Abstract
Purpose
99mTcN-MPO ([99mTcN(mpo)(PNP5)]+: mpo=2-mercaptopyridine oxide and PNP5=N-ethoxyethyl-N,N-bis[2-(bis(3-methoxypropyl)phosphino)ethyl]amine) is a cationic 99mTc-nitrido complex, which has favorable biodistribution and myocardial uptake with rapid liver clearance in Sprague Dawley rats. The objective of this study was to compare the biodistribution and pharmacokinetics of 99mTcN-MPO and 99mTc-Sestamibi in normal dogs, and to evaluate the potential of 99mTcN-MPO as a myocardial perfusion agent in canines with acute myocardial infarction.
Methods
Five normal mongrel dogs were injected intravenously with 99mTcN-MPO. Venous blood samples were collected via a femoral vein catheter at 0.5, 1, 2, 3, 4, 5, 10, 20, 30, 40, 60, and 90 min post-injection (p.i.). Anterior-posterior planar images were acquired by γ-camera at 10, 20, 30, 60, 90, and 120 min p.i. Regions of interest (ROIs) were drawn around the heart, liver, and lungs. The heart/liver and heart/lung ratios were calculated by dividing the mean counts in heart ROI by the mean counts in the liver and lung ROI, respectively. For comparison, 99mTc-sestamibi was also evaluated in the same five dogs. The interval period between the two examinations was 1 week to eliminate possible interference between these two radiotracers. In addition, single positron emission computed tomography (SPECT) images in the canine infarct model were collected 24 h after myocardial infarction at 30 and 60 min after the administration of 99mTcN-MPO (n=4) or 99mTc-Sestamibi (n=4).
Results
It was found that 99mTcN-MPO and 99mTc-Sestamibi displayed very similar blood clearance characteristics during the first 90 min p.i. Both 99mTcN-MPO and 99mTc-Sestamibi had a rapid blood clearance with less than 50% of initial radioactivity remaining at 1 min and less than 5% at 30 min p.i. 99mTcN-MPO and 99mTc-Sestamibi both showed good heart/lung contrast. The heart/liver ratio of 99mTcN-MPO increased with time (0.53±0.06 at 10 min, 0.90±0.062 at 30 min, and 1.22±0.06 at 60 min p.i.), whereas the heart/liver ratio of 99mTc-Sestamibi remained low at all time points (0.50±0.03 at 10 min, 0.64±0.03 at 30 min, and 0.60±0.02 at 60 min p.i.). SPECT imaging studies in canines with acute myocardial infarction indicated that good visualization of the left ventricular wall and perfusion defects could be achieved at 30 min after administration of 99mTcN-MPO but not after 99mTc-Sestamibi.
Conclusion
The combination of reasonable heart uptake with rapid hepatobiliary excretion makes 99mTcN-MPO a promising new radiotracer for myocardial perfusion imaging.
Keywords: 99mTcN-MPO, Myocardial perfusion imaging, Biodistribution, Liver clearance, Myocardial infarction, SPECT
Introduction
Myocardial perfusion imaging (MPI) is a valuable imaging modality for non-invasive evaluations of patients with known or suspected coronary artery disease [1, 2]. Ideally, a perfusion radiotracer should have the following characteristics: myocardial uptake directly proportional to blood flow, high extraction fraction, high target-to-background (T/B) ratio, and good myocardial retention. 99mTc-Sestamibi has been widely used for MPI in nuclear cardiology. However, it does not meet the requirements of an ideal perfusion imaging agent, due to its high liver uptake [3] and roll-off at higher blood flow levels (and consequent inability to track increases in myocardial blood flow well). The intense liver uptake makes it difficult to interpret the heart activity in the inferior and left ventricular wall [4]. Despite intensive efforts to reduce this interference, photon scattering from high liver activity remains a challenge for accurate diagnosis of heart disease by SPECT. Therefore, it would be of great benefit to develop a new perfusion radiotracer with improved biodistribution and/or extraction properties [5, 6].
