Abstract
Objective
The amount of hemorrhage observed on a brain computed tomography scan, or a patient's Fisher grade (FG), is a powerful risk factor for development of shunt dependent hydrocephlaus (SDHC). However, the influence of treatment modality (clipping versus coiling) on the rate of SDHC development has not been thoroughly investigated. Therefore, we compared the risk of SDHC in both treatment groups according to the amount of subarachnoid hemorrhage (SAH).
Methods
We retrospectively reviewed 839 patients with aneurysmal SAH for a 5-year-period. Incidence of chronic SDHC was analyzed using each treatment modality according to the FG system. In addition, other well known risk factors for SDHC were also evaluated.
Results
According to our data, Hunt-Hess grade, FG, acute hydrocephalus, and intraventricular hemorrhage were significant risk factors for development of chronic SDHC. Coiling group showed lower incidence of SDHC in FG 2 patients, and clipping groups revealed a significantly lower rate in FG 4 patients.
Conclusion
Based on our data, treatment modality might have an influence on the incidence of SDHC. In FG 4 patients, the clipping group showed lower incidence of SDHC, and the coiling group showed lower incidence in FG 2 patients. We suggest that these findings could be a considerable factor when deciding on a treatment modality for aneurysmal SAH patients, particularly when the ruptured aneurysm can be occluded by either clipping or coiling.
Keywords: Shunt dependent hydrocephalus, Fisher grading system, Subarachnoid hemorrhage
INTRODUCTION
Hydrocephalus after aneurysmal subarachnoid hemorrhage (SAH) is a well known complication, and has been reported to range from 6 to 67%17). Suggested risk factors for development of shunt dependent hydrocephalus (SDHC) include location of the aneurysm, Fisher grade (FG) on computed tomography (CT) scan, intraventricular hemorrhage (IVH), and so on7,13,15,17,20). Similar to vasospasm, development of hydrocephalus has a strong association with the amount of blood spilled into the subarachnoid space and ventricular system3-5,8).
Due to technical advancement in performance of the endovascular procedure on aneurysmal SAH patients, a comparison of SDHC rate in patients treated using microsurgical clipping with those who underwent endovascular coiling is important. Whether or not incidence of SDHC differs significantly between surgical clipping and endovascular coiling is currently unknown. However, several authors have reported results that demonstrated fewer occurrences of SDHC in the surgical treatment group3-5,19) Another study revealed no difference between the two groups8).
Early evacuation of cisternal clots during surgery is a possible mechanism for lowering SDHC in the surgical group8); however, this is hypothesized only under thick hemorrhagic conditions. In SAH patients with scanty hemorrhaging, the merit of an open surgical procedure is decreased due to the risk of hydrocephalus.
Therefore, we retrospectively analyzed the incidence of SDHC between the clipping and coiling groups according to the FG system. In addition, we analyzed other risk factors for development of SDHC after aneurysmal SAH.
MATERIALS AND METHODS
Patient population
A total of 839 patients with confirmed SAH who were admitted to our hospital from January 2004 to December 2009 comprised the basis of the present study. Fifty-five patients with angiographically negative SAH were excluded. Twenty-eight patients who died during the acute stage and 20 patients who refused treatment were also excluded. Finally, the present study was composed of 736 patients diagnosed with aneurysmal SAH by brain CT scan; the ruptured aneurysm was confirmed by 3D CT angiography and digital subtraction angiography. Age of patients ranged from 21 to 86 years. The female to male ratio was 1.8 : 1. Clinical characteristics of the patients were obtained from their medical records and imaging studies. Our institutional review board did not require approval or patient informed consent for this study.
Determination of treatment modality
Treatment modality was determined according to the patient's clinical state, location of the aneurysm, and the angioarchitecture of the aneurysm by a vascular team composed of a vascular neurosurgeon and an interventional neuroradiologist.
