Abstract
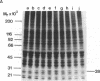
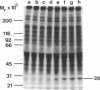
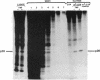
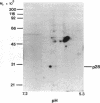
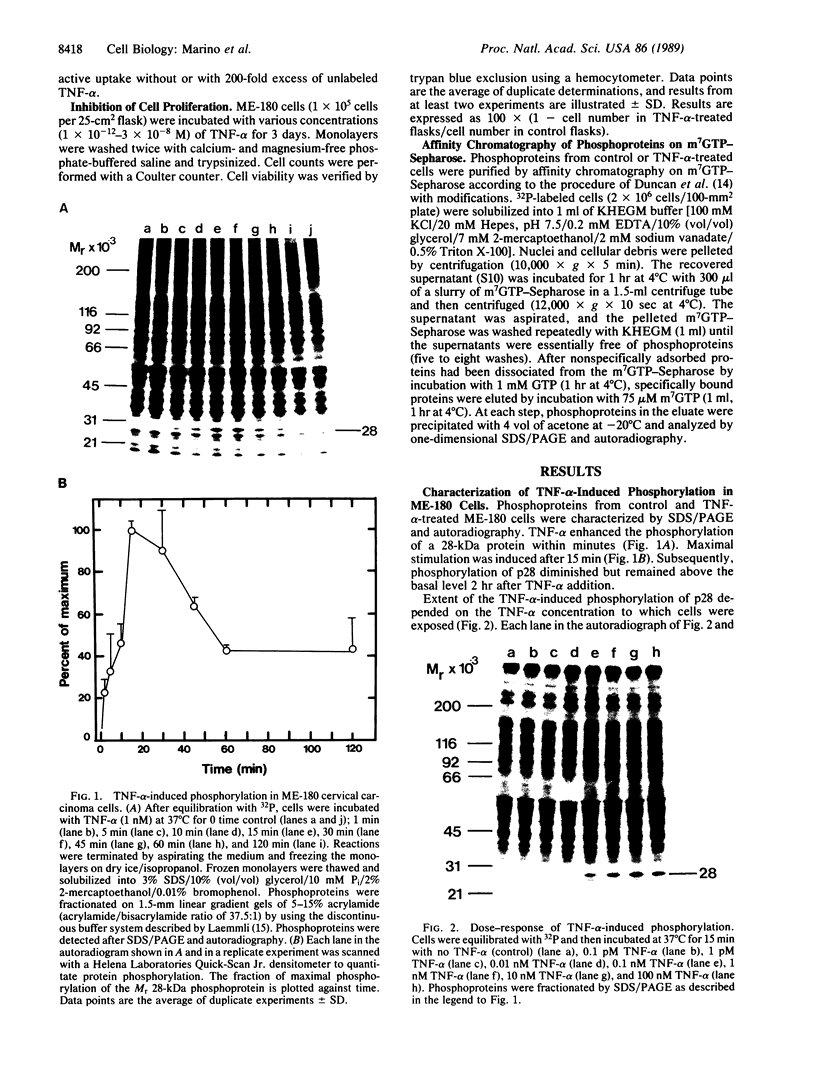
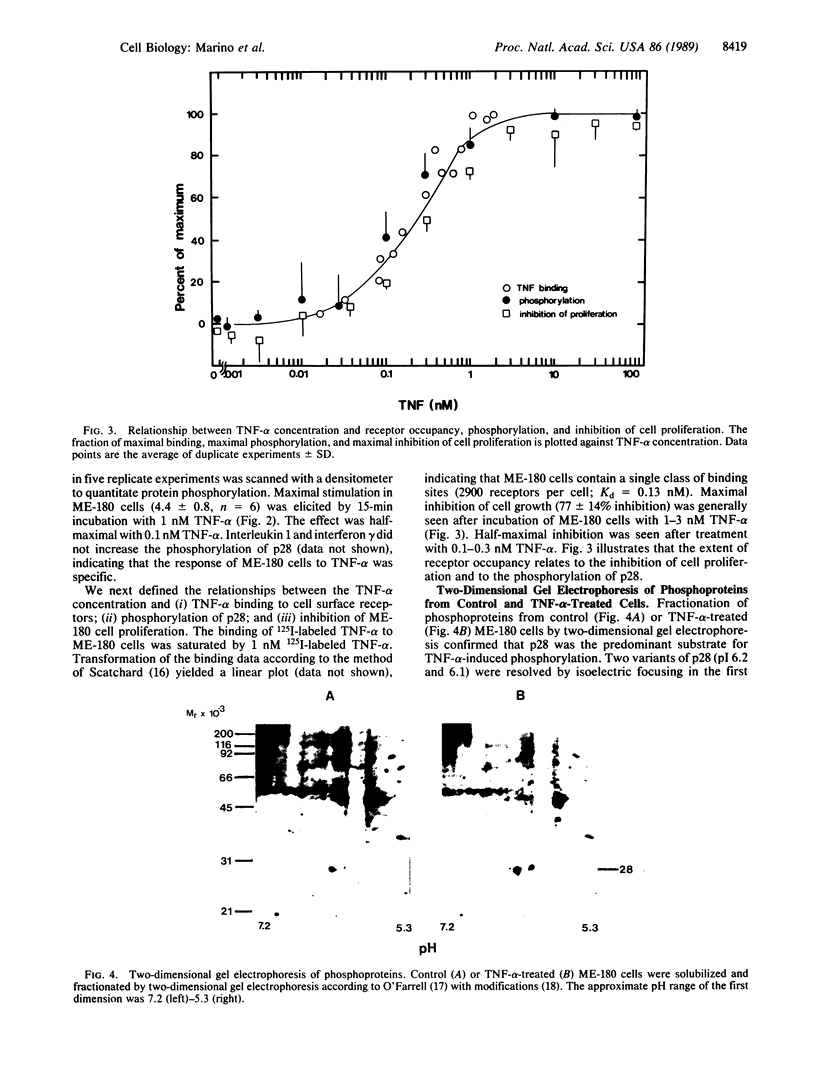
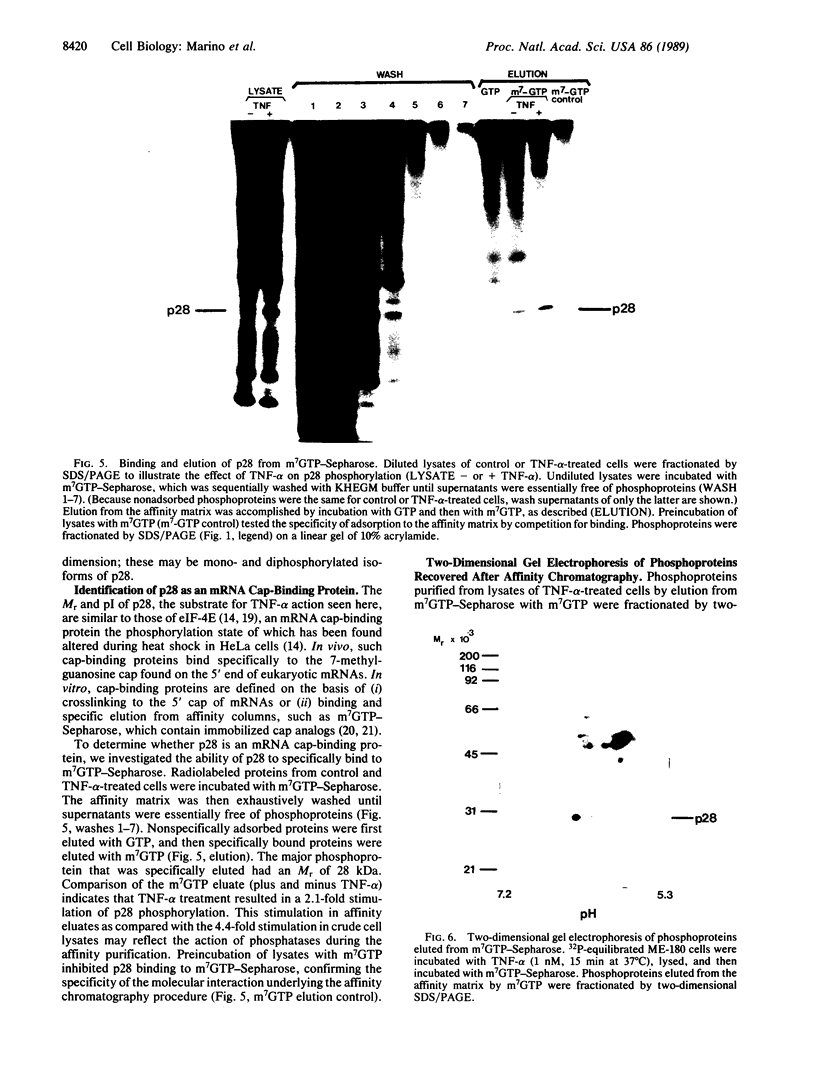
Tumor necrosis factor alpha (TNF-alpha) stimulated the phosphorylation of a 28-kDa protein (p28) in the ME-180 line of human cervical carcinoma cells. The effect of TNF-alpha on the phosphorylation state of p28 was rapid (4-fold increase within 15 min) and persistent, remaining above the basal level for at least 2 hr. The specific binding of 125I-labeled TNF-alpha to cell-surface binding sites, the stimulation of p28 phosphorylation by TNF-alpha, and the inhibition of cell proliferation by TNF-alpha occurred with nearly identical dose-response relationships. Two-dimensional SDS/PAGE resolved p28 into two isoforms having pI values of 6.2 and 6.1. A phosphorylated cap-binding protein was substantially enriched from lysates of control or TNF-alpha-treated ME-180 cells by affinity chromatography with 7-methylguanosine 5'-triphosphate-Sepharose. The phosphoprotein recovered from this procedure was the substrate for TNF-alpha-promoted phosphorylation, p28. Thus, TNF-alpha stimulates the phosphorylation of this mRNA cap-binding protein, which may be involved in the transduction of TNF-alpha-receptor binding into cellular responses.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aggarwal B. B., Eessalu T. E. Induction of receptors for tumor necrosis factor-alpha by interferons is not a major mechanism for their synergistic cytotoxic response. J Biol Chem. 1987 Jul 25;262(21):10000–10007. [PubMed] [Google Scholar]
- Aggarwal B. B., Traquina P. R., Eessalu T. E. Modulation of receptors and cytotoxic response of tumor necrosis factor-alpha by various lectins. J Biol Chem. 1986 Oct 15;261(29):13652–13656. [PubMed] [Google Scholar]
