Abstract
Objective
The aim of this study was to analyze the correlation between thromboembolic complications and antiplatelet drugs before and after neurointervention.
Methods
Blood samples and radiographic data of patients who received a neurointervention (coil embolization, stent placement or both) were collected prospectively. Rapid platelet function assay-aspirin (RPFA-ASA) was used to calculate aspirin resistance in aspirin reaction units (ARU). For clopidogrel resistance, a P2Y12 assay was used to analyze the percentage of platelet inhibition. ARU > 550 and platelet inhibition < 40% were defined as aspirin and clopidogrel resistance, respectively.
Results
Both aspirin and clopidogrel oral pills were administered in fifty-three patients before and after neurointerventional procedures. The mean resistance values of all patients were 484 ARU and < 39%. Ten (17.0%) of 53 patients showed resistance to aspirin with an average of 597 ARU, and 33 (62.3%) of 53 patients showed resistance to clopidogrel with an average of < 26%. Ten patients demonstrated resistance to both drugs, 5 of which suffered a thromboembolic complication after neurointervention (mean values : 640 ARU and platelet inhibition < 23%). Diabetic patients and patients with hypercholesterolemia displayed mean aspirin resistances of 513.7 and 501.8 ARU, and mean clopidogrel resistances of < 33.8% and < 40.7%, respectively.
Conclusion
Identifying individuals with poor platelet inhibition using standard regimens is of great clinical importance and may help prevent cerebral ischemic events in the future. Neurointerventional research should focus on ideal doses, timing, choices, safety, and reliable measurements of antiplatelet drug therapy, as well as confirming the clinical relevance of aggregometry in cerebrovascular patients.
Keywords: Aspirin, Clopidogrel, Resistance, Neurointervention
INTRODUCTION
Recent developments in biomedical devices and pharmaceuticals have led to milestones in cerebrovascular neurointervention. However, thromboembolic events have always been a major problem in coil embolization of cerebral aneurysms or stent insertions in patients with carotid artery stenosis22). Recently, perioperative aspirin and clopidogrel dual therapy has been considered as "standard of care" following the insertion of stents in cerebrovascular disease entities. Anti-platelet agents such as aspirin and clopidogrel are cornerstones that have allowed and increased the use of minimally invasive treatment of cerebrovascular diseases. Aspirin irreversibly inhibits full platelet aggregation by inhibiting cyclooxgenase-1 (COX-1) dependent synthesis of thromboxane A2 (TXA2), while clopidogrel, a thienopyridine, reduces platelet activation by irreversibly blocking ADP (P2Y12) receptor-dependent pathways11).
Oral aspirin and/or clopidogrel could reduce unfortunate thromboembolic events; however, drug resistance to anti-platelet agents has been a long-term problem that has not yet to be fully understood11). The term "drug resistance" by definition is when "a drug is incompetent to reach its pharmacologic target due to reduced bioavailability, in vivo inactivation, negative interaction with other substances or alterations of the target"4). Aspirin resistance would therefore mean the inability to inhibit COX-1-dependent TXA2 production and its byproducts. Clopidogrel resistance occurs along with variations in its absorption, metabolism, and genetic variations in the P2Y12 receptor3).
This study investigated aspirin and clopidogrel resistance rates of patients medicated prior to and after a neurointervention, including those who suffered thromboembolic complications.
MATERIALS AND METHODS
A prospective study was done on 53 patients (24 male, 29 female) from January 2007 to July 2008. All patients received either a coil embolization, stent insertion, or both for : intracranial arterial stenosis (7 patients), carotid arterial stenosis (15 patients), cerebrovascular dissection (3 patients), and intracranial aneurysm (28 patients). Eleven (39.3%) of 28 patients with aneurysms received coil embolization without balloon or stent, while 17 patients received stent-assisted coiling. The remaining patients were treated with stents only. All patients were pre-medicated with 100mg of aspirin and 75 mg of clopidogrel for at least 72 hours before the procedure. An aspirin/clopidogrel resistance test based on pharmaceutical doses was conducted after taking the medicine for 3 days. Clinical and angiographic examinations were done before and 6 months after the intervention.