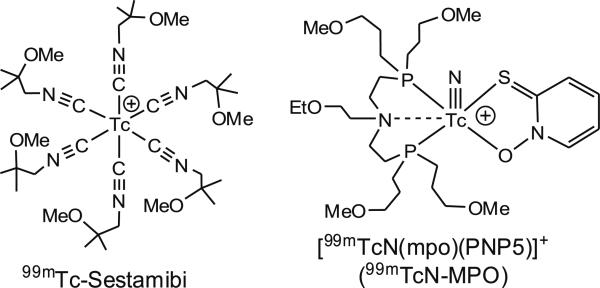
Many ether-containing cationic 99mTc complexes have been reported to have the improved T/B ratios [7–15]. 99mTcN-DBODC5 ([99mTcN(DBODC)(PNP5)]+: PNP5=N-ethoxyethyl-N,N-bis[2-(bis(3-methoxypropyl)phosphino) ethyl]amine and DBODC=N,N-bis(ethoxyethyl)-dithiocarbamato) is under clinical investigation as a new single positron emission computed tomography (SPECT) radiotracer for MPI [16]. Recently, we reported the evaluation of a cationic 99mTc radiotracer [99mTcN(mpo)(PNP5)]+ (Fig. 1: 99mTcN-MPO) in Sprague Dawley (SD) rats [17]. It was found that 99mTcN-MPO has a heart uptake between that of 99mTc-Sestamibi and 99mTcN-DBODC5. The heart/liver ratio of 99mTcN-MPO (12.75±3.34) was twice as much as that of 99mTcN-DBODC5 (6.01±1.45), and was >4 times better than that of 99mTc-Sestamibi (2.90±0.22) at 30 min p. i. Planar imaging studies in SD rats indicated that clear heart images could be obtained as early as 15 min p.i. when 99mTcN-MPO was used as the radiotracer. It was also demonstrated that 99mTc-Sestamibi and 99mTcN-MPO share the same myocardial localization mechanism with almost identical subcellular distribution characteristics [18].
Fig. 1.
Structures of 99mTcN-MPO and 99mTc-Sestamibi.
In this study, we determined the blood clearance kinetics of 99mTcN-MPO in normal dogs and evaluated 99mTcNMPO's potential as a myocardial perfusion radiotracer in canines with acute myocardial infarction. The main objective of this study was to further confirm its fast liver clearance kinetics and to demonstrate its capability, in conjunction with SPECT, to detect myocardial perfusion defects in canines with acute myocardial infarction.
Materials and Methods
Chemicals, such as 1,2-diaminopropane-N,N,N′,N′-tetraacetic acid (PDTA), 2-mercaptopyridine N-oxide (mpo; sodium salt) and succinic dihydrazide (SDH), were purchased from Sigma-Aldrich (St. Louis, MO, USA). PNP5 (N-ethoxyethyl-N,N-bis[2-(bis(3-methoxypropyl)phosphino)ethyl]amine) was prepared according to literature methods [10, 12] Na99mTcO4 was obtained from a Technelite® 99Mo/99mTc generator (Beijing Senke Ltd). 99mTc-Sestamibi was obtained from the China Institute of Atomic Energy.
99mTcN-MPO
99mTcO4- solution (500-750 MBq) was added into a lyophilized mixture of 5 mg of SDH, 5 mg of PDTA, and 25 μg of SnCl2·2H2O. The reaction mixture (pH=7.5-8.0) was kept at room temperature for 15-30 min to form the 99mTc-nitrido intermediate. 0.5 mL 50% ethanol was added to a separate vial containing 2 mg of PNP5 and 2 mg of mpo. The resulting solution was added to the vial containing the 99mTc-nitrido intermediate. The mixture was heated at 100°C for ~15 min. After cooling to room temperature, a sample of the resulting solution was analyzed by radio-TLC (radiolabeling yield>90%). Doses for imaging studies were made by dissolving the reaction mixture to a concentration of 200-400 MBq/mL with saline, before being injected into animals [17].
Animal Preparation
All experiments were performed in accordance with the NIH animal experiment guidelines (Principles of Laboratory Animal Care, NIH Publication No. 86-23, revised 1985). The protocols for animal studies have been approved by the Harbin Medical University Animal Care and Use Committee (Harbin, China). A total of 13 adult mongrel dogs (19.5±0.9 kg, ranging from 18.7 to 21 kg, fasted overnight) were used in this study. The same five normal dogs were used for planar imaging and eight infarcted dogs were used for the SPECT study (four for 99mTcN-MPO and four for 99mTc-Sestamibi). All dogs were anesthetized by an intravenous (i.v.) injection of 25 mg/kg sodium pentobarbital (25-35 mg/kg), intubated with a cuffed endotracheal tube, and ventilated on a respirator with a positive end-expiratory pressure of 4 cm H2O [19]. Additional sodium pentobarbital was provided via i.v. injection to maintain anesthesia as needed.
