here due to under-sampling of the GPi. In addition, the specific algorithm and statistical criteria used for burst detection could have biased our classification of bursts, thereby altering the identification of relationships between bursts and oscillations. This work, however, employed detection methods for bursts and oscillatory activity that are both widely-accepted and frequently used to characterize pathophysiologic activity in the parkinsonian state (see above). Thus, even with these potential confounds, the current findings are likely to be relevant to our current understanding of bursts and oscillatory activity in the basal ganglia, and how they contribute to the pathophysiology of PD.
Oscillatory firing, burstiness, and firing rate
Oscillations and bursts were not only found to be relatively independent of each other, but were also differently related to other properties of neural activity, such as firing rate. OF cells exhibited higher mean firing rates than NOF cells, consistent with the idea that BG oscillations are adopted from cortical oscillations when dopamine deficiency leads to a higher background firing rate (Levy et al., 2002a). That observation is also consistent with the premise that OF superimposed on a high frequency baseline firing rate is a feature of the parkinsonian BG (Starr et al., 2005).
The higher firing rates observed in OF cells may be partly due to inherent biases of our detection methods, however. Oscillatory firing is more likely to be detected in cells with higher FR due to increased signal. Moreover, the statistical power for detecting OF is inherently weaker in cells with lower firing rates, especially given the short duration of many of our recordings. While these potential confounds are important to consider, the high firing rates of all GPi cells makes it unlikely that differences in firing rate dramatically biased the detection of OF.
In contrast to OF, mean firing rates were negatively correlated with burstiness. That result was somewhat surprising, as bursting activity is known to coexist with oscillatory activity and increased baseline firing in the parkinsonian GPi (Filion and Tremblay, 1991; Raz et al., 2000; Starr et al., 2005; Wichmann and Soares, 2006). Importantly, however, this finding is consistent with the idea that oscillations and bursts are unrelated phenomena in the parkinsonian GPi.
Cells with α-range OF differ from other cells in burstiness and burst morphology
While oscillations and bursts emerged as generally independent properties, GPi cells with α-range oscillatory activity were found to be much burstier than all other cells. The morphology of bursts in these cells differed markedly from the typical burst firing described for the parkinsonian GPi (Wichmann and Soares, 2006). One previous study mentioned a similar form of burst firing in the GPi of PD patients ("GPi tonic bursters", Taha et al., 1997). To our knowledge, the present study is the first to characterize this type of burst activity in detail, observe that it is strongly associated with α-range OF, and find that it is not attributable to differences in spectral power or firing rate. Cells with α-range OF also exhibited burst firing with decrementing action potentials, a well-established characteristic of a sub-population of neurons in the external segment of the globus pallidus [GPe, “LFD-B” neurons, (DeLong, 1971)]. Although only 5/132 cells displayed α-range OF, the increased burstiness and burst morphology of these five cells was remarkably similar to each other and substantially different from all other cells. It is unlikely that this form of burst firing was some type of injury discharge because its presence was stable across the duration of recordings. Alpha-range OF was found in five different patients and thus was not an anomaly specific to on individual.
Future studies are needed to explore the role of α-range oscillatory cells in the pathophysiology of PD. Modeling studies (Rubin and Terman, 2004; Terman et al., 2002) and in vivo experiments (Kojima and Doupe, 2009; Person and Perkel, 2005) suggest that BG-recipient thalamic neurons are particularly susceptible to driving by a burst-pause pattern of BG input such as that exhibited by α-range OF cells. Indeed, several studies indicate that the suppression of α-range oscillations in the BG is associated with therapeutic benefit (Heimer et al., 2006; McCairn and Turner, 2009).
