Abstract
Urocortin (Ucn) peptides are the endogenous ligands for the corticotropin-releasing factor type 2 receptor (CRFR2). They have potentially important roles in cardiovascular physiology in health and disease, and show promise as therapeutics for congestive heart failure. Analysis of canine heart tissue showed mRNA expression of Ucn 1, Ucn 3 and CRFR2 in all heart chambers. Immunohistochemistry also demonstrated Ucn 1 and 3 expression in cardiomyocytes. To assess the potential usefulness of circulating Ucns as markers of heart disease, plasma samples from 45 dogs with cardiac disease and 15 controls were analysed by radioimmunoassay. Both Ucn 1 and 3 were measurable but the presence of cardiac disease did not alter their concentrations. Therefore, whilst Ucns are expressed in canine myocardium (where they may play a role in the endogenous neurohumoral response to cardiac disease or failure) they do not appear to be sensitive biomarkers of cardiac disease in our canine patient population.
Keywords: Urocortin, Dog, Corticotropin-releasing factor receptor, Heart, Cardiac disease
Introduction
Urocortins (Ucns 1, 2 and 3) are peptide hormones (Vaughan et al., 1995; Hsu and Hsueh, 2001; Lewis et al., 2001; Reyes et al., 2001) structurally and biologically related to corticotropin-releasing factor (CRF) (Fekete and Zorrilla, 2007). In contrast to CRF, Ucns are expressed in peripheral tissues including the heart and vasculature (Baigent and Lowry, 2000; Hsu and Hsueh, 2001; Lewis et al., 2001) and are under investigation as therapeutic agents for heart failure. Ucns protect cardiomyocytes from hypoxic injury in experimental myocardial ischemia reperfusion models (Brar et al., 2004; Lawrence et al., 2004), and increase cardiac output via potent positive inotropic actions. Beneficial effects of Ucns are observed in animal models of heart failure (Parkes et al., 1997; Bale et al., 2004; Rademaker et al., 2005a, b; Rademaker et al., 2006).
The role of endogenous Ucns in the pathophysiology of heart failure is unknown. Expression of Ucn peptides has been demonstrated in rodent (Okosi et al., 1998; Pournajafi-Nazarloo et al., 2007) and human hearts (Hsu and Hsueh, 2001; Kimura et al., 2002; Takahashi et al., 2004). Plasma concentrations of Ucn 1 are increased in human heart failure patients (Ng et al., 2004; Wright et al., 2009; Gruson et al, 2010), and sheep with experimentally-induced heart failure also have increased circulating levels (Charles et al., 2006). Endogenous concentrations of Ucns 2 and 3 have not been described in heart failure.
The CRF receptor type 2 (CRFR2) is specific for Ucn signalling and is expressed in peripheral tissues including heart and vasculature (Kishimoto et al., 1995; Perrin et al., 1995). Several receptor isoforms exist (CRFR2α, β, γ) and their localisation varies with species and tissue. In man, CRFR2α is present in all heart chambers, whereas CRFR2β expression is restricted to the atria (Kimura et al., 2002). In rodents, CRFR2β is the major isoform in heart (Lovenberg et al., 1995; Nishikimi et al., 2000). No studies on the expression of Ucns or CRFR2 have been performed in the dog.
Given the importance of canine heart failure in veterinary practice and the current interest in Ucns as potential therapeutics for cardiac conditions, we have characterised the expression of Ucns and CRFR2 in the dog heart. We also determined plasma Ucn levels in dogs with heart disease and controls to assess their potential usefulness as a marker of heart disease in this species.
Materials and methods
Heart tissue sample collection
Studies were approved by the University of Edinburgh Ethics Committee. Cardiac tissues were obtained from healthy dogs euthanased for behavioural reasons, with no history or evidence of cardiovascular disease on ante-mortem clinical examination. Estimated ages ranged from 18 months to 10 years. There were four entire female and four entire male dogs. Breeds were two terrier-cross, one Staffordshire Bull terrier, two Staffordshire Bull terrier-cross, one Yorkshire terrier, one Rottweiler and one cross-breed dog. Tissues were collected within 30-60 min of euthanasia from the following standardised sites: right atrium (RA), right ventricular free wall (RVFW), left atrium (LA), left ventricular free wall (LVFW), interventricular septum (IVS) and left ventricular papillary muscle (LVPM). All tissues were macroscopically normal. Tissue for mRNA extraction was collected in RNAlater (Ambion), stored at 4 °C for 24 h and then −80 °C until analysis. Tissue for immunohistochemistry was stored at −80 °C until sectioning.
Plasma sample collection and heart disease classification
Plasma was obtained from 45 dogs investigated for cardiac diseases at the University of Edinburgh Hospital for Small Animals (UK) and the Koret School of Veterinary Medicine (Israel) with owner consent. There were 24 males, 3 neutered males, 9 females, and 9 neutered females. Ages ranged from 9 months to 15 years (mean 7.9 years). Control plasma was from 15 dogs with non-critical non-cardiac diseases comprising 5 males, 3 neutered males, 1 female, 6 neutered females, with ages raging from 1 to 13 years (mean 5.8 years). Blood was collected by jugular venepuncture into EDTA collection tubes, separated by centrifugation, and plasma stored at −80 °C until analysis.