Blood Clearance Kinetics in Normal Dogs
Under sodium pentobarbital anesthesia, each animal was administered 370 MBq of 99mTcN-MPO or 99mTc-Sestamibi via a femoral vein. Venous blood samples (1 mL) were collected via a femoral vein catheter at 0.5, 1, 2, 3, 4, 5, 10, 20, 30, 40, 60, and 90 min p.i. The collected blood samples were weighed and counted for radioactivity in a gamma counter (FT-646, Beijing Nuclear Instrument Factory, China). The radioactivity counts were corrected for background and decay.
Planar Imaging in Normal Dogs
After the animal was under anesthesia, it was placed supine on a two-head Siemens γ-camera (E. cam Duet®) equipped with a parallel hole, low energy, high resolution collimator, and 20% window centered on the 140-keV 99mTc photo peak. After administration of 370 MBq of 99mTcN-MPO via the contralateral femoral vein, anterior and posterior planar images were collected for 2 min at the specified time points (10, 20, 30, 60, 90, and 120 min) using a 256×1,024 image matrix. The acquisition count limits were set at 500 K. SPECT images were acquired at 40, 70, 90, and 120 min p.i. and were stored digitally in a 128×128 matrix. After the completion of image acquisition, animals were allowed to recover. To quantify the images (n=5/group), ROIs were drawn around the heart (normal left circumflex artery area), liver (gall-bladder area excluded), lung (left lower lobe area), and the kidneys on each image acquired at each time point. The raw activity in each ROI was expressed as counts per minute (cpm)/pixel (decay corrected to injection time). Different organ time-radioactivity curves were thus obtained. The heart/liver and heart/lung ratios were calculated by dividing the mean counts in heart ROI by the mean counts in liver and lung ROI, respectively, for each individual dog. For comparison purposes, 99mTc-Sestamibi was evaluated using the same protocol as for 99mTcN-MPO in the same five adult mongrel dogs. The time interval between the two imaging studies in the same dogs was at least 7 days to allow radiation decay and to eliminate possible interference between these two radiotracers.
SPECT in Canines with Myocardial Infarction
Eight anesthetized dogs underwent femoral artery cannulation and digital subtraction angiography (DSA). Prophylactic lidocaine was administered intravenously to prevent lethal arrhythmia [20]. An acute anterior myocardial infarction animal model was induced on anesthetized dogs by intra-vessel embolism through the second diagonal branch (D2) of the anterior descending artery (LAD) with spongia gelatinosa (1.0 mm×1.0 mm) [21, 22]. Interruption of the arterial blood stream was affirmed by DSA. The myocardial damage was proved by observing elevated levels of serum aspartate amino-transferase, lactate dehydrogenase, and creatine paosphokinase. SPECT images were acquired on the myocardial infarct dogs at 24 h after acute myocardial infarction. After the administration of 370 MBq of 99mTcN-MPO (n=4) or 99mTc-Sestamibi (n=4) via contralateral femoral vein, SPECT images were collected for 15 min at 30 min and 60 min p.i., while the animals were still under anesthesia, using 128×128 projections over 360°. The raw data were stored digitally in a 128×128 matrix. Myocardial tomograms of vertical major axis, horizontal major axis, and minor axis were reconstructed using a standard filtered backprojection algorithm with a three-dimensional Butterworth filter. The cutoff frequency was at 0.28 cycles/pixel and the slice thickness was 3.9 mm.
Statistical Analysis
Quantitative data were expressed as mean±SD. Means were compared using one-way analysis of variance and Student's t test. P values<0.05 were considered statistically significant.