Conclusions
Parkinsonism has long been associated with pathologically-increased levels of oscillatory and bursty activity in the GPi. Therefore, those two features of neuronal activity have often been lumped together as linked contributors to the pathophysiology of PD. By examining the prevalence of bursts and oscillations on a single cell level, however, we found that oscillatory and bursty activity are generally independent properties of spiking activity in the parkinsonian GPi. Therefore, bursts and oscillations may well play functionally-distinct roles in the genesis of PD motor signs, and may even represent separate therapeutic targets or indicators of responsiveness to therapy. In contrast to the general independence of bursts and oscillatory firing, the activity of cells with α-range oscillations was uniquely bursty with a distinct burst morphology. The present work emphasizes the importance of considering bursts and oscillations independently in future studies of the neurophysiologic correlates of PD. Further studies are needed to relate bursts and oscillations independently to the development and resolution of specific parkinsonian signs.
Research Highlights
-
*
Bursts and oscillatory activity in the parkinsonian GPi occur independently
-
*
Cells with α-range oscillations are very bursty
-
*
Cells with α-range oscillations have a distinct burst morphology
Acknowledgements
This research was supported by the National Institute of Neurological Disorders and Stroke at the National Institutes of Health, grant number K08-NS02201 to PAS, grant number R21-NS55197 to RST, by the National Institutes of Health, grant number T32 NS007433 in support of Vanessa Chan, and by the Parkinson's Disease Research, Education, and Clinical Center at San Francisco Veteran's Affairs Medical Center.
Glossary
- OF
oscillatory firing
- NOF
non-oscillatory firing
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Bergman H, Wichmann T, Karmon B, DeLong MR. The primate subthalamic nucleus. II. Neuronal activity in the MPTP model of parkinsonism. J Neurophysiol. 1994;72:507–520. doi: 10.1152/jn.1994.72.2.507. [DOI] [PubMed] [Google Scholar]
- 2.Boraud T, Bezard E, Bioulac B, Gross C. High frequency stimulation of the internal Globus Pallidus (GPi) simultaneously improves parkinsonian symptoms and reduces the firing frequency of GPi neurons in the MPTP-treated monkey. Neurosci Lett. 1996;215:17–20. doi: 10.1016/s0304-3940(96)12943-8. [DOI] [PubMed] [Google Scholar]
- 3.Boraud T, Bezard E, Guehl D, Bioulac B, Gross C. Effects of L-DOPA on neuronal activity of the globus pallidus externalis (GPe) and globus pallidus internalis (GPi) in the MPTP-treated monkey. Brain Res. 1998;787:157–160. doi: 10.1016/s0006-8993(97)01563-1. [DOI] [PubMed] [Google Scholar]
- 4.Boraud T, Bezard E, Stutzmann JM, Bioulac B, Gross CE. Effects of riluzole on the electrophysiological activity of pallidal neurons in the 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-treated monkey. Neurosci Lett. 2000;281:75–78. doi: 10.1016/s0304-3940(00)00780-1. [DOI] [PubMed] [Google Scholar]
- 5.Brown P, Oliviero A, Mazzone P, Insola A, Tonali P, Di Lazzaro V. Dopamine dependency of oscillations between subthalamic nucleus and pallidum in Parkinson's disease. J Neurosci. 2001;21:1033–1038. doi: 10.1523/JNEUROSCI.21-03-01033.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen MT, Morales M, Woodward DJ, Hoffer BJ, Janak PH. In vivo extracellular recording of striatal neurons in the awake rat following unilateral 6-hydroxydopamine lesions. Exp Neurol. 2001;171:72–83. doi: 10.1006/exnr.2001.7730. [DOI] [PubMed] [Google Scholar]