Endovascular procedures
Endovascular procedures were carried out under general anesthesia in 28 (52.8%) of the 53 patients and under sedation in 8 (15.0%) of them. Arterial access was achieved via the femoral artery in all patients. A bolus of heparin was administered with 5,000 IU, maintaining the clotting time during the procedure at no more than 250 seconds during the procedure. A guiding catheter was placed in the internal carotid artery. We used an A 6-F guiding catheter system (Envoy; Codman Neurovascular, Miami Lakes, FL, USA) through the femoral artery via the vascular sheath. Cerebral angiography was done using this guiding catheter with heparin infusion (clotting time between 250 to 300 seconds) during the intervention. Then a microcatheter (Excelsior SL-10; Boston Scientific, Natick, MA, USA; Echelon-10; ev3, Irvine, CA, USA) was advanced and placed into the aneurysm using Synchro and Agility 0.014-inch microwires (Boston Scientific, Natick, MA, USA) via conventional technique. We also used two-types of self-expandable stents (Neuroform 3; Boston Scientific, Natick, MA, USA; Enterprise stent; Codman Neurovascular, Miami, FL, USA), Guglielmi detachable coils (GDCs; Boston Scientific), and other bare platinum coils (MicroPlex; Microvention, Aliso Viejo, CA, USA; Trufill-DCS; Codman Neurovascular, Miami Lakes, FL, USA) for aneurysms. For the stenting procedure, placement of the embolic protection device into a distal artery occurred after advancement of a Shuttle catheter (Cook : Bloomington, IN, USA). Balloon dilatation was optional. Stent insertion was carried out using the following stents : Precise (Codman Neurovascular, Miami, FL, USA), Protege (ev3, Irvine, CA, USA) and Wallstent (Boston Scientific, Natick, MAM USA). Vascular accesses were sealed up using the Angio-Seal closure device (St. Jude Medical, Minnetonka, MN, USA).
Assessments of drug resistance
VerifyNow Rapid platelet function assay-aspirin (RPFA-ASA) (Accumetrics, San Diego, CA, USA) was used to calculate aspirin resistance in aspirin reaction units (ARU). The VerifyNow P2Y12 assay (Accumetrics, San Diego, CA, USA) was used to analyze the percentage of platelet inhibition for clopidogrel resistance. ARU > 550 and a platelet inhibition < 40% were defined as aspirin and clopidogrel resistance, respectively.
Statistical analysis
Statistical analyses were done using commercial software (SPSS, version 15.0, SPSS Inc.) Uni- (Fisher's exact test) and multi-variate (binary logistic regression) analysis were used to explore associations of variable factors with aspirin and/or clopidogrel resistance. Statistical analysis on the correlations between complication occurrence and aspirin (> 550) and clopidogrel resistance (< 40%) were performed using the Mann-Whitney U test. p values < 0.05 were considered statistically significant.
RESULTS
Patients ranged in age from 52 to 81 years (mean ± SD; 60.3 ± 12 years), including 24 male and 29 female patients. Thirteen patients were diabetic and 10 had hypercholesterolemia. Mean follow-up period was 18.2 ± 7.84 months. Mean drug resistance levels for all patients were 484 ± 85.6 ARU for aspirin and < 39 ± 20.1% for clopidogrel. Ten patients (17%) showed resistance to aspirin (597 ± 80.97 ARU), 33 patients (62.3%) to clopidogrel (< 26 ± 10.35%), and 10 patients (17%) were resistant to both drugs.
There were 5 (9.4%) cases of thromboembolic complications (2 embolic and 3 thrombotic), and all 5 showed resistance to both agents (640 ± 34.8 ARU for aspirin and < 23 ± 2.3% for clopidogrel). The remaining 48 non-complicated patients had drug resistance levels of 468 ± 71.8 ARU for aspirin and < 40 ± 20.4% for clopidogrel. Only 5 non-complicated patients (10.4%) were aspirin resistant, while 28 (52.8%) presented with clopidogrel resistance.