- Bachwich P. R., Chensue S. W., Larrick J. W., Kunkel S. L. Tumor necrosis factor stimulates interleukin-1 and prostaglandin E2 production in resting macrophages. Biochem Biophys Res Commun. 1986 Apr 14;136(1):94–101. doi: 10.1016/0006-291x(86)90881-8. [DOI] [PubMed] [Google Scholar]
- Beutler B., Cerami A. Cachectin and tumour necrosis factor as two sides of the same biological coin. Nature. 1986 Apr 17;320(6063):584–588. doi: 10.1038/320584a0. [DOI] [PubMed] [Google Scholar]
- Bonneau A. M., Sonenberg N. Involvement of the 24-kDa cap-binding protein in regulation of protein synthesis in mitosis. J Biol Chem. 1987 Aug 15;262(23):11134–11139. [PubMed] [Google Scholar]
- Buckley B., Ehrenfeld E. Two-dimensional gel analyses of the 24-kDa cap binding protein from poliovirus-infected and uninfected HeLa cells. Virology. 1986 Jul 30;152(2):497–501. doi: 10.1016/0042-6822(86)90155-8. [DOI] [PubMed] [Google Scholar]
- Carswell E. A., Old L. J., Kassel R. L., Green S., Fiore N., Williamson B. An endotoxin-induced serum factor that causes necrosis of tumors. Proc Natl Acad Sci U S A. 1975 Sep;72(9):3666–3670. doi: 10.1073/pnas.72.9.3666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darlington G. J., Wilson D. R., Lachman L. B. Monocyte-conditioned medium, interleukin-1, and tumor necrosis factor stimulate the acute phase response in human hepatoma cells in vitro. J Cell Biol. 1986 Sep;103(3):787–793. doi: 10.1083/jcb.103.3.787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan R., Milburn S. C., Hershey J. W. Regulated phosphorylation and low abundance of HeLa cell initiation factor eIF-4F suggest a role in translational control. Heat shock effects on eIF-4F. J Biol Chem. 1987 Jan 5;262(1):380–388. [PubMed] [Google Scholar]
- Greengard P. Phosphorylated proteins as physiological effectors. Science. 1978 Jan 13;199(4325):146–152. doi: 10.1126/science.22932. [DOI] [PubMed] [Google Scholar]