Dogs with cardiac disease were characterised by clinical examination, thoracic radiography, electrocardiography and two-dimensional and colour-flow Doppler echocardiography. Diagnoses were as follows: dilated cardiomyopathy (DCM) (n = 15), mitral valve disease (MVD) (n = 18), subaortic stenosis (SAS) (n = 6), or ‘other’ cardiac disease (pericardial effusion n = 1, third degree AV block n = 1, tricuspid dysplasia n = 1, patent ductus arteriosus n = 1, primary supraventricular tachycardia n = 1, heart base tumour n = 1). MVD was defined as a systolic heart murmur of grade 3/6 or greater over the left apex with fractional shortening (FS) > 30%. SAS was classified as velocity > 2.5 m/s. DCM was defined as a systolic heart murmur grade 3/6 or less over the left apex with FS < 25%.
RNA analysis
Total RNA was isolated with Trizol (Ambion), digested with RNase-Free DNase (Qiagen) and reverse transcribed (RT) to generate cDNA (SuperScript II, Invitrogen). Primers (Table 1) were designed from published nucleotide sequences in the Ensembl database. CRFR2α and CRFR2β PCRs amplified sequence spanning an intron. S16 was used as a housekeeping gene. Genomic DNA from canine liver was included as a positive control for Ucn PCRs. RT negative controls were included for all reactions.
Table 1.
PCR primers sequences used in this study.
| Gene | Transcript ID | Primer | Sequence | Product |
|---|---|---|---|---|
| UCN 1 | ENSCAFT0000003593 7 |
Sense Antisens e |
5′-AGGACCCAAGTCTGCGCTA 5′- CGAATATGATGCGGTTCTGCTC |
258 bp |
| UCN 2 | ENSCAFT0000001979 0 |
Sense Antisens e |
5′-ATGACCAGGTGGGCTTTG 5′-GTTGGTGGTGGCCTGCTC |
306 bp |
| UCN 3 | ENSCAFT0000000847 3 |
Sense Antisens e |
5′- CAGAAGTTCCACCCAGGAAA 5′-GTATTTGTACCGGGCGCTTA |
240 bp |
| CRFR2α | ENSCAFT0000000497 1 |
Sense Antisens e |
5′-AGGAGCTGCTCCTGGACGG 5′-TGCGGTAGTGCAGGTCATAC |
285 bp |
| CRFR2β | ENSCAFT0000000497 2 |
Sense Antisens e |
5′-GAGCCAGGCGCAGATACAT 5′- AAACGCAGTGACCCAGGTAG |
356 bp |
| S16 | ENSCAFT0000000550 1 |
Sense Antisens e |
5′-CAAGGGTCCTCTGCAGTCC 5′-GTCCCATGACAGCGGTTTAT |
485 bp |
The GC-RICH PCR System (Roche) was used for RT-PCR for Ucns and CRFR2. Conditions for Ucn 2, Ucn 3 and CRFR2β were as follows: 50 μL reaction with 1X GC-Rich Buffer, 0.2 mM dNTP, 2.5 mM MgCl2, 0.4 μM sense primer, 0.4 μM antisense primer, 2U GC-Rich Taq, cDNA equivalent to 25 ng tRNA (1μL) and PCR cycling 95 °C 3 min, then 95 °C 30 s, 56 °C 30 s, 72 °C 1 min for 40 cycles, and a final elongation step 72 °C for 7 min. For Ucn 1 and CRFR2α conditions were as follows: 50 μL reaction with 1X GC-Rich Buffer, 0.2 mM dNTP, 5 mM MgCl2, 5% DMSO, 0.8 μM sense primer, 0.8 μM antisense primer, 1 μL GC-Rich Taq, 1 μL cDNA and cycled as above with annealing temperature 58 °C. For S16 conditions were: 50 μL reaction with 1X Hotmaster Taq buffer, 0.2 mM dNTP, 0.4 μM sense primer, 0.4 μM antisense primer, 1U Hotmaster Taq Polymerase (Eppendorf), 1 μL cDNA, cycled as above for 30 cycles with annealing temperature of 58 °C.
Amplification products were subjected to electrophoresis in a 2% agarose gel, stained with ethidium bromide and photographed under UV illumination. PCR products were purified (High Pure PCR Product Purification kit, Roche) and submitted for DNA sequencing to verify specificity of product.
Immunohistochemistry
The LVFW and LA of four dogs were analysed. Ucn antisera were raised in rabbit (The Salk Institute; anti-Ucn 1 PBL 5779, anti-Ucn 2 6488, anti-Ucn 3 6570). Frozen tissue sections (5 μm) were fixed in ice-cold acetone 10 min, washed 3X in PBS 5 min, blocked with Protein Block (DAKO Corp) 10 min, washed briefly, blocked with goat serum 20 min at room temperature, incubated with primary antibody at 1:50 concentration in DAKO antibody diluent at room temperature for 1 h, washed 3X in PBS for 5 min, blocked again with sera for 10 min, incubated with HRP goat anti-rabbit antibodies for 30 min at room temperature, and the PBS washes were repeated. Sections were then incubated with a diaminobenzidine solution and finally washed with water. A light haematoxylin counterstain was performed, and sections dehydrated with ascending grades of alcohol, cleared in xylene and mounted in Pertex.