A total of 497 patients underwent clipping of aneurysms by pterional or posterior fossa craniotomy. Most patients underwent surgery by the pterional approach (467/498, 93.8%). If necessary, hematoma evacuation was undertaken during the procedure. Clot removal was performed only to the extent that it was possible. Aggressive means of clot removal6,9,11,21), such as cisternal lavage, cisternal drainage, and use of thrombolytic agents were not used with our patients.
The endovascular procedure was performed using coil embolization in 239 patients with ruptured aneurysmal SAH. Due to antiplatelet medication administered during or following the procedure, we preferred neither stent nor balloon assisted techniques for treatment of ruptured intracranial aneurysms. For the purpose of minimizing in-hospital rebleeding, all patients underwent aneurysm clipping surgery or coil embolization of the aneurysm within 24 hours from admission.
Criteria for external ventricular drainage and ventriculoperitoneal shunt
Hydrocephalus was defined as confirmation of a significantly enlarged temporal horn or greater than 30% of the frontal horn index (frontal horn to maximal biparietal diameter) on brain CT scans. Patients presenting with alteration of consciousness or radiologic evidence of acute hydrocephalus underwent immediate external ventricular drainage (EVD). Patients with EVD kept the drain for at least 7 days after SAH, and opening pressure was progressively elevated. If elevation of opening pressure resulted in neurological deterioration or deterioration of hydrocephalus on the control brain CT, the patient underwent ventriculoperitoneal shunt insertion. Asymptomatic hydrocephalus was considered for patients who revealed hydrocephalus on brain CT but showed no neurological signs.
Statistical analysis
In a comparison of the shunt dependent group and the non-dependent group, we analyzed the following factors for statistical significance using χ2-test : 1) age, 2) sex, 3) initial Hunt & Hess grade (H-H grade), 4) initial IVH, 5) initial FG, 6) presence of acute hydrocephalus, and 7) location of the aneurysm. The patient's data were then categorized further by treatment modality (microsurgical clipping versus endovascular coiling). Risk factor analysis for each subgroup was performed, and, according to the FG system, shunt dependency was assessed for both groups, also used χ2-test.
Homogeneity of odds ratio (OR) in each level of FG system about shunt dependency was assessed using Breslow-Day test, and the result was statistically significant (p = 0.002). Therefore, we performed logistic regression analysis for each FG system and estimated adjusted OR according to FG system (OR was adjusted for age, H-H grade, IVH and acute hydrocephalus).
Data analysis was performed using the Statistical Package for the Social Sciences for Windows (Version 18.0; SPSS, Inc.).
RESULTS
Risk factor analysis for chronic hydrocephalus after SAH
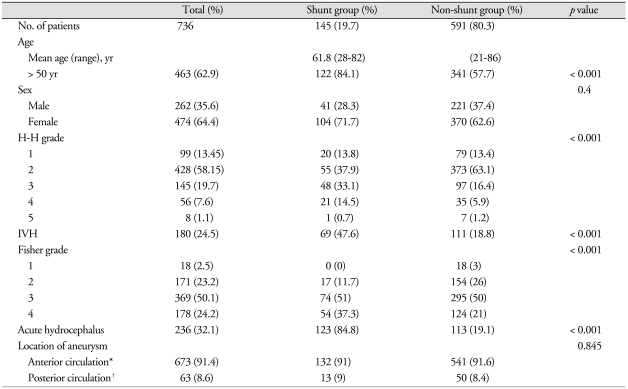
Number of SDHC was significantly associated with old age (> 50 years), H-H grade, Fisher grade, IVH, and presence of acute hydrocephalus (Table 1). Of the 145 shunted patients, 22 (15.2%) patients presented with H-H grades 4 and 5. With regard to FG, 51% (74/145) of shunt dependent patients presented with grade III. Rate of IVH in the group of shunt-dependent patients was 47.6% (69/145). Among the 145 patients who required shunt placement, 123 (84.8%) patients had acute hydrocephalus on admission.