In the diabetic subgroup, aspirin resistance was 513.7 ± 102.54 ARU and clopidogrel resistance was < 33.8%. For patients with hypercholesterolemia, means of 501.8 ± 93.81 ARU for aspirin and < 40.7% for clopidogrel were measured. Of the 13 patients with diabetes, 3 (23%) presented with complications, while 3 (30%) of the 10 patients with hypercholesterolemia experienced a thromboembolic event.
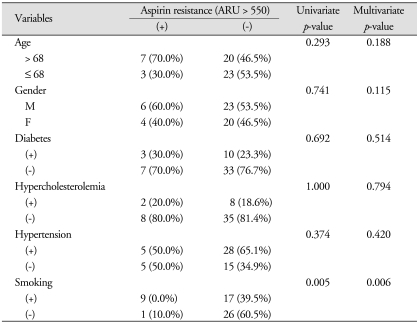
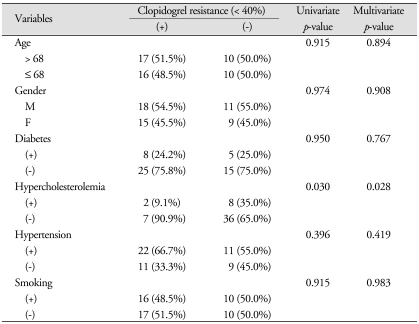
In this study, uni- and multi-variate analysis revealed that smoking was the only factor associated with aspirin resistance (p = 0.005 and 0.006, respectively), while hypercholesterolemia was the only factor associated with clopidogrel resistance (p = 0.030 and 0.028, respectively) (Table 1, 2).
Table 1.
Univariate and multivariate analysis of the correlation between variable factors and aspirin resistance
Table 2.
Univariate and multivariate analysis of the correlation between variable factors and clopidogrel resistance
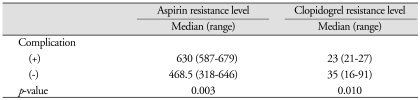
Associations between the occurrence of post-procedural complications and each aspirin and/or clopidogrel resistance levels were analyzed. Mean aspirin resistance levels with and without complication were 630 (range, 587-679) and 468.5 (range, 318-646), respectively (p = 0.003). Clopidogrel resistance levels with and without post-procedural complication were 23 (range : 21-27) and 35 (range : 16-91), respectively (p = 0.010) (Table 3).
Table 3.
The correlation between post-procedural complication and each resistance of aspirin and clopidogrel
DISCUSSION
The ultimate goal of cerebrovascular diseases is to keep cerebrovascular blood flow hemodynamically stable so that no occlusion or hemorrhage occurs31). Self-expandable stents, therefore, are used after a coil embolization in order to maintain cerebral blood flow. The insertion of a bare metal stent or coil has always required the need for anti-thrombotic agents to prevent thrombosis and occlusion24). Thrombotic agents such as anti-coagulants (heparin and warfarin), anti-platelet drugs (aspirin, clopidogrel, and dipyridamole), and thrombolytics have been used for more than 2 decades23). In general, antiplatelet agents are considered most beneficial for thrombotic strokes and anticoagulants are most effective for cardio-embolic strokes1). Overdosage with these agents, however, increases risk of hemorrhage. Therefore, careful dosing is needed. Finding the optimal dosage to reach suitable therapeutic levels has been difficult because of drug resistance.
According to previous clinical trial, clopidogrel resistance is more frequent than aspirin resistance16,25). In a study by Lee et al.14), poor response to clopidogrel was quite prevalent (42.9%), whereas just a few patients had poor response to aspirin (2%). Mean clopidogrel resistance of all patients (39 ± 20.1%) was also high in this study, compared to aspirin (484 ± 85.6 ARU), supporting the reason why more than half of the patients (62.3%) in the study showed clopidogrel resistance. Aspirin inhibits serum thromboxane B2 up to 95%, and resistance implies less sensitive inactivation of cyclooxygenase-1, whereas clopidogrel is highly variable and reflects the bioavailability of the active metabolite, not "resistance" of the receptor to inhibition10). Extrinsic factors (patient non-compliance, underdosing, drug interactions, and variability in intestinal absorption/hepatic metabolism), patient conditions (diabetes and hypercholesterolemia) that increase platelet reactivity, and intrinsic factors (variability in P2Y12 receptor affinity and binding properties) also contribute to clopidogrel resistance13,33).