Urocortin radioimmunoassays
Ucn 1 and Ucn 3 were measured in plasma using RIAs we developed and with a protocol similar to that for inhibin subunits (Vaughan et al., 1989). Samples were acidified and extracted as described, except elution of octadecyl silica cartridges was with 75% acetonitrile/25% triethylammonium formate, pH 3.0 (Vale et al., 1986). For Ucn 1 RIA, rabbit anti-rat Ucn 1 serum (PBL 5779) was used at 1:700,000 final dilution, with [125 I]DTyr° rUcn 1 used as tracer, and rUcn 1 as standard. For Ucn 3 RIA, rabbit anti-mouse TyrGlyUcn 3 serum (PBL 6598) was used at a 1:75,000 final dilution, [125I]Tyr0Nle12,35 mUcn 3 was the tracer, and mUcn 3 was used as standard. Samples were tested at two dose levels. Free tracer was separated from antibody-bound tracer with sheep anti-rabbit γ-globulins and 10% (wt/vol) polyethylene glycol. Results were calculated using a logit/log RIA data processing program.
The EC50 and minimum detectable dose per tube were 30 pg and 1.5 pg for Ucn 1, and 25 pg and 2 pg for Ucn 3, respectively. This corresponds to a minimal detectable level of 0.5 pmol/L for Ucn 1 and 0.8 pmol/L for Ucn 3 in samples. Closely related CRF peptide family members r/h CRF, hUcn 2, mUcn 2, hUcn 3, and mUcn 3 at doses to 100 ng/tube failed to displace the tracer in the rUcn 1 RIA. The mUcn 3 RIA is highly specific for the murine Ucn 3 homolog and cross-reactivity with related peptides has been previously reported (Li et al., 2003).
Statistical analysis
Statistical analysis was performed with Minitab 15 for Windows. Ucn 1 and Ucn 3 concentrations below the lowest detectable limit of the assay were ascribed a value just over half the limit (Ucn 1, 0.27 pmol/L; Ucn 3, 0.48 pmol/L) for analysis. Data were assessed graphically and with the Kolmogorov-Smirnov test, and were found not to be normally distributed. Therefore, the Kruskal-Wallis test was used to make comparisons between the cardiac disease and the control groups. Statistical significance was set at P < 0.05. Data are displayed as the median (minimum/maximum) concentrations.
Results
Ucn peptide sequences
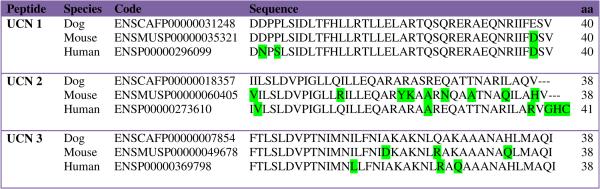
A species comparison between the published precursor and predicted mature peptide sequences of canine Ucns (1, 2 and 3) is presented in Tables 2 and 3. Canine Ucn 1 shows 93% and 98% homology to the human and mouse peptides, respectively (Table 3), whilst Ucn 3 has 92% homology to both human and mouse sequences. Canine Ucn 2 is 76% homologous to murine Ucn 2 but has 92% homology with human Ucn 2. Notably, the canine Ucn 2 precursor has a glycine residue followed by two basic amino acids at the C-terminus, which could be predicted to allow amidation and cleavage to a 38 amino acid peptide like in rodents.
Table 2.
Canine urocortin precursor peptide sequences. Underlined regions represent the predicted mature peptide sequence.
| Peptide Sequence |
|---|
|
UCN 1 MKQRGRAALLMALLLLAQLRPGSSQWSREAAAAGVQDPSLRWNPGARNQDGGARAL LLLLAERFPRRAGPGRWGSRTAGERPRRDDPPLSIDLTFHLLRTLLELARTQSQRERAEQ NRIIFESVGK |
|
UCN 2 MTRWALLVLMVLTSGRALLVTGTPSPAFQLLPQNPPQATPCPVTSESTTATTTGPSTAW G HPSPGPRSGPRIILSLDVPIGLLQILLEQARARASREQATTNARILAQVGRR |
|
UCN 3 MLMPAYLLLLLLLLPGIPQPGLSQKFHPGKSFFSCINTALWEARQSPLEDAPLLSKRSFPY LPSQDPSSGEDEEKEEEEEDKKKRTFPGFGGGNGAVSARYKYLSPAQLKGRLHQDKAKS DRRTKFTLSLDVPTNIMNILFNIAKAKNLQAKAAANAHLMAQIGRKK |
Table 3.
Homology of canine urocortin peptides to mouse and human homologues. Boxed residues differ from the canine sequences.
|
mRNA expression of Ucns and CRFR2 in canine heart
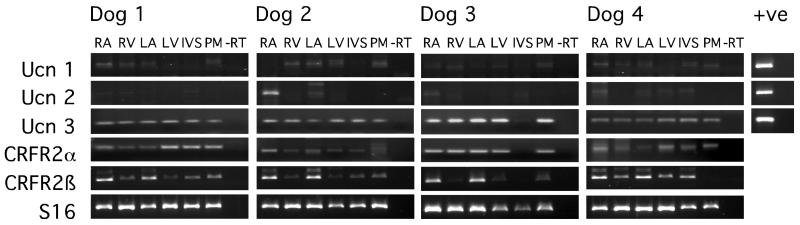
mRNA expression of all three Ucns was demonstrated in canine heart (Fig. 1). Ucn 3 was the major transcript with robust expression in all cardiac tissues at similar levels. Ucn 1 and Ucn 2 were expressed at barely detectable levels. However, while Ucn 1 mRNA was variably present in all heart regions, Ucn 2 mRNA showed higher levels in RA of 3/4 dogs. The CRFR2α splice variant was expressed in all cardiac tissues with no clear differences in regional distribution. However, CRFR2β expression was clearly higher in atrial tissue and weak elsewhere (LVFW, RVFW, IVS and LVPM) (Fig. 1).