Table 1.
Relating factors of shunt dependency in aneurysmal subarachnoid hemorrhage patients
*Anterior circulation : anterior cerebral artery, anterior communicating artery, posterior communicating artery,internal carotid artery, middle cerebral artery, †Posterior circulation : posterior cerebral artery, basilar artery, vertebral artery, posterior-inferior cerebellar artery, anterior-inferior cerebellar artery, superior cerebellar artery. H-H grade : Hunt & Hess grade, IVH : intraventricular hemorrhage
Shunt-dependent hydrocephalus according to the Fisher grading system (clipping group versus coiling group)
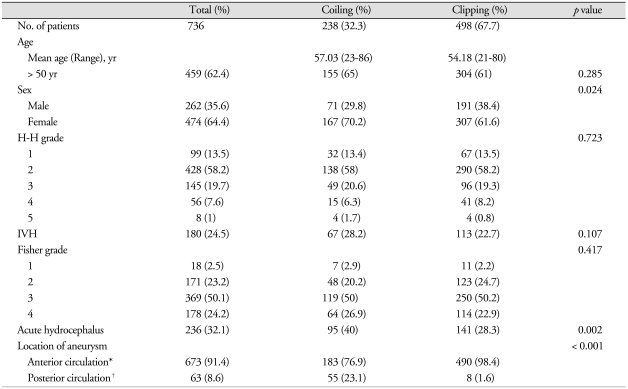
Overall rate of SDHC for all patients was 19.7% (145/736). Most factors, such as age, H-H grade, IVH, and FG were similar between the two groups. On the other hand, the incidence of acute hydrocephalus and posterior circulation aneurysms were more frequent in the coiling group. Among significant variables for shunt dependency, only acute hydrocephalus was higher in the coiling group than in the clipping group (Table 2). Rate of shunt dependency was 18.7% (93/498) in the clipping group and 21.8% (52/238) in the coiling group, which was not statistically significant (p = 0.311).
Table 2.
Comparison of patient demographics and characteristics in both treatment groups
*Anterior circulation : anterior cerebral artery, anterior communicating artery, posterior communicating artery,internal carotid artery, middle cerebral artery, †Posterior circulation : posterior cerebral artery, basilar artery, vertebral artery, posterior-inferior cerebellar artery, anterior-inferior cerebellar artery, superior cerebellar artery. H-H grade : Hunt & Hess grade, IVH : intraventricular hemorrhage
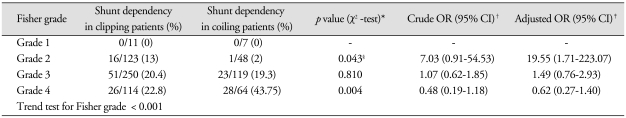
However, significant differences in both groups were revealed according to FG. In FG 1 patients, none of the patients needed shunt surgery; therefore, no differences were found. In FG 2 patients, the clipping group had a more frequent SDHC rate compared with the coiling group [clipping : 16/123 (13%), coiling : 1/48 (2%); p = 0.043]. In FG 3 patients, there was no statistical significance [clipping : 51/250 (20.4%), coiling : 23/119 (19.3%); p = 0.81]. In FG 4 patients, a significantly lower risk of SDHC in the clipping group was observed when compared with the coiling group [clipping : 26/114 (22.8%), coiling : 28/64 (43.7%); p = 0.004].
A logistic regression analysis was shown that clipping group showed a more frequent SDHC rate compared with the coiling group in FG 2 patients (adjusted OR : 19.55, CI 1.71-223.07) and lower rate in FG 4 patients (adjusted OR : 0.62, CI 0.27-1.40). However, only FG 2 patients group revealed statistical significance. Additionally, trend test for the influence of FG to SDHC demonstrated statistically significant (p < 0.001) (Table 3).
Table 3.