The percentage of our patients with aspirin resistance was similar to those with resistance to both aspirin and clopidogrel. This could be explained by the association of aspirin resistance with platelet hyper-reactivity16), collagen, and adenosine diphosphate (ADP) sensitivity30). In one study, 50% of patients with aspirin resistance were also resistant to clopidogrel, while only about 20% of aspirin-sensitive patients were resistant to clopidogrel20). Aspirin-resistant patients displayed a reduced degree of platelet aggregation, P-selectin expression, and less inhibition of GP IIb/IIIa receptor activation in response to clopidogrel16).
In our study, all patients with a complication after neurointervention displayed resistance to both aspirin and clopidogrel. These patients may have unpredictable heritable factors, indirectly related to platelet cyclooxygenase inactivation, and bioavailability of clopidogrel susceptible to drug resistance10).
The patient group with diabetes and hypercholesterolemia showed resistance to aspirin (513.7, 501.8 ARU), but only the diabetic group revealed resistant to clopidogrel (< 33.8%). Diabetes plays a role in decreasing the effectiveness of anti-platelet agents by producing reactive oxidant species6), increases platelet aggregation, activation2) by insulin resistance and increases P2Y12 signaling9), increases platelet turnover, alteres platelet membrane structure, increases intracellular calcium, and abnormal glycation17,28,32). These mechanisms could explain why diabetics were more clopidogrel resistant than hypercholesterolemia in our study. Prabhakaran et al.22), found multivariable models of diabetes were independently predictive of poor platelet inhibition in clopidogrel-treated patients. Likewise, hypercholesterolemia attenuates aspirin's effect on thrombin27). Similar to diabetes, hypercholesterolemia is a pathophysiological condition that promotes atherosclerosis, thus increasing oxygen-free radicals and isoprostane levels8) that contribute to aspirin resistance6).
Previous studies have suggested a strong association of smoking with aspirin resistance19). Our analysis also revealed correlation of smoking with aspirin resistance. Although our results show different result to the existing researches, smoking has been thought to have a "paradoxical effect" with clopidogrel. Interestingly, recent studies reported that cigarette smoking is one of the factors associated with a prompt antiplatelet response to clopidogrel5).
Although recent clinical trials have shown a protocol using the "dual therapy" of aspirin and clopidogrel to prevent thrombotic and embolic complication during/after a neurointervention18,26,35), antithrombotic therapy still remains an unproven benefit in many clinical settings of cerebrovascular disease23). This elicited the need for monitoring the response to anti-platelet agents to help elucidate the problem of drug resistance. Currently, there are several suggested drug regimens. First of all, extrinsic factors can be modified. Non-compliance of the patient can be monitored thoroughly7). And, increasing dosage of aspirin and clopidogrel can shorten duration of antiplatelet therapy. For example, increasing the loading dose from 300 mg to 600 mg or changing the maintenance dose from 75 mg to 150 mg lowered the frequency of clopidogrel resistance29), though this may be associated with a higher bleeding risk12). Short-term administration of dual therapy resulted in a relatively stable level of inhibition14). Avoiding the use of non-steroidal anti-inflammatory drugs (competitor of COX inhibition) with aspirin could also help avoid aspirin resistance. Secondly, the medical condition of patients can be controlled beforehand. Diabetes regulated on a daily basis and a low-fat diet with more exercise decreased blood cholesterol levels. Thirdly, measurement of resistance level should be standardized. At the present time, many laboratory methods are used to measure aspirin and clopidogrel resistance. However, results can be different according to method of measurement. Unified and verified testing is needed. Finally, administering a whole new anti-platelet drug or supplementing an additional anti-platelet agent to conventional dual therapy, is recommended. New anti-platelet agents, known as CS-747 (Prasugrel), intravenous P2Y12 inhibitor agent (Cangrelor)21), and oral ADP antagonist (AZD6140) are now in clinical trials34); in particular, triple anti-platelet therapy with the addition of Cilostazol is thought to be better in preventing stent thrombosis than conventional treatment15). Cilostazol is a selective inhibitor of phosphodiesterase type 3. This action results in increased cyclic adenosine monophosphate (cAMP). An increase in cAMP causes an increase in the active form of protein kinase A, which is associated with an inhibition of platelet aggregation. Many studies have suggested not only efficacy but also safety of cilostazol. As a result, cilostazol has been in the spotlight for combination therapy with aspirin and clopidogrel.