Results
Blood Clearance Kinetics
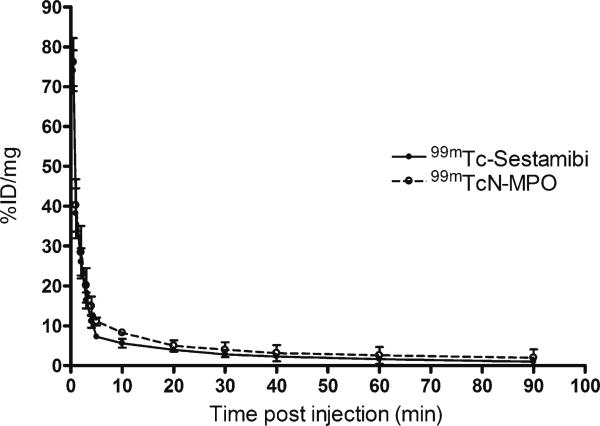
Figure 2 shows the blood radioactivity clearance curves of 99mTcN-MPO and 99mTc-Sestamibi in normal mongrel dogs during the first 90 min following tracer administration. The radioactivity was expressed as a percentage of the initial radioactivity level (defined as the value 30 s after administration of the tracer). Clearly, 99mTcN-MPO and 99mTc-Sestamibi each had a rapid blood clearance, with a circulation half-life of 0.90 and 1.0 min, respectively. Less than 5% of the initial radioactivity remained in the circulation at 30 min and <1% of initial radioactivity remained at 90 min.
Fig. 2.
Blood clearance curves of 99mTcN-MPO (blank circle) and 99mTc-Sestamibi (filled circle) in normal mongrel dogs. Both tracers showed rapid blood clearance with less than 5% of initial radioactivity remaining at 30 min p.i.
Planar Imaging
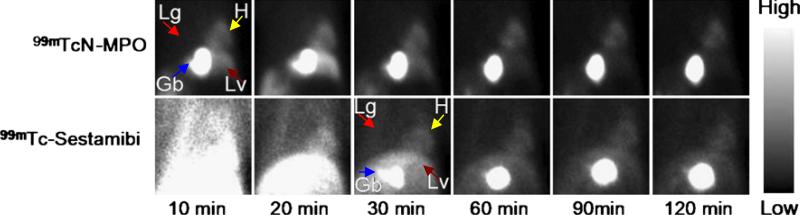
Figure 3 illustrates planar images of the normal dogs, following administration of either 99mTcN-MPO (A) or 99mTc-Sestamibi (B). Good-quality images of heart were obtained between 30 min and 2 h after 99mTcN-MPO administration. By contrast, clear heart images were not achieved until 60 min after 99mTc-Sestamibi administration.
Fig. 3.
Planar images of normal dogs administered 99mTcN-MPO or 99mTc-Sestamibi. Both radiotracers had reasonable myocardial uptake. The liver clearance of 99mTcN-MPO was faster than that of 99mTc-Sestamibi. Gb gallbladder, H heart, Lg lung, Lv liver.
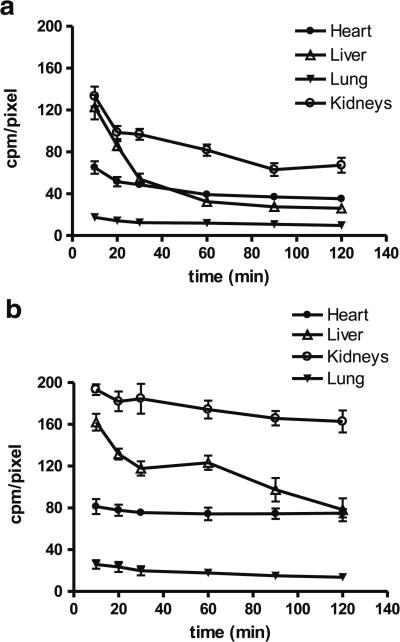
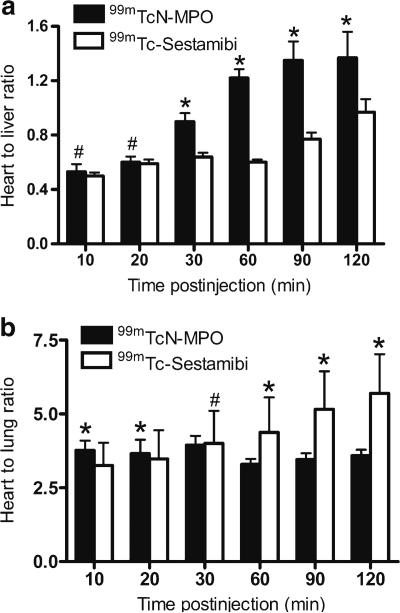
Figure 4 shows the organ distribution kinetics derived from planar images of normal dogs following administration of either 99mTcN-MPO or 99mTc-Sestamibi. 99mTcN-MPO had a slightly lower initial myocardial radioactivity accumulation than 99mTc-Sestamibi (67.6±6.2 cpm/pixel vs. 81.2±7.13 cpm/pixel at 10 min p.i., p<0.05). The retention of 99mTcN-MPO in the myocardium was not as good as with 99mTc-Sestamibi. The myocardium 99mTcN-MPO radioactivity decreased to 40.8±2.9 and 36.6±2.3 cpm/pixel at 60 and 120 min p.i., respectively. On the contrary, 99mTc-Sestamibi showed prolonged retention (74.9±6.0 and 74.3±4.1 cpm/pixel at 60 and 120 min p.i.). However, 99mTcNMPO had significantly lower liver uptake than 99mTc-Sestamibi at all time points examined (p<0.05). The 99mTcN-MPO radioactivity in the liver decreased rapidly from 127.5±12.1 cpm/pixel at 10 min, to 50.5±3.5 cpm/pixel at 30 min, and 40.8±2.9 cpm/pixel at 60 min p.i. By contrast, 