Incidence of shunt dependent hydrocephalus in each treatment modality group according to Fisher grading system
*p values were calculated by χ2-test or Fisher's exact test, †OR and 95% CI were calculated by simple and multiple logistic regressionanalysis. ‡Adjusted for age, Hunt & Hess grade, intraventricular hemorrhage and acute hydrocephalus, §Fisher's exact test. OR : odds ratio, CI : confidence interval
DISCUSSION
Development of hydrocephalus after SAH can result in poor neurological outcome and cognitive deficit, which is a well known complication of ruptured cerebral aneurysms1). The amount of blood in the subarachnoid space showed special significance in association with development of SDHC. Dorai et al.5) stated that 1.3% of shunt-treated patients were in FG 1, whereas 86% of patients were reported as FG 3. Also, the influence of the quantity of blood in the subarachnoid space was supported by results from many other studies7,8,12,18). Our results showed a total rate of SDHC of 19.7%, which was consistent with results from other series found in the literature4,5,8,16). Based on our data, 0% of shunt-treated patients were in FG 1, with 11.7% in FG 2, 51% in FG 3, and 37.3% in FG 4.
In contrast to clipping surgery, removal of a subarachnoid clot is not possible during an endovascular procedure. Several studies have compared the incidence of SDHC according to treatment modality. Most reports revealed that endovascular coiling could be attributed to an increased rate of SDHC4,5,19). However, Gruber et al.8) reported that the endovascular treatment group did not suffer SDHC more frequently than the surgical treatment group.
Based upon our data, treatment modality for subarachnoid hemorrhage does not affect overall risk of development of SDHC (microsurgical clipping; 18.7%, endovascular coiling; 21.8%, p = 0.31). However, when considering the amount of hemorrhage, there were some differences in the incidence of SDHC according to the FG system. The authors further categorized patients according to FG and found some significant results. The coiling group was associated with a lower risk of SDHC in FG 2 patients. By contrast, clipping was associated with a lower risk in FG 4 patients. In addition, there were no differences in FG 1 and 3 patients.
Possible mechanisms have been suggested for lowering shunt dependent hydrocephalus in the surgical clipping group, from which early open surgery can remove blood clots, and lamina terminalis fenestration was possible during surgery1). On the other hand, endovascular coiling can lower the incidence of SDHC from minimal brain manipulation and heparinization during and after embolization2). According to the report, heparin may reach the CSF space under conditions of blood brain barrier disruption following SAH; however, because heparin may not have lysed previously formed subarachnoid clots, the effect of heparinization on the rate of shunt dependency was questionable2,8,11,14).
According to our data, there was no difference in the incidence of SDHC in FG 1 patients according to treatment modality. However, due to the limited number of patients in FG 1, affirming the significance of the result was difficult. In FG 3 patients, our data also showed no statistical significance for the two treatment modalities. Because our center does not advocate use of aggressive means, such as cisternal lavage or drainage, for clot removal during surgical clipping, and we do not perform routine fenestration of lamina terminalis, statistical significance in FG 3 patients may not be attained. In FG 2 and 4 patients, our data showed differences in development of SDHC according to treatment modality. In univariate analysis (χ2-test), a statistical significance was obtained in FG 2 and 4 patients. And, the same tendency was observed in FG 2 and 4 patients in multivariate analysis (logistic regression analysis), however, statistical significance was only shown in FG 2 patients.
In FG 2 patients, minimal manipulation of the brain during coil embolization is considered more beneficial with regard to incidence of SDHC. On the other hand, evacuation of intracerebral hematoma and drainage of IVH during microsurgical clipping may be an influencing factor in FG 4 patients.
Our study has some limitations. This is a retrospective study; therefore, selection bias and protocol deviations were inevitable. Subjects in both groups were not similar, such that there were 498 patients in the clipping group and 238 patients in the coiling group. Moreover, location of aneurysms was not evenly distributed in either group. The aforementioned limitations could also have originated from the retrospective nature of the present study. Therefore, a prospective, multicenter, and well stratified trial will be needed for confirmation of the effect of treatment modality on development of SDHC.