CONCLUSION
Identifying individuals with poor platelet inhibition using standard regimens may be of great clinical importance and will help prevent cerebral ischemic events in the future. Neurointerventional research should focus on optimal doses, timing, choices, safety, and reliable measurement of antiplatelet drug therapy, as well as confirming the clinical relevance of aggregometry in cerebrovascular patients.
Acknowledgements
This study was supported by a faculty research grand of Yonsei University College of Medicine for 6-2007-0165.
References
- 1.Albers GW. Antithrombotic agents in cerebral ischemia. Am J Cardiol. 1995;75:34B–38B. doi: 10.1016/0002-9149(95)80008-g. [DOI] [PubMed] [Google Scholar]
- 2.Angiolillo DJ, Fernandez-Ortiz A, Bernardo E, Ramírez C, Sabate M, Jimenez-Quevedo P, et al. Platelet function profiles in patients with type 2 diabetes and coronary artery disease on combined aspirin and clopidogrel treatment. Diabetes. 2005;54:2430–2435. doi: 10.2337/diabetes.54.8.2430. [DOI] [PubMed] [Google Scholar]
- 3.Cairns JA, Eikelboom J. Clopidogrel resistance : more grist for the mill. J Am Coll Cardiol. 2008;51:1935–1937. doi: 10.1016/j.jacc.2008.01.051. [DOI] [PubMed] [Google Scholar]
- 4.Cattaneo M. Laboratory detection of 'aspirin resistance' : what test should we use (if any)? Eur Heart J. 2007;28:1673–1675. doi: 10.1093/eurheartj/ehm232. [DOI] [PubMed] [Google Scholar]
- 5.Coma-Canella I, Valasc A. Variability in individual responsiveness to aspirin : clinical implications and treatment. Cardiovasc Hematol Disord Drug Targets. 2007;7:274–287. doi: 10.2174/187152907782793590. [DOI] [PubMed] [Google Scholar]
- 6.Csiszar A, Stef G, Pacher P, Ungvari Z. Oxidative stress-induced isoprostane formation may contribute to aspirin resistance in platelets. Prostaglandins Leukot Essent Fatty Acids. 2002;66:557–558. doi: 10.1054/plef.2002.0399. [DOI] [PubMed] [Google Scholar]
- 7.Dalen JE. Aspirin resistance : is it real? Is it clinically significant? Am J Med. 2007;120:1–4. doi: 10.1016/j.amjmed.2006.08.023. [DOI] [PubMed] [Google Scholar]
- 8.Davi G, Gresele P, Violi F, Basili S, Catalano M, Giammarresi C, et al. Diabetes mellitus, hypercholesterolemia, and hypertension but not vascular disease per se are associated with persistent platelet activation in vivo. Evidence derived from the study of peripheral arterial disease. Circulation. 1997;96:69–75. doi: 10.1161/01.cir.96.1.69. [DOI] [PubMed] [Google Scholar]
- 9.Ferreira IA, Mocking AI, Feijge MA, Gorter G, van Haeften TW, Heemskerk JW, et al. Platelet inhibition by insulin is absent in type 2 diabetes mellitus. Arterioscler Thromb Vasc Biol. 2006;26:417–422. doi: 10.1161/01.ATV.0000199519.37089.a0. [DOI] [PubMed] [Google Scholar]
- 10.Fitzgerald DJ, Maree A. Aspirin and clopidogrel resistance. Hematology Am Soc Hematol Educ Program. 2007:114–120. doi: 10.1182/asheducation-2007.1.114. [DOI] [PubMed] [Google Scholar]
- 11.Grau AJ, Reiners S, Lichy C, Buggle F, Ruf A. Platelet function under aspirin, clopidogrel, and both after ischemic stroke : a case-crossover study. Stroke. 2003;34:849–854. doi: 10.1161/01.STR.0000064326.65899.AC. [DOI] [PubMed] [Google Scholar]