- 7.Coban A, Hanagasi HA, Karamursel S, Barlas O. Comparison of unilateral pallidotomy and subthalamotomy findings in advanced idiopathic Parkinson's disease. Br J Neurosurg. 2009;23:23–29. doi: 10.1080/02688690802507775. [DOI] [PubMed] [Google Scholar]
- 8.de Bie RM, de Haan RJ, Nijssen PC, Rutgers AW, Beute GN, Bosch DA, Haaxma R, Schmand B, Schuurman PR, Staal MJ, Speelman JD. Unilateral pallidotomy in Parkinson's disease: a randomised, single-blind, multicentre trial. Lancet. 1999;354:1665–1669. doi: 10.1016/S0140-6736(99)03556-4. [DOI] [PubMed] [Google Scholar]
- 9.Degos B, Deniau JM, Chavez M, Maurice N. Chronic but not acute dopaminergic transmission interruption promotes a progressive increase in cortical beta frequency synchronization: relationships to vigilance state and akinesia. Cereb Cortex. 2009;19:1616–1630. doi: 10.1093/cercor/bhn199. [DOI] [PubMed] [Google Scholar]
- 10.DeLong MR. Activity of pallidal neurons during movement. J Neurophysiol. 1971;34:414–427. doi: 10.1152/jn.1971.34.3.414. [DOI] [PubMed] [Google Scholar]
- 11.Dogali M, Beric A, Sterio D, Eidelberg D, Fazzini E, Takikawa S, Samelson DR, Devinsky O, Kolodny EH. Anatomic and physiological considerations in pallidotomy for Parkinson's disease. Stereotact Funct Neurosurg. 1994;62:53–60. doi: 10.1159/000098597. [DOI] [PubMed] [Google Scholar]
- 12.Filion M, Tremblay L. Abnormal spontaneous activity of globus pallidus neurons in monkeys with MPTP-induced parkinsonism. Brain Res. 1991;547:142–151. [PubMed] [Google Scholar]
- 13.Foffani G, Ardolino G, Egidi M, Caputo E, Bossi B, Priori A. Subthalamic oscillatory activities at beta or higher frequency do not change after high-frequency DBS in Parkinson's disease. Brain Res Bull. 2006;69:123–130. doi: 10.1016/j.brainresbull.2005.11.012. [DOI] [PubMed] [Google Scholar]
- 14.Galvan A, Wichmann T. Pathophysiology of parkinsonism. Clin Neurophysiol. 2008;119:1459–1474. doi: 10.1016/j.clinph.2008.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gatev P, Darbin O, Wichmann T. Oscillations in the basal ganglia under normal conditions and in movement disorders. Mov Disord. 2006;21:1566–1577. doi: 10.1002/mds.21033. [DOI] [PubMed] [Google Scholar]
- 16.Hahn PJ, Russo GS, Hashimoto T, Miocinovic S, Xu W, McIntyre CC, Vitek JL. Pallidal burst activity during therapeutic deep brain stimulation. Exp Neurol. 2008;211:243–251. doi: 10.1016/j.expneurol.2008.01.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Heimer G, Rivlin-Etzion M, Bar-Gad I, Goldberg JA, Haber SN, Bergman H. Dopamine replacement therapy does not restore the full spectrum of normal pallidal activity in the 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine primate model of Parkinsonism. J Neurosci. 2006;26:8101–8114. doi: 10.1523/JNEUROSCI.5140-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hutchison WD, Lozano AM, Davis KD, Saint-Cyr JA, Lang AE, Dostrovsky JO. Differential neuronal activity in segments of globus pallidus in Parkinson's disease patients. Neuroreport. 1994;5:1533–1537. doi: 10.1097/00001756-199407000-00031. [DOI] [PubMed] [Google Scholar]
- 19.Hutchison WD, Lozano AM, Tasker RR, Lang AE, Dostrovsky JO. Identification and characterization of neurons with tremor-frequency activity in human globus pallidus. Exp Brain Res. 1997;113:557–563. doi: 10.1007/pl00005606. [DOI] [PubMed] [Google Scholar]
