Abstract
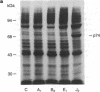
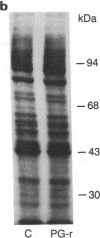
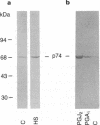
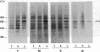
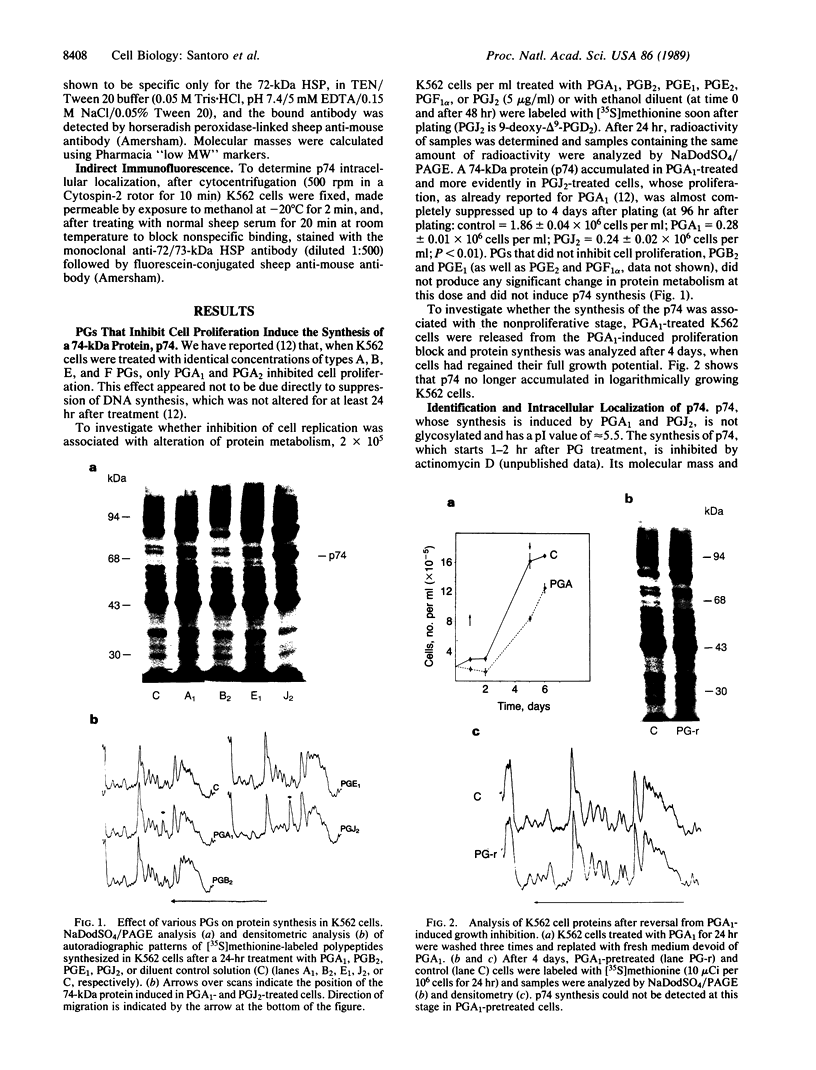
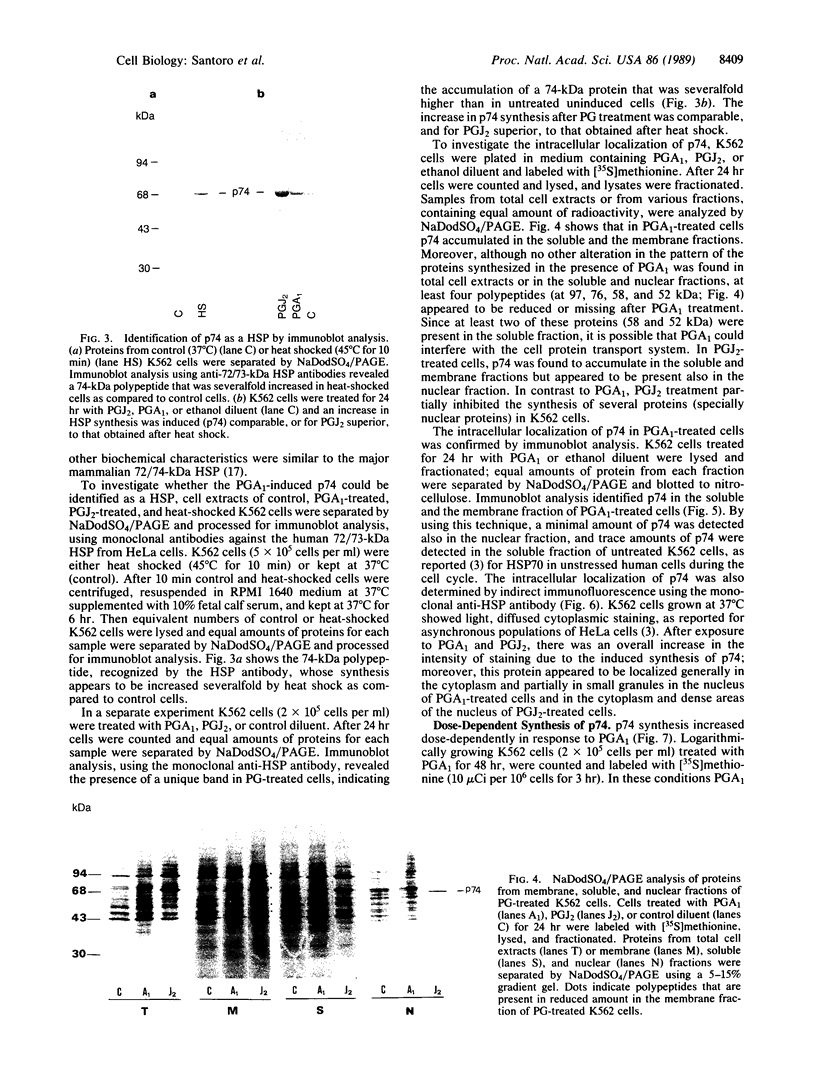
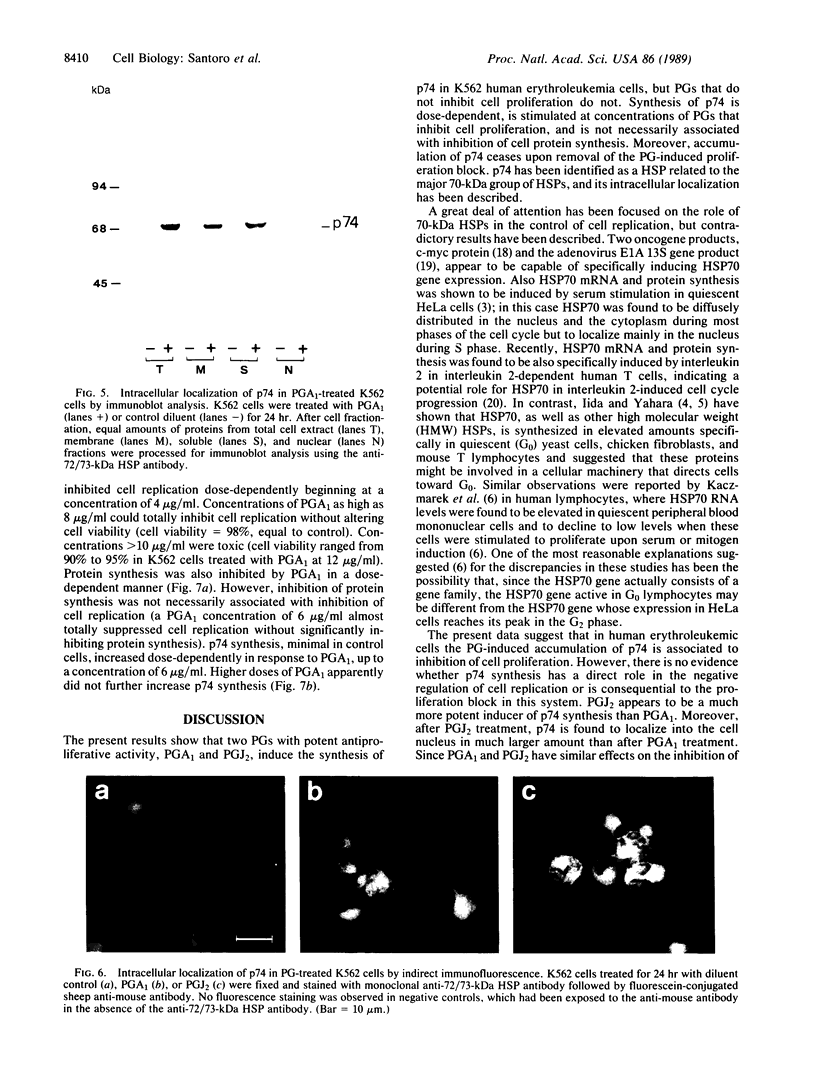
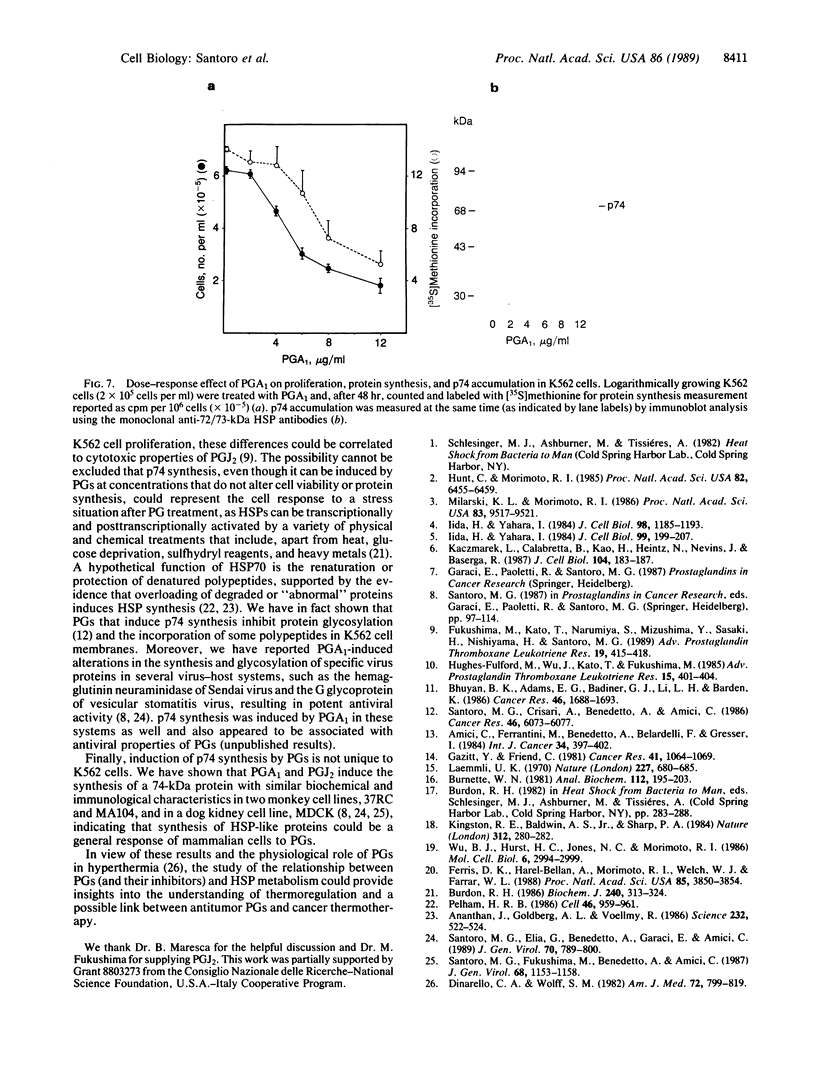
Prostaglandins (PGs) A1 and J2 were found to potently suppress the proliferation of human K562 erythroleukemia cells and to induce the synthesis of a 74-kDa protein (p74) that was identified as a heat shock protein related to the major 70-kDa heat shock protein group. p74 synthesis was stimulated at doses of PGA1 and PGJ2 that inhibited cell replication, and its accumulation ceased upon removal of the PG-induced proliferation block. PGs that did not affect K562 cell replication did not induce p74 synthesis. p74 was found to be localized mainly in the cytoplasm of PG-treated cells, but moderate amounts were found also in dense areas of the nucleus after PGJ2 treatment. p74 synthesis was not necessarily associated with cytotoxicity or with inhibition of cell protein synthesis. The results described support the hypothesis that synthesis of the 70-kDa heat shock proteins is associated with changes in cell proliferation. The observation that PGs can induce the synthesis of heat shock proteins expands our understanding of the mechanism of action of these compounds whose regulatory role is well known in many physiological phenomena, including the control of fever production.