Fig. 1. RT-PCR analysis for Ucns and CRFR2 isoforms in canine cardiac tissue.
RA, right atrium; RV, right ventricle; LA, left atrium; LV, left ventricle; IVS, interventricular septum; PM, left ventricular papillary muscle; -RT, tRNA subjected to the RT protocol without reverse transcriptase enzyme. S16 was used as an internal control. Genomic DNA was used as a positive control for Ucn PCR reactions.
Immunohistochemistry
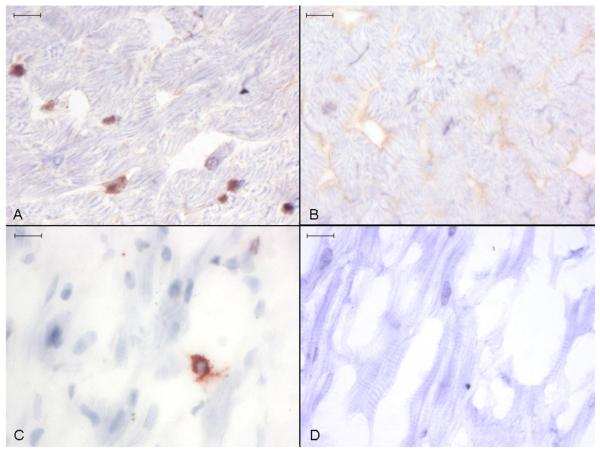
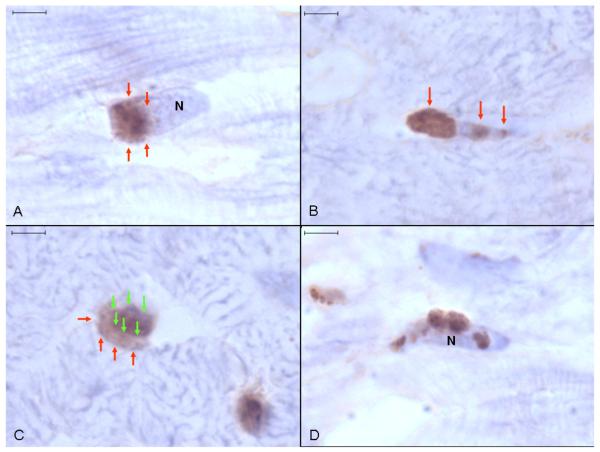
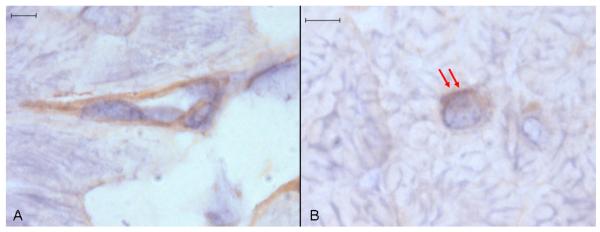
Ucn 1 positive staining occurred intracellularly throughout atrial and ventricular myocardium as discreet round or irregular foci, usually in close proximity with cardiomyocyte nuclei (Fig. 2). Staining was more abundant in ventricular tissue. Specific Ucn 1 staining appeared to be outside of nuclei or on the nuclear membrane surface, often localised to one nuclear pole (Fig. 3a). Additionally, there were often smaller bands of staining perpendicular to the long axis of cardiomyocyte nuclei (Fig. 3b). The apparent perinuclear (rather than intranuclear) localisation of Ucn 1 was obvious in sectional planes where the Ucn 1-positive staining clearly extended beyond the haematoxylin-stained nuclear boundaries (Fig. 3c). Irregular, asymmetrical granular Ucn 1 staining, apparently adjacent to cardiomyocyte nuclear membranes was also commonly seen (Fig. 3d).
Fig. 2. Anti-Ucn 1 immunohistochemistry of canine ventricular myocardium.
Discreet foci of Ucn 1 positive staining are seen in ventricular (A) and atrial (C) tissue. No staining occurred in ventricular (B) and atrial (D) tissue incubated with control non-immune rabbit serum. Representative image, size bars are 10 μm, light haematoxylin counterstain.
Fig. 3. Anti-Ucn 1 immunohistochemistry of canine ventricular myocardium.
(A) A round focus of Ucn 1-positive staining (arrows) adjacent to one pole of a haematoxylin stained cardiomyocyte nucleus. (B) Associated with a cardiomyocyte nucleus is a prominent focus of peripolar staining (left arrow) and several small bands of Ucn 1-positive staining perpendicular to the long axis of the nucleus (other arrows). (C) Ucn 1-positive staining (red arrows) extends beyond the boundaries of the cardiomyocyte nucleus (green arrows). (D) Irregular, random granular Ucn 1-positive staining appears to be adherent to a cardiomyocyte nucleus (N). Representative image, size bars are 4 μm, light haematoxylin counterstain.