CONCLUSION
Treatment modality showed no statistical significance on the overall rate of SDHC. However, in the FG system, treatment modality could have an influence on the incidence of SDHC according to the amount of SAH. In FG 1 and 3 patients, no differences in shunt dependency according to treatment modality were observed. On the contrary, surgical clipping may be associated with a lower risk for shunt dependency in FG 4 patients, and endovascular coiling may be associated with a lower risk in FG 2 patients.
In addition, we suggest that these findings might be a considerable factor when deciding on treatment modality in aneurysmal SAH patients, particularly under conditions where the ruptured aneurysm can be occluded by either clipping or coiling.
References
- 1.Auer LM, Mokry M. Disturbed cerebrospinal fluid circulation after subarachnoid hemorrhage and acute aneurysm surgery. Neurosurgery. 1990;26:804–808. doi: 10.1097/00006123-199005000-00012. discussion 808-809. [DOI] [PubMed] [Google Scholar]
- 2.Blasberg R, Johnson D, Fenstermacher J. Absorption resistance of cerebrospinal fluid after subarachnoid hemorrhage in the monkey; Effects of heparin. Neurosurgery. 1981;9:686–691. doi: 10.1227/00006123-198112000-00012. [DOI] [PubMed] [Google Scholar]
- 3.de Oliveira JG, Beck J, Setzer M, Gerlach R, Vatter H, Seifert V, et al. Risk of shunt-dependent hydrocephalus after occlusion of ruptured intracranial aneurysms by surgical clipping or endovascular coiling : a single-institution series and meta-analysis. Neurosurgery. 2007;61:924–933. doi: 10.1227/01.neu.0000303188.72425.24. discussion 933-934. [DOI] [PubMed] [Google Scholar]
- 4.Dehdashti AR, Rilliet B, Rufenacht DA, de Tribolet N. Shunt-dependent hydrocephalus after rupture of intracranial aneurysms : a prospective study of the influence of treatment modality. J Neurosurg. 2004;101:402–407. doi: 10.3171/jns.2004.101.3.0402. [DOI] [PubMed] [Google Scholar]
- 5.Dorai Z, Hynan LS, Kopitnik TA, Samson D. Factors related to hydrocephalus after aneurysmal subarachnoid hemorrhage. Neurosurgery. 2003;52:763–771. doi: 10.1227/01.neu.0000053222.74852.2d. [DOI] [PubMed] [Google Scholar]
- 6.Findlay JM, Weir BK, Steinke D, Tanabe T, Gordon P, Grace M. Effect of intrathecal thrombolytic therapy on subarachnoid clot and chronic vasospasm in a primate model of SAH. J Neurosurg. 1988;69:723–735. doi: 10.3171/jns.1988.69.5.0723. [DOI] [PubMed] [Google Scholar]
- 7.Graff-Radford NR, Torner J, Adams HP, Kassell NF. Factors associated with subarachnoid hemorrhage. A report of the Cooperative Aneurysm Study. Arch Neurol. 1989;46:744–752. doi: 10.1001/archneur.1989.00520430038014. [DOI] [PubMed] [Google Scholar]
- 8.Gruber A, Reinprecht A, Bavinzski G, Czech T, Richling B. Chronic shunt dependent hydrocephalus after early surgical and early endovascular treatment of ruptured intracranial aneurysms. Neurosurgery. 1999;44:503–509. doi: 10.1097/00006123-199903000-00039. discussion 509-512. [DOI] [PubMed] [Google Scholar]