99mTc-Sestamibi had a slow decrease from 162.1±8.0 cpm/pixel at 10 min to 123.2±1.8 cpm/pixel at 30 min and to 117.8±6.8 cpm/pixel at 60 min p.i. These results indicated a rapid liver clearance of 99mTcN-MPO, which led to a favorable heart/liver ratio as early as 30 min after injection. The heart/liver ratio of 99mTcN-MPO (Fig. 5a) increased from 0.53±0.06 at 10 min p.i. to 0.90±0.06 at 30 min p.i. (p<0.01) and to 1.37±0.19 at 120 min p.i. (p<0.01). On the other hand, the heart/liver ratio of 99mTc-Sestamibi was rather low at early time points (0.50±0.03 at 10 min and 0.60±0.02 at 60 min) and remained less than 1.0 at 120 min (0.90±0.10). The gallbladder activity of 99mTcN-MPO increased rapidly; but increased slowly for 99mTc-Sestamibi over the same period.
Fig. 4.
Organ clearance kinetics from imaging quantification in normal dogs administered 99mTcN-MPO (a) or 99mTc-Sestamibi (b). The liver activity of 99mTcN-MPO was markedly decreased within the first 60 min, whereas 99mTc-Sestamibi had a slower reduction in liver activity over time. A mild myocardial washout was observed in the dogs administered 99mTcN-MPO. No significant myocardial washout was seen in the dogs administered 99mTc-Sestamibi.
Fig. 5.
Comparison of heart/liver (a) and heart/lung (b) ratios for 99mTcN-MPO and 99mTc-Sestamibi in normal dogs. Both tracers had good heart/lung contrast. The heart/liver ratio of 99mTcN-MPO increased rapidly over the 2 h study period, whereas the heart/liver ratio of 99mTc-Sestambi improved only slightly with time (single number sign P>0.05;asterisk P<0.05).
Both 99mTcN-MPO and 99mTc-Sestamibi had good heart/lung ratios (Fig. 5b) over the 2 h study period (99mTcNMPO: 3.77±0.33 at 10 min p.i. and 3.59±0.20 at 120 min p. i.; 99mTc-Sestamibi: 3.25±0.77 at 10 min p.i. and 5.70±1.33 at 120 min p.i.).
SPECT Imaging
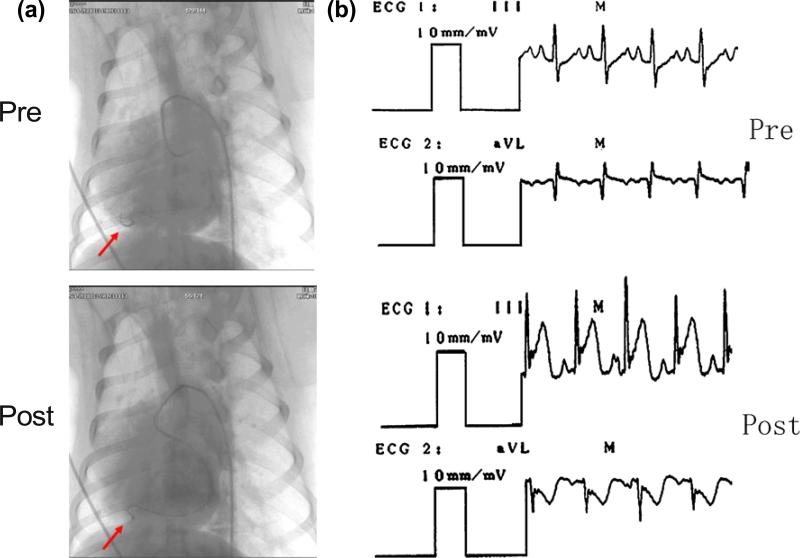
Acute myocardial infarction in anesthetized mongrel dogs was induced by femoral artery cannulation. The interruption of arterial blood stream was confirmed by DSA (Fig. 6a). The myocardial damage was proved by observing the elevated levels of serum aspartate amino-transferase (31.4±1.6 pre-infarction vs. 57.8±1.9 U/l post-infarction, p<0.05), lactate dehydrogenase (135.6±1.9 pre vs. 717.6±87.0 U/l post, p<0.05) and creatine phosphokinase (56.8±1.5 pre vs. 384.2±24.4 U/l post, p<0.05). The following electrocardiographical changes were used as evidence for acute myocar-dial infarction: appearance of ST elevation, negative T waves, and pathological Q-waves (Fig. 6b).