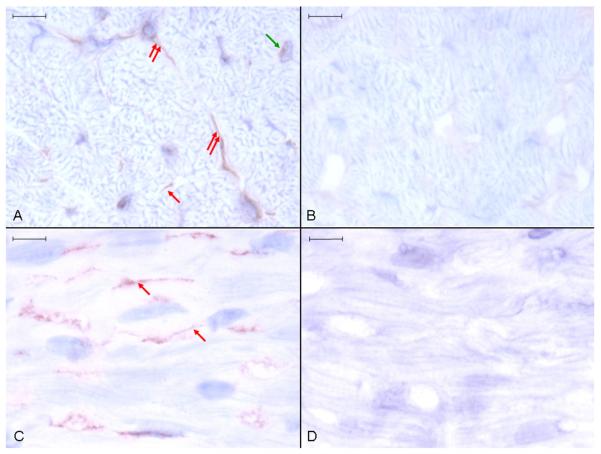
Ucn 3 positive staining occurred throughout the atrial and ventricular myocardium in three patterns. The most abundant pattern was uniform, short linear structures, which appeared to be within the endomysium and not within cardiomyocytes (Fig. 4). The second pattern was uniform cytoplasmic staining of stellate or fusiform cells within the endomysium. The least abundant pattern was within cardiomyocytes adjacent to nuclei. Rarely, the first pattern (short linear endomysial) could be resolved into two parallel lines or triangles around a central lumen suggesting that most or all of this was cytoplasmic staining of capillary endothelial cells (Fig. 5a) but inadequate resolution of cryostat sections precluded confirmation of this. Specific Ucn 3 perinuclear staining resembled that seen for Ucn 1, being localised eccentrically adjacent to the nucleus (Fig. 5b). No positive staining for Ucn 2 was seen in any sections (data not shown).
Fig. 4. Anti-Ucn 3 immunohistochemistry of canine ventricular myocardium.
Ucn 3-positive staining is seen in ventricular (A) and atrial (C) tissue. No staining occurred in ventricular (B) and atrial (D) tissue incubated with control non-immune rabbit serum. Three patterns of Ucn 3-positive staining are discerned. The first is short linear structures, which appear to be in the endomysium (single red arrows). The second is diffuse cytoplasmic staining of fusiform or stellate cells within the endomysium (double red arrows). The third is perinuclear within cardiomyocytes (green arrows). Representative image, size bars are 10 μm, light haematoxylin counterstain.
Fig. 5. Anti-Ucn 3 immunohistochemistry of canine ventricular myocardium.
(A) Ucn 3-positive staining of an apparent capillary endothelial cell. (B) Ucn 3-positive staining adjacent to a haematoxylin counterstained cardiomyocyte nucleus (red arrows). Representative image, size bars are 4 μm, light haematoxylin counterstain.
Plasma concentrations of Ucn 1 and Ucn 3
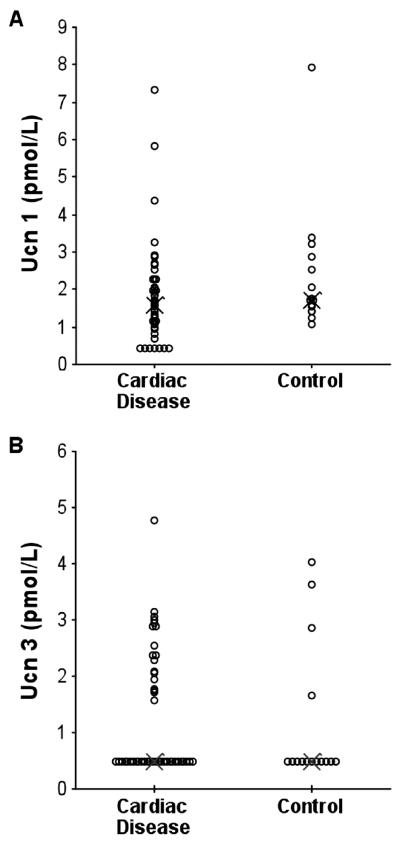
Ucn 1 and Ucn 3 were measurable in plasma from dogs with cardiac disease and controls (Fig. 6). Ucn 1 was detectable in all control dogs (median level 1.71 [range: 1.08-7.92] pmol/L), and in 39/35 dogs with cardiac disease (median level 1.59 [0.42-7.33] pmol/L). In contrast, Ucn 3 was below the assay detection level in 11/15 control dogs (median 0.48 [0.48-4.01] pmol/L) and 18/45 dogs with cardiac disease (median 0.48 [0.48-4.76] pmol/L). There was no statistical difference between the levels of Ucn 1 or Ucn 3 in controls and dogs with cardiac disease (Fig. 6). The numbers in this study did not allow for an adequately powered statistical analysis comparing specific cardiac conditions or class of heart failure, and no evidence of a trend for differences in Ucn levels was observed when data were categorised in this manner (data not shown).
Fig.6.
Plasma concentrations of (A) Ucn 1 and (B) Ucn 3 (pmol/L) in dogs with cardiac disease (n = 45) and a control group (n = 15). Data are presented as a dot plot where X represents the median value.