- 20.Kaneoke Y, Vitek JL. Burst and oscillation as disparate neuronal properties. J Neurosci Methods. 1996;68:211–223. doi: 10.1016/0165-0270(96)00081-7. [DOI] [PubMed] [Google Scholar]
- 21.Kojima S, Doupe AJ. Activity propagation in an avian basal ganglia-thalamocortical circuit essential for vocal learning. J Neurosci. 2009;29:4782–4793. doi: 10.1523/JNEUROSCI.4903-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Leblois A, Meissner W, Bioulac B, Gross CE, Hansel D, Boraud T. Late emergence of synchronized oscillatory activity in the pallidum during progressive Parkinsonism. Eur J Neurosci. 2007;26:1701–1713. doi: 10.1111/j.1460-9568.2007.05777.x. [DOI] [PubMed] [Google Scholar]
- 23.Legendy CR, Salcman M. Bursts and recurrences of bursts in the spike trains of spontaneously active striate cortex neurons. J Neurophysiol. 1985;53:926–939. doi: 10.1152/jn.1985.53.4.926. [DOI] [PubMed] [Google Scholar]
- 24.Levy R, Ashby P, Hutchison WD, Lang AE, Lozano AM, Dostrovsky JO. Dependence of subthalamic nucleus oscillations on movement and dopamine in Parkinson's disease. Brain. 2002;125:1196–1209. doi: 10.1093/brain/awf128. [DOI] [PubMed] [Google Scholar]
- 25.Levy R, Dostrovsky JO, Lang AE, Sime E, Hutchison WD, Lozano AM. Effects of apomorphine on subthalamic nucleus and globus pallidus internus neurons in patients with Parkinson's disease. J Neurophysiol. 2001;86:249–260. doi: 10.1152/jn.2001.86.1.249. [DOI] [PubMed] [Google Scholar]
- 26.Levy R, Hutchison WD, Lozano AM, Dostrovsky JO. Synchronized neuronal discharge in the basal ganglia of parkinsonian patients is limited to oscillatory activity. J Neurosci. 2002;22:2855–2861. doi: 10.1523/JNEUROSCI.22-07-02855.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lozano A, Hutchison W, Kiss Z, Tasker R, Davis K, Dostrovsky J. Methods for microelectrode-guided posteroventral pallidotomy. J Neurosurg. 1996;84:194–202. doi: 10.3171/jns.1996.84.2.0194. [DOI] [PubMed] [Google Scholar]
- 28.Mallet N, Pogosyan A, Sharott A, Csicsvari J, Bolam JP, Brown P, Magill PJ. Disrupted dopamine transmission and the emergence of exaggerated beta oscillations in subthalamic nucleus and cerebral cortex. J Neurosci. 2008;28:4795–4806. doi: 10.1523/JNEUROSCI.0123-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.McCairn KW, Turner RS. Deep brain stimulation of the globus pallidus internus in the parkinsonian primate: local entrainment and suppression of low-frequency oscillations. J Neurophysiol. 2009;101:1941–1960. doi: 10.1152/jn.91092.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Miller WC, DeLong MR. Altered tonic activity of neurons in the globus pallidus and subthalamic nucleus in the primate MPTP model of parkinsonism. In: Carpenter MBJA, editor. The Basal Ganglia II. New York: Plenum Press; 1987. pp. 415–427. [Google Scholar]
- 31.Person AL, Perkel DJ. Unitary IPSPs drive precise thalamic spiking in a circuit required for learning. Neuron. 2005;46:129–140. doi: 10.1016/j.neuron.2004.12.057. [DOI] [PubMed] [Google Scholar]
- 32.Plenz D, Kitai ST. A basal ganglia pacemaker formed by the subthalamic nucleus and external globus pallidus. Nature. 1999;400:677–682. doi: 10.1038/23281. [DOI] [PubMed] [Google Scholar]
- 33.Raz A, Bergman H, Eimerl D, Israel Z. Propofol induced changes in the neuronal activity of subthalamic nucleus neurons. Movement Disorders. 2008;23:S117. [Google Scholar]