Fig. 6.
Validation of acute myocardial infarction in mongrel dogs. a Digital subtraction angiography (DSA) shows that the D2 of the anterior descending artery (LAD) was interrupted after infarction. Arrow points to D2. b Electrocardiograms indicated ST segment elevation in lead III and ST segment depression in lead aVL after infarction.
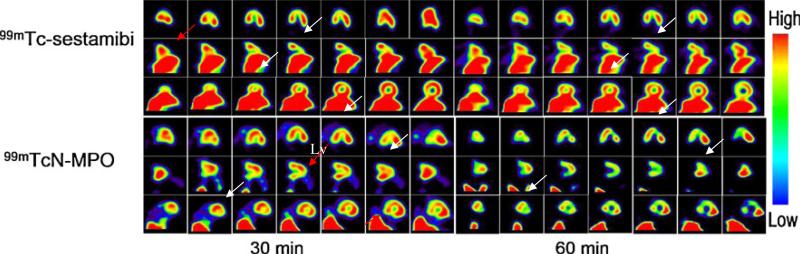
Figure 7 illustrates SPECT images of a dog with acute myocardial infarction at 30 and 60 min p.i. A good visualization of the left ventricular wall and perfusion defect could be achieved as early as 30 min after administration of 99mTcN-MPO without a stress agent such as adenosine. There was an excellent separation between the heart and surrounding organs. The perfusion defects remained well-defined for >120 min p.i. By contrast, there was substantial interference from the liver radioactivity at 30 and 60 min p.i. in the dog administered 99mTc-Sestamibi.
Fig. 7.
SPECT images of dogs with acute myocardial infarction administered 99mTc-Sestamibi or 99mTcN-MPO at 30 and 60 min p.i. The perfusion defects are indicated by white arrows. Arrows show the liver and gallbladder. Anterior myocardial infarctions near the apex are visible.
Discussion
The combination of SPECT MPI with a 99mTc-labeled compound is a mainstream imaging modality for detecting and estimating the severity of ischemic heart disease [23, 24]. Since contrast with neighboring tissues or organs is a key measure of SPECT MPI image quality, there has been a great deal of effort in the past decade to optimize the biodistribution properties of 99mTc radiotracers [25].
The in vivo biodistribution behavior of a 99mTc-compound is mainly determined by its physical and chemical properties, such as lipophilicity, molecular shape, size, and oxidation state. As previously reported, lipophilicity remains the most important factor affecting both heart uptake and T/B ratios. Crown ethers are very useful functional groups to improve the liver clearance of cationic 99mTc-nitrido complexes. It is the combination of the appropriate DTCs and bisphosphines that results in high heart uptake and fast liver clearance of cationic 99mTc-nitrido complexes.
The principal finding of this study is that 99mTcN-MPO has the desirable characteristics of reasonable heart uptake and fast washout from non-target organs surrounding the heart region. Low liver uptake and rapid hepatobiliary excretion resulted in favorable heart-to-liver ratios (0.90±0.06) as early as 30 min p.i. The liver and heart time-activity curves of 99mTcN-MPO crossed at 26.5 min p.i. After 26.5 min p.i., the hepatic activity curve was lower than that of the heart (Fig. 4a), whereas time-radioactivity curves of 99mTc-Sestamibi showed that the hepatic activity curve was always above that of the heart (Fig. 4b).