Discussion
While Ucn 1 and Ucn 3 peptides are highly conserved across species, Ucn 2 is more divergent and canine Ucn 2 shows higher homology to human Ucn 2 than rodent Ucn 2. However, unlike human Ucn 2, which lacks a consensus proteolytic cleavage site for C-terminal processing to the bioactive peptide (Reyes et al., 2001), the canine Ucn 2 sequence is predicted to produce a bioactive peptide. Nevertheless, Ucn 1 and Ucn 3 are the main mRNA transcripts in canine heart, with only barely detectable Ucn 2 mRNA levels present in the right atrium. As in humans, CRFR2α is expressed throughout the heart, while CRFR2β is expressed at higher levels in atrial tissue (Kimura et al., 2002). The significance of these species-specific patterns of expression is unclear but probably represents evolutionary selection of ligands for CRFR2.
Immunohistochemistry was consistent with mRNA expression, with Ucn 1 and Ucn 3 detected in cardiomyocytes. Stronger immunostaining for Ucn 1 in contrast to the PCR results is most likely due to relative differences in antibody affinities and PCR reaction efficiencies. The cellular distribution of Ucn positive immunostaining is interesting. Detailed ultrastructural studies are needed to definitively identify the Ucn immunoreactive subcellular structures, but the perinuclear staining is consistent with distribution in the Golgi apparatus of cardiomyocytes (Kobayashi et al., 2006), and with previous reports of Ucn 1 localisation in neurons (Kozicz et al., 2002; Swinny et al., 2002). Ucn 3 was also present in structures which may represent endothelial cells, a known target for CRFR2 action (Davidson and Yellon, 2009) which express mRNAs for Ucns (Kageyama et al., 2009).
Having established that Ucn 1 and Ucn 3 are expressed in the canine heart, we measured circulating concentrations in dogs with cardiac disease. Clearly if these potent regulators of cardiovascular physiology are expressed endogenously, their concentrations may be altered by cardiac disease or stress. This has been demonstrated for Ucn 1 in experimental heart failure models (Nishikimi et al., 2000; Charles et al., 2006) and in clinical studies in humans (Ng et al., 2004; Wright et al., 2009; Gruson et al., 2010). However, the results of these studies have shown that Ucn 1 is an inferior biomarker of heart failure to NT-proBNP (Wright et al., 2009) and that concentrations do not correlate significantly with other well established heart failure biomarkers (Gruson et al., 2010). Trans-organ arteriovenous sampling in sheep has suggested Ucn 1 release from kidney, brain, liver and/or gastrointestinal tract, but surprisingly not from cardiac tissue either normally or in heart failure (Takahashi et al., 2004; Charles et al., 2006). Thus, the site of production of circulating Ucns may be a significant confounding factor.
We found that whereas both Ucn 1 and Ucn 3 were measurable in canine plasma, concentrations were highly variable both in dogs with heart disease and in control dogs and that considerable overlap exists with no significant difference between the two populations. While it is possible that increasing sample numbers may have detected altered Ucn concentrations, and that Ucn expression is altered in canine heart disease at the tissue level, our aim was to assess the usefulness of Ucns as clinical markers for cardiac disease. Ucns may be relevant to the cardiac humoral response to heart failure and modulate cardiovascular functions in health and disease but studies correlating circulating Ucn levels and heart function (including the current work) demonstrate a lack of sensitivity, and Ucns are unlikely to be useful biomarkers for cardiac disease or function or offer any advantage over serum natriuretic peptides.
Conclusions
Ucn 1 and Ucn 3 are expressed in canine heart tissue and are measurable in canine plasma but they do not appear to be sensitive biomarkers of cardiac disease in the canine population evaluated in this study.
Acknowledgements
The authors thank Jordana Wingate, Rod Carter, Margaret Paterson and Bob Morris for excellent technical assistance, Brendan Corcoran and Richard Han for tissue collections, Darren Shaw for advice on data handling, and Ann Hedley for bioinformatics support. This study was supported by BSAVA Petsavers (GFV, DGO, AJF, DGB, GJC, ATF, PMJ), the Caja Madrid Foundation (GFV), the Clayton Medical Research Foundation, Inc. and Award Number P01 DK26741-30 from the National Institute of Diabetes and Digestive and Kidney Diseases (WWV, JMV).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest
WWV is a co-founder, member of the Board of Directors, and shareholder of Neurocrine Biosciences, a company developing small molecule antagonists of corticotropin releasing factor and which has licensed urocortin 2 as a potential treatment for acute congestive heart failure. The work described in this manuscript is completely independent of Neurocrine Biosciences. The other authors of this paper do not have a financial or personal relationship with other people or organisations that could inappropriately influence or bias the content of the paper.