- Helson L., Green S., Carswell E., Old L. J. Effect of tumour necrosis factor on cultured human melanoma cells. Nature. 1975 Dec 25;258(5537):731–732. doi: 10.1038/258731a0. [DOI] [PubMed] [Google Scholar]
- Hepburn A., Demolle D., Boeynaems J. m., Fiers W., Dumont J. E. Rapid phosphorylation of a 27 kDa protein induced by tumor necrosis factor. FEBS Lett. 1988 Jan 25;227(2):175–178. doi: 10.1016/0014-5793(88)80892-5. [DOI] [PubMed] [Google Scholar]
- Kaur P., Saklatvala J. Interleukin 1 and tumour necrosis factor increase phosphorylation of fibroblast proteins. FEBS Lett. 1988 Dec 5;241(1-2):6–10. doi: 10.1016/0014-5793(88)81019-6. [DOI] [PubMed] [Google Scholar]
- Kohase M., Henriksen-DeStefano D., May L. T., Vilcek J., Sehgal P. B. Induction of beta 2-interferon by tumor necrosis factor: a homeostatic mechanism in the control of cell proliferation. Cell. 1986 Jun 6;45(5):659–666. doi: 10.1016/0092-8674(86)90780-4. [DOI] [PubMed] [Google Scholar]
- Kull F. C., Jr, Jacobs S., Cuatrecasas P. Cellular receptor for 125I-labeled tumor necrosis factor: specific binding, affinity labeling, and relationship to sensitivity. Proc Natl Acad Sci U S A. 1985 Sep;82(17):5756–5760. doi: 10.1073/pnas.82.17.5756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Mackiewicz A., Ganapathi M. K., Schultz D., Samols D., Reese J., Kushner I. Regulation of rabbit acute phase protein biosynthesis by monokines. Biochem J. 1988 Aug 1;253(3):851–857. doi: 10.1042/bj2530851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munker R., Gasson J., Ogawa M., Koeffler H. P. Recombinant human TNF induces production of granulocyte-monocyte colony-stimulating factor. Nature. 1986 Sep 4;323(6083):79–82. doi: 10.1038/323079a0. [DOI] [PubMed] [Google Scholar]
- O'Farrell P. H. High resolution two-dimensional electrophoresis of proteins. J Biol Chem. 1975 May 25;250(10):4007–4021. [PMC free article] [PubMed] [Google Scholar]