- 9.Ito U, Tomita H, Yamazaki S, Takada Y, Inaba Y. Enhanced cisternal drainage and cerebral vasospasm in early aneurysm surgery. Acta Neurochir (Wien) 1986;80:18–23. doi: 10.1007/BF01809552. [DOI] [PubMed] [Google Scholar]
- 10.Kassell NF, Torner JC, Adams HP., Jr Antifibrinolytic therapy in the acute period following aneurysmal subarachnoid hemorrhage. Preliminary observations from the Cooperative Aneurysm Study. J Neurosurg. 1984;61:225–230. doi: 10.3171/jns.1984.61.2.0225. [DOI] [PubMed] [Google Scholar]
- 11.Kawakami Y, Shimamura Y. Cisternal drainage after early operation of ruptured intracranial aneurysms. Neurosurgery. 1987;20:8–14. doi: 10.1227/00006123-198701000-00003. [DOI] [PubMed] [Google Scholar]
- 12.Kinugasa K, Kamata I, Hirotsune N, Tokunaga K, Sugiu K, Handa A, et al. Early treatment of subarachnoid hemorrhage after preventing rerupture of an aneurysm. J Neurosurg. 1995;83:34–41. doi: 10.3171/jns.1995.83.1.0034. [DOI] [PubMed] [Google Scholar]
- 13.Kwon JH, Sung SK, Song YJ, Choi HJ, Huh JT, Kim HD. Predisposing factors related to shunt dependent chronic hydrocephalus after aneurismal subarachnoid hemorrhage. J Korean Neurosurg Soc. 2008;43:177–181. doi: 10.3340/jkns.2008.43.4.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Park BE. Spontaneous subarachnoid hemorrhage complicated by communicating hydrocephalus : epsilon-amino caproic acid as a possible predisposing factor. Surg Neurol. 1979;11:73–80. [PubMed] [Google Scholar]
- 15.Pietilä TA, Heimberger KC, Palleske H, Brock M. Influence of aneurysm location on the development of chronic hydrocephalus following SAH. Acta Neurochir (Wien) 1995;137:70–73. doi: 10.1007/BF02188784. [DOI] [PubMed] [Google Scholar]
- 16.Sethi H, Moore A, Dervin J, Clifton A, MacSweeney JE. Hydrocephalus : comparison of clipping and embolization in aneurysm treatment. J Neurosurg. 2000;92:991–994. doi: 10.3171/jns.2000.92.6.0991. [DOI] [PubMed] [Google Scholar]
- 17.Spallone A, Gagliardi FM. Hydrocephalus following aneurysmal SAH. Zentralbl Neurochir. 1983;44:141–150. [PubMed] [Google Scholar]
- 18.Vale FL, Bradley EL, Fisher WS., 3rd The relationship of subarachnoid hemorrhage and the need for postoperative shunting. J Neurosurg. 1997;86:462–466. doi: 10.3171/jns.1997.86.3.0462. [DOI] [PubMed] [Google Scholar]
- 19.Varelas P, Helms A, Sinson G, Spanaki M, Hacein-Bey L. Clipping or coiling of ruptured cerebral aneurysms and shunt-dependent hydrocephalus. Neurocrit Care. 2006;4:223–228. doi: 10.1385/NCC:4:3:223. [DOI] [PubMed] [Google Scholar]
- 20.Vermeij FH, Hasan D, Vermeulen M, Tanghe HL, van Gijn J. Predictive factors for deterioration from hydrocephalus after subarachnoid hemorrhage. Neurology. 1994;44:1851–1855. doi: 10.1212/wnl.44.10.1851. [DOI] [PubMed] [Google Scholar]
- 21.Zabramski JM, Spetzler RF, Lee KS, Papadopoulos SM, Bovill E, Zimmerman RS, et al. Phase 1 trial of tissue plasminogen activator for the prevention of vasospasm in patients with aneurysmal subarachnoid hemorrhage. J Neurosurg. 1991;75:189–196. doi: 10.3171/jns.1991.75.2.0189. [DOI] [PubMed] [Google Scholar]