References
- Baigent SM, Lowry PJ. mRNA expression profiles for corticotrophin-releasing factor (CRF), urocortin, CRF receptors and CRF-binding protein in peripheral rat tissues. Journal of Molecular Endocrinology. 2000;25:43–52. doi: 10.1677/jme.0.0250043. [DOI] [PubMed] [Google Scholar]
- Bale TL, Hoshijima M, Gu Y, Dalton N, Anderson KR, Lee KF, Rivier J, Chien KR, Vale WW, Peterson KL. The cardiovascular physiologic actions of urocortin II: acute effects in murine heart failure. Procedings of the National Academy of Sciences USA. 2004;101:3697–3702. doi: 10.1073/pnas.0307324101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brar BK, Jonassen AK, Egorina EM, Chen A, Negro A, Perrin MH, Mjos OD, Latchman DS, Lee KF, Vale W. Urocortin-II and urocortin-III are cardioprotective against ischemia reperfusion injury: an essential endogenous cardioprotective role for corticotropin releasing factor receptor type 2 in the murine heart. Endocrinology. 2004;145:24–35. doi: 10.1210/en.2003-0689. [DOI] [PubMed] [Google Scholar]
- Charles CJ, Rademaker MT, Richards AM, Yandle TG. Plasma urocortin 1 in sheep: regional sampling and effects of experimental heart failure. Peptides. 2006;27:1801–1805. doi: 10.1016/j.peptides.2005.12.010. [DOI] [PubMed] [Google Scholar]
- Davidson SM, Yellon DM. Urocortin: a protective peptide that targets both the myocardium and vasculature. Pharmacological Reports. 2009;61:172–182. doi: 10.1016/s1734-1140(09)70019-2. [DOI] [PubMed] [Google Scholar]
- Fekete EM, Zorrilla EP. Physiology, pharmacology, and therapeutic relevance of urocortins in mammals: ancient CRF paralogs. Frontiers in Neuroendocrinology. 2007;28:1–27. doi: 10.1016/j.yfrne.2006.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruson D, Ahn SA, Ketelslegers JM, Rousseau MF. Circulating levels of stress associated peptide Urocortin in heart failure patients. Peptides. 2010;31:354–356. doi: 10.1016/j.peptides.2009.11.023. [DOI] [PubMed] [Google Scholar]
- Hsu SY, Hsueh AJ. Human stresscopin and stresscopin-related peptide are selective ligands for the type 2 corticotropin-releasing hormone receptor. Nature Medicine. 2001;7:605–611. doi: 10.1038/87936. [DOI] [PubMed] [Google Scholar]
- Kageyama K, Hanada K, Suda T. Differential regulation of urocortins1-3 mRNA in human umbilical vein endothelial cells. Regulatory Peptides. 2009;155:131–138. doi: 10.1016/j.regpep.2009.02.012. [DOI] [PubMed] [Google Scholar]
- Kimura Y, Takahashi K, Totsune K, Muramatsu Y, Kaneko C, Darnel AD, Suzuki T, Ebina M, Nukiwa T, Sasano H. Expression of urocortin and corticotropin-releasing factor receptor subtypes in the human heart. Journal of Clinical Endocrinology and Metabolism. 2002;87:340–346. doi: 10.1210/jcem.87.1.8160. [DOI] [PubMed] [Google Scholar]
- Kishimoto T, Pearse RV, 2nd, Lin CR, Rosenfeld MG. A sauvagine/corticotropin-releasing factor receptor expressed in heart and skeletal muscle. Procedings of the National Academy of Sciences USA. 1995;92:1108–1112. doi: 10.1073/pnas.92.4.1108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi Y, Katanosaka Y, Iwata Y, Matsuoka M, Shigekawa M, Wakabayashi S. Identification and characterization of GSRP-56, a novel Golgi-localized spectrin repeat-containing protein. Experimental Cell Research. 2006;312:3152–3164. doi: 10.1016/j.yexcr.2006.06.026. [DOI] [PubMed] [Google Scholar]
- Kozicz T, Arimura A, Maderdrut JL, Lazar G. Distribution of urocortin-like immunoreactivity in the central nervous system of the frog Rana esculenta. Journal of Comparative Neurology. 2002;453:185–198. doi: 10.1002/cne.10403. [DOI] [PubMed] [Google Scholar]
- Lawrence KM, Townsend PA, Davidson SM, Carroll CJ, Eaton S, Hubank M, Knight RA, Stephanou A, Latchman DS. The cardioprotective effect of urocortin during ischaemia/reperfusion involves the prevention of mitochondrial damage. Biochemical and Biophysical Research Communications. 2004;321:479–486. doi: 10.1016/j.bbrc.2004.06.170. [DOI] [PubMed] [Google Scholar]
- Lewis K, Li C, Perrin MH, Blount A, Kunitake K, Donaldson C, Vaughan J, Reyes TM, Gulyas J, Fischer W, Bilezikjian L, Rivier J, Sawchenko PE, Vale WW. Identification of urocortin III, an additional member of the corticotropin-releasing factor (CRF) family with high affinity for the CRF2 receptor. Procedings of the National Academy of Sciences USA. 2001;98:7570–7575. doi: 10.1073/pnas.121165198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li C, Chen P, Vaughan J, Blount A, Chen A, Jamieson PM, Rivier J, Smith MS, Vale W. Urocortin III is expressed in pancreatic beta-cells and stimulates insulin and glucagon secretion. Endocrinology. 2003;144:3216–3224. doi: 10.1210/en.2002-0087. [DOI] [PubMed] [Google Scholar]
- Lovenberg TW, Chalmers DT, Liu C, De Souza EB. CRF2 alpha and CRF2 beta receptor mRNAs are differentially distributed between the rat central nervous system and peripheral tissues. Endocrinology. 1995;136:4139–4142. doi: 10.1210/endo.136.9.7544278. [DOI] [PubMed] [Google Scholar]