Full text
PDF
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amici C., Ferrantini M., Benedetto A., Belardelli F., Gresser I. Biologic and biochemical differences between in vitro and in vivo passaged Friend erythroleukemia cells. II. Changes in cell surface glycoproteins associated with a highly malignant phenotype. Int J Cancer. 1984 Sep 15;34(3):397–402. doi: 10.1002/ijc.2910340317. [DOI] [PubMed] [Google Scholar]
- Ananthan J., Goldberg A. L., Voellmy R. Abnormal proteins serve as eukaryotic stress signals and trigger the activation of heat shock genes. Science. 1986 Apr 25;232(4749):522–524. doi: 10.1126/science.3083508. [DOI] [PubMed] [Google Scholar]
- Bhuyan B. K., Adams E. G., Badiner G. J., Li L. H., Barden K. Cell cycle effects of prostaglandins A1, A2, and D2 in human and murine melanoma cells in culture. Cancer Res. 1986 Apr;46(4 Pt 1):1688–1693. [PubMed] [Google Scholar]
- Burdon R. H. Heat shock and the heat shock proteins. Biochem J. 1986 Dec 1;240(2):313–324. doi: 10.1042/bj2400313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnette W. N. "Western blotting": electrophoretic transfer of proteins from sodium dodecyl sulfate--polyacrylamide gels to unmodified nitrocellulose and radiographic detection with antibody and radioiodinated protein A. Anal Biochem. 1981 Apr;112(2):195–203. doi: 10.1016/0003-2697(81)90281-5. [DOI] [PubMed] [Google Scholar]
- Dinarello C. A., Wolff S. M. Molecular basis of fever in humans. Am J Med. 1982 May;72(5):799–819. doi: 10.1016/0002-9343(82)90548-4. [DOI] [PubMed] [Google Scholar]
- Ferris D. K., Harel-Bellan A., Morimoto R. I., Welch W. J., Farrar W. L. Mitogen and lymphokine stimulation of heat shock proteins in T lymphocytes. Proc Natl Acad Sci U S A. 1988 Jun;85(11):3850–3854. doi: 10.1073/pnas.85.11.3850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukushima M., Kato T., Narumiya S., Mizushima Y., Sasaki H., Terashima Y., Nishiyama Y., Santoro M. G. Prostaglandin A and J: antitumor and antiviral prostaglandins. Adv Prostaglandin Thromboxane Leukot Res. 1989;19:415–418. [PubMed] [Google Scholar]
- Gazitt Y., Friend C. Synthesis and phosphorylation of plasma membrane proteins of Friend erythroleukemia cells induced to differentiate. Cancer Res. 1981 Mar;41(3):1064–1069. [PubMed] [Google Scholar]
- Hughes-Fulford M., Wu J., Kato T., Fukushima M. Inhibition of DNA synthesis and cell cycle by prostaglandins independent of cyclic AMP. Adv Prostaglandin Thromboxane Leukot Res. 1985;15:401–404. [PubMed] [Google Scholar]
- Hunt C., Morimoto R. I. Conserved features of eukaryotic hsp70 genes revealed by comparison with the nucleotide sequence of human hsp70. Proc Natl Acad Sci U S A. 1985 Oct;82(19):6455–6459. doi: 10.1073/pnas.82.19.6455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iida H., Yahara I. Durable synthesis of high molecular weight heat shock proteins in G0 cells of the yeast and other eucaryotes. J Cell Biol. 1984 Jul;99(1 Pt 1):199–207. doi: 10.1083/jcb.99.1.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iida H., Yahara I. Specific early-G1 blocks accompanied with stringent response in Saccharomyces cerevisiae lead to growth arrest in resting state similar to the G0 of higher eucaryotes. J Cell Biol. 1984 Apr;98(4):1185–1193. doi: 10.1083/jcb.98.4.1185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaczmarek L., Calabretta B., Kao H. T., Heintz N., Nevins J., Baserga R. Control of hsp70 RNA levels in human lymphocytes. J Cell Biol. 1987 Feb;104(2):183–187. doi: 10.1083/jcb.104.2.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kingston R. E., Baldwin A. S., Jr, Sharp P. A. Regulation of heat shock protein 70 gene expression by c-myc. Nature. 1984 Nov 15;312(5991):280–282. doi: 10.1038/312280a0. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Milarski K. L., Morimoto R. I. Expression of human HSP70 during the synthetic phase of the cell cycle. Proc Natl Acad Sci U S A. 1986 Dec;83(24):9517–9521. doi: 10.1073/pnas.83.24.9517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelham H. R. Speculations on the functions of the major heat shock and glucose-regulated proteins. Cell. 1986 Sep 26;46(7):959–961. doi: 10.1016/0092-8674(86)90693-8. [DOI] [PubMed] [Google Scholar]
- Santoro M. G., Amici C., Elia G., Benedetto A., Garaci E. Inhibition of virus protein glycosylation as the mechanism of the antiviral action of prostaglandin A in Sendai virus-infected cells. J Gen Virol. 1989 Apr;70(Pt 4):789–800. doi: 10.1099/0022-1317-70-4-789. [DOI] [PubMed] [Google Scholar]
- Santoro M. G., Crisari A., Benedetto A., Amici C. Modulation of the growth of a human erythroleukemic cell line (K562) by prostaglandins: antiproliferative action of prostaglandin A. Cancer Res. 1986 Dec;46(12 Pt 1):6073–6077. [PubMed] [Google Scholar]
- Santoro M. G., Fukushima M., Benedetto A., Amici C. PGJ2, a new antiviral prostaglandin: inhibition of Sendai virus replication and alteration of virus protein synthesis. J Gen Virol. 1987 Apr;68(Pt 4):1153–1158. doi: 10.1099/0022-1317-68-4-1153. [DOI] [PubMed] [Google Scholar]
- Wu B. J., Hurst H. C., Jones N. C., Morimoto R. I. The E1A 13S product of adenovirus 5 activates transcription of the cellular human HSP70 gene. Mol Cell Biol. 1986 Aug;6(8):2994–2999. doi: 10.1128/mcb.6.8.2994. [DOI] [PMC free article] [PubMed] [Google Scholar]