- 12.Gurbel PA, Bliden KP, Hayes KM, Yoho JA, Herzog WR, Tantry US. The relation of dosing to clopidogrel responsiveness and the incidence of high post-treatment platelet aggregation in patients undergoing coronary stenting. J Am Coll Cardiol. 2005;45:1392–1396. doi: 10.1016/j.jacc.2005.01.030. [DOI] [PubMed] [Google Scholar]
- 13.Gurbel PA, Tantry US. Clopidogrel resistance? Thromb Res. 2007;120:311–321. doi: 10.1016/j.thromres.2006.08.012. [DOI] [PubMed] [Google Scholar]
- 14.Lee DH, Arat A, Morsi H, Shaltoni H, Harris JR, Mawad ME. Dual antiplatelet therapy monitoring for neurointerventional procedures using a point-of-care platelet function test : a single-center experience. AJNR Am J Neuroradiol. 2008;29:1389–1394. doi: 10.3174/ajnr.A1070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lee SW, Park SW, Hong MK, Kim YH, Lee BK, Song JM, et al. Triple versus dual antiplatelet therapy after coronary stenting : impact on stent thrombosis. J Am Coll Cardiol. 2005;46:1833–1837. doi: 10.1016/j.jacc.2005.07.048. [DOI] [PubMed] [Google Scholar]
- 16.Lev EI, Patel RT, Maresh KJ, Guthikonda S, Granada J, DeLao T, et al. Aspirin and clopidogrel drug response in patients undergoing percutaneous coronary intervention : the role of dual drug resistance. J Am Coll Cardiol. 2006;47:27–33. doi: 10.1016/j.jacc.2005.08.058. [DOI] [PubMed] [Google Scholar]
- 17.Li Y, Woo V, Bose R. Platelet hyperactivity and abnormal Ca(2+) homeostasis in diabetes mellitus. Am J Physiol Heart Circ Physiol. 2001;280:H1480–H1489. doi: 10.1152/ajpheart.2001.280.4.H1480. [DOI] [PubMed] [Google Scholar]
- 18.Mehta SR, Yusuf S, Peters RJ, Bertrand ME, Lewis BS, Natarajan MK, et al. Effects of pretreatment with clopidogrel and aspirin followed by long-term therapy in patients undergoing percutaneous coronary intervention : the PCI-CURE study. Lancet. 2001;358:527–533. doi: 10.1016/s0140-6736(01)05701-4. [DOI] [PubMed] [Google Scholar]
- 19.Motovska Z, Widimsky P, Petr R, Bilkova D, Marinov I, Simek S, et al. Factors influencing clopidogrel efficacy in patients with stable coronary artery disease undergoing elective percutaneous coronary intervention : statin's advantage and the smoking "paradox". J Cardiovasc Pharmacol. 2009;53:368–372. doi: 10.1097/FJC.0b013e31819d616b. [DOI] [PubMed] [Google Scholar]
- 20.Oqueli E, Hiscock M, Dick R. Clopidogrel resistance. Heart Lung Circ. 2007;16(Suppl 3):S17–S28. doi: 10.1016/j.hlc.2007.03.012. [DOI] [PubMed] [Google Scholar]
- 21.Patrono C, Bachmann F, Baigent C, Bode C, De Caterina R, Charbonnier B, et al. Expert consensus document on the use of antiplatelet agents. The task force on the use of antiplatelet agents in patients with atherosclerotic cardiovascular disease of the European society of cardiology. Eur Heart J. 2004;25:166–181. doi: 10.1016/j.ehj.2003.10.013. [DOI] [PubMed] [Google Scholar]
- 22.Prabhakaran S, Wells KR, Lee VH, Flaherty CA, Lopes DK. Prevalence and risk factors for aspirin and clopidogrel resistance in cerebrovascular stenting. AJNR Am J Neuroradiol. 2008;29:281–285. doi: 10.3174/ajnr.A0818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rothrock JF, Hart RG. Antithrombotic therapy in cerebrovascular disease. Ann Intern Med. 1991;115:885–895. doi: 10.7326/0003-4819-115-11-885. [DOI] [PubMed] [Google Scholar]