- Old L. J. Tumor necrosis factor (TNF). Science. 1985 Nov 8;230(4726):630–632. doi: 10.1126/science.2413547. [DOI] [PubMed] [Google Scholar]
- Schütze S., Scheurich P., Pfizenmaier K., Krönke M. Tumor necrosis factor signal transduction. Tissue-specific serine phosphorylation of a 26-kDa cytosolic protein. J Biol Chem. 1989 Feb 25;264(6):3562–3567. [PubMed] [Google Scholar]
- Sonenberg N. Cap-binding proteins of eukaryotic messenger RNA: functions in initiation and control of translation. Prog Nucleic Acid Res Mol Biol. 1988;35:173–207. doi: 10.1016/s0079-6603(08)60614-5. [DOI] [PubMed] [Google Scholar]
- Sugarman B. J., Aggarwal B. B., Hass P. E., Figari I. S., Palladino M. A., Jr, Shepard H. M. Recombinant human tumor necrosis factor-alpha: effects on proliferation of normal and transformed cells in vitro. Science. 1985 Nov 22;230(4728):943–945. doi: 10.1126/science.3933111. [DOI] [PubMed] [Google Scholar]
- Torti F. M., Dieckmann B., Beutler B., Cerami A., Ringold G. M. A macrophage factor inhibits adipocyte gene expression: an in vitro model of cachexia. Science. 1985 Aug 30;229(4716):867–869. doi: 10.1126/science.3839597. [DOI] [PubMed] [Google Scholar]
- Vilcek J., Palombella V. J., Henriksen-DeStefano D., Swenson C., Feinman R., Hirai M., Tsujimoto M. Fibroblast growth enhancing activity of tumor necrosis factor and its relationship to other polypeptide growth factors. J Exp Med. 1986 Mar 1;163(3):632–643. doi: 10.1084/jem.163.3.632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webb N. R., Chari R. V., DePillis G., Kozarich J. W., Rhoads R. E. Purification of the messenger RNA cap-binding protein using a new affinity medium. Biochemistry. 1984 Jan 17;23(2):177–181. doi: 10.1021/bi00297a001. [DOI] [PubMed] [Google Scholar]
- Yamada K., Lipson K. E., Marino M. W., Donner D. B. Effect of growth hormone on protein phosphorylation in isolated rat hepatocytes. Biochemistry. 1987 Feb 10;26(3):715–721. doi: 10.1021/bi00377a009. [DOI] [PubMed] [Google Scholar]