- Ng LL, Loke IW, O'Brien RJ, Squire IB, Davies JE. Plasma urocortin in human systolic heart failure. Clinical Science. 2004;106:383–388. doi: 10.1042/CS20030311. [DOI] [PubMed] [Google Scholar]
- Nishikimi T, Miyata A, Horio T, Yoshihara F, Nagaya N, Takishita S, Yutani C, Matsuo H, Matsuoka H, Kangawa K. Urocortin, a member of the corticotropin-releasing factor family, in normal and diseased heart. American Journal of Physiology Heart and Circulatory Physiology. 2000;279:H3031–3039. doi: 10.1152/ajpheart.2000.279.6.H3031. [DOI] [PubMed] [Google Scholar]
- Okosi A, Brar BK, Chan M, D'Souza L, Smith E, Stephanou A, Latchman DS, Chowdrey HS, Knight RA. Expression and protective effects of urocortin in cardiac myocytes. Neuropeptides. 1998;32:167–171. doi: 10.1016/s0143-4179(98)90033-6. [DOI] [PubMed] [Google Scholar]
- Parkes DG, Vaughan J, Rivier J, Vale WW, May CN. Cardiac inotropic actions of urocortin in conscious sheep. American Journal of Physiology Heart and Circulatory Physiology. 1997;41:H2115–2122. doi: 10.1152/ajpheart.1997.272.5.H2115. [DOI] [PubMed] [Google Scholar]
- Perrin M, Donaldson C, Chen R, Blount A, Berggren T, Bilezikjian L, Sawchenko P, Vale W. Identification of a second corticotropin-releasing factor receptor gene and characterization of a cDNA expressed in heart. Procedings of the National Academy of Sciences USA. 1995;92:2969–2973. doi: 10.1073/pnas.92.7.2969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pournajafi-Nazarloo H, Partoo L, Sanzenbacher L, Azizi F, Carter CS. Modulation of corticotropin-releasing hormone type 2 receptor and urocortin 1 and urocortin 2 mRNA expression in the cardiovascular system of prairie voles following acute or chronic stress. Neuroendocrinology. 2007;86:17–25. doi: 10.1159/000103587. [DOI] [PubMed] [Google Scholar]
- Rademaker MT, Cameron VA, Charles CJ, Richards AM. Integrated hemodynamic, hormonal, and renal actions of urocortin 2 in normal and paced sheep: beneficial effects in heart failure. Circulation. 2005a;112:3624–3632. doi: 10.1161/CIRCULATIONAHA.105.561308. [DOI] [PubMed] [Google Scholar]
- Rademaker MT, Charles CJ, Espiner EA, Frampton CM, Lainchbury JG, Richards AM. Four-day urocortin-I administration has sustained beneficial haemodynamic, hormonal, and renal effects in experimental heart failure. European Heart Journal. 2005b;26:2055–2062. doi: 10.1093/eurheartj/ehi351. [DOI] [PubMed] [Google Scholar]
- Rademaker MT, Cameron VA, Charles CJ, Richards AM. Urocortin 3: haemodynamic, hormonal, and renal effects in experimental heart failure. European Heart Journal. 2006;27:2088–2098. doi: 10.1093/eurheartj/ehl138. [DOI] [PubMed] [Google Scholar]
- Reyes TM, Lewis K, Perrin MH, Kunitake KS, Vaughan J, Arias CA, Hogenesch JB, Gulyas J, Rivier J, Vale WW, Sawchenko PE. Urocortin II: a member of the corticotropin-releasing factor (CRF) neuropeptide family that is selectively bound by type 2 CRF receptors. Procedings of the National Academy of Sciences USA. 2001;98:2843–2848. doi: 10.1073/pnas.051626398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swinny JD, Kalicharan D, Gramsbergen A, van der Want JJ. The localisation of urocortin in the adult rat cerebellum: a light and electron microscopic study. Neuroscience. 2002;114:891–903. doi: 10.1016/s0306-4522(02)00311-1. [DOI] [PubMed] [Google Scholar]
- Takahashi K, Totsune K, Murakami O, Saruta M, Nakabayashi M, Suzuki T, Sasano H, Shibahara S. Expression of urocortin III/stresscopin in human heart and kidney. Journal of Clinical Endocrinology and Metabolism. 2004;89:1897–1903. doi: 10.1210/jc.2003-031663. [DOI] [PubMed] [Google Scholar]
- Vale W, Vaughan J, Jolley D, Yamamoto G, Bruhn T, Seifert H, Perrin M, Thorner M, Rivier J. Assay of growth hormone-releasing factor. Methods in Enzymology. 1986;124:389–401. doi: 10.1016/0076-6879(86)24030-6. [DOI] [PubMed] [Google Scholar]
- Vaughan JM, Rivier J, Corrigan AZ, McClintock R, Campen CA, Jolley D, Voglmayr JK, Bardin CW, Rivier C, Vale W. Detection and purification of inhibin using antisera generated against synthetic peptide fragments. Methods in Enzymology. 1989;168:588–617. doi: 10.1016/0076-6879(89)68044-5. [DOI] [PubMed] [Google Scholar]
- Vaughan J, Donaldson C, Bittencourt J, Perrin MH, Lewis K, Sutton S, Chan R, Turnbull AV, Lovejoy D, Rivier C, et al. Urocortin, a mammalian neuropeptide related to fish urotensin I and to corticotropin-releasing factor. Nature. 1995;378:287–292. doi: 10.1038/378287a0. [DOI] [PubMed] [Google Scholar]
- Wright SP, Doughty RN, Frampton CM, Gamble GD, Yandle TG, Richards AM. Plasma urocortin 1 in human heart failure. Circulation and Heart Failure. 2009;2:465–471. doi: 10.1161/CIRCHEARTFAILURE.108.840207. [DOI] [PubMed] [Google Scholar]