- 34.Raz A, Vaadia E, Bergman H. Firing patterns and correlations of spontaneous discharge of pallidal neurons in the normal and the tremulous 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine vervet model of parkinsonism. J Neurosci. 2000;20:8559–8571. doi: 10.1523/JNEUROSCI.20-22-08559.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rivlin-Etzion M, Marmor O, Heimer G, Raz A, Nini A, Bergman H. Basal ganglia oscillations and pathophysiology of movement disorders. Curr Opin Neurobiol. 2006;16:629–637. doi: 10.1016/j.conb.2006.10.002. [DOI] [PubMed] [Google Scholar]
- 36.Rivlin-Etzion M, Ritov Y, Heimer G, Bergman H, Bar-Gad I. Local shuffling of spike trains boosts the accuracy of spike train spectral analysis. J Neurophysiol. 2006;95:3245–3256. doi: 10.1152/jn.00055.2005. [DOI] [PubMed] [Google Scholar]
- 37.Rubin JE, Terman D. High frequency stimulation of the subthalamic nucleus eliminates pathological thalamic rhythmicity in a computational model. J Comput Neurosci. 2004;16:211–235. doi: 10.1023/B:JCNS.0000025686.47117.67. [DOI] [PubMed] [Google Scholar]
- 38.Starr PA. Placement of deep brain stimulators into the subthalamic nucleus or Globus pallidus internus: technical approach. Stereotact Funct Neurosurg. 2002;79:118–145. doi: 10.1159/000070828. [DOI] [PubMed] [Google Scholar]
- 39.Starr PA, Rau GM, Davis V, Marks WJ, Jr, Ostrem JL, Simmons D, Lindsey N, Turner RS. Spontaneous pallidal neuronal activity in human dystonia: comparison with Parkinson's disease and normal macaque. J Neurophysiol. 2005;93:3165–3176. doi: 10.1152/jn.00971.2004. [DOI] [PubMed] [Google Scholar]
- 40.Taha JM, Favre J, Burchiel KJ. Infrequent types of pallidal discharges during pallidotomy. Stereotact Funct Neurosurg. 1997;68:231–235. doi: 10.1159/000099930. [DOI] [PubMed] [Google Scholar]
- 41.Terman D, Rubin JE, Yew AC, Wilson CJ. Activity patterns in a model for the subthalamopallidal network of the basal ganglia. J Neurosci. 2002;22:2963–2976. doi: 10.1523/JNEUROSCI.22-07-02963.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Vitek JL, Bakay RA, Freeman A, Evatt M, Green J, McDonald W, Haber M, Barnhart H, Wahlay N, Triche S, Mewes K, Chockkan V, Zhang JY, DeLong MR. Randomized trial of pallidotomy versus medical therapy for Parkinson's disease. Ann Neurol. 2003;53:558–569. doi: 10.1002/ana.10517. [DOI] [PubMed] [Google Scholar]
- 43.Vitek JL, Bakay RA, Hashimoto T, Kaneoke Y, Mewes K, Zhang JY, Rye D, Starr P, Baron M, Turner R, DeLong MR. Microelectrode-guided pallidotomy: technical approach and its application in medically intractable Parkinson's disease. J Neurosurg. 1998;88:1027–1043. doi: 10.3171/jns.1998.88.6.1027. [DOI] [PubMed] [Google Scholar]
- 44.Weinberger M, Mahant N, Hutchison WD, Lozano AM, Moro E, Hodaie M, Lang AE, Dostrovsky JO. Beta oscillatory activity in the subthalamic nucleus and its relation to dopaminergic response in Parkinson's disease. J Neurophysiol. 2006;96:3248–3256. doi: 10.1152/jn.00697.2006. [DOI] [PubMed] [Google Scholar]
- 45.Wichmann T, Bergman H, Starr PA, Subramanian T, Watts RL, DeLong MR. Comparison of MPTP-induced changes in spontaneous neuronal discharge in the internal pallidal segment and in the substantia nigra pars reticulata in primates. Exp Brain Res. 1999;125:397–409. doi: 10.1007/s002210050696. [DOI] [PubMed] [Google Scholar]
- 46.Wichmann T, Soares J. Neuronal firing before and after burst discharges in the monkey basal ganglia is predictably patterned in the normal state and altered in parkinsonism. J Neurophysiol. 2006;95:2120–2133. doi: 10.1152/jn.01013.2005. [DOI] [PubMed] [Google Scholar]