- 24.Saratzis A, Saratzis N, Melas N, Kiskinis D. Pharmacotherapy before and after endovascular repair of abdominal aortic aneurysms. Curr Vasc Pharmacol. 2008;6:240–249. doi: 10.2174/157016108785909689. [DOI] [PubMed] [Google Scholar]
- 25.Shim CY, Yoon SJ, Park S, Kim JS, Choi JR, Ko YG, et al. The clopidogrel resistance can be attenuated with triple antiplatelet therapy in patients undergoing drug-eluting stents implantation. Int J Cardiol. 2009;134:351–355. doi: 10.1016/j.ijcard.2008.02.016. [DOI] [PubMed] [Google Scholar]
- 26.Steinhubl SR, Berger PB, Mann JT, 3rd, Fry ET, DeLago A, Wilmer C, et al. Early and sustained dual oral antiplatelet therapy following percutaneous coronary intervention : a randomized controlled trial. JAMA. 2002;288:2411–2420. doi: 10.1001/jama.288.19.2411. [DOI] [PubMed] [Google Scholar]
- 27.Szczeklik A, Musial J, Undas A, Gajewski P, Góra P, Swadzba J, et al. Inhibition of thrombin generation by simvastatin and lack of additive effects of aspirin in patients with marked hypercholesterolemia. J Am Coll Cardiol. 1999;33:1286–1293. doi: 10.1016/s0735-1097(99)00023-6. [DOI] [PubMed] [Google Scholar]
- 28.Tschoepe D, Roesen P, Esser J, Schwippert B, Nieuwenhuis HK, Kehrel B, et al. Large platelets circulate in an activated state in diabetes mellitus. Semin Thromb Hemost. 1991;17:433–438. doi: 10.1055/s-2007-1002650. [DOI] [PubMed] [Google Scholar]
- 29.von Beckerath N, Kastrati A, Wieczorek A, Pogatsa-Murray G, Sibbing D, Graf I, et al. A double-blind, randomized study on platelet aggregation in patients treated with a daily dose of 150 or 75 mg of clopidogrel for 30 days. Eur Heart J. 2007;28:1814–1819. doi: 10.1093/eurheartj/ehl489. [DOI] [PubMed] [Google Scholar]
- 30.Wang TH, Bhatt DL, Topol EJ. Aspirin and clopidogrel resistance : an emerging clinical entity. Eur Heart J. 2006;27:647–654. doi: 10.1093/eurheartj/ehi684. [DOI] [PubMed] [Google Scholar]
- 31.Weksler BB, Lewin M. Anticoagulation in cerebral ischemia. Stroke. 1983;14:658–663. doi: 10.1161/01.str.14.5.658. [DOI] [PubMed] [Google Scholar]
- 32.Winocour PD, Watala C, Perry DW, Kinlough-Rathbone RL. Decreased platelet membrane fluidity due to glycation or acetylation of membrane proteins. Thromb Haemost. 1992;68:577–582. [PubMed] [Google Scholar]
- 33.Wiviott SD. Clopidogrel response variability, resistance, or both? Am J Cardiol. 2006;98:18N–24N. doi: 10.1016/j.amjcard.2006.09.010. [DOI] [PubMed] [Google Scholar]
- 34.Wiviott SD, Braunwald E, McCabe CH, Montalescot G, Ruzyllo W, Gottlieb S, et al. Prasugrel versus clopidogrel in patients with acute coronary syndromes. N Engl J Med. 2007;357:2001–2015. doi: 10.1056/NEJMoa0706482. [DOI] [PubMed] [Google Scholar]
- 35.Yusuf S, Zhao F, Mehta SR, Chrolavicius S, Tognoni G, Fox KK Clopidogrel in Unstable Angina to Prevent Recurrent Events Trial Investigators. Effects of clopidogrel in addition to aspirin in patients with acute coronary syndromes without ST-segment elevation. N Engl J Med. 2001;345:494–502. doi: 10.1056/NEJMoa010746. [DOI] [PubMed] [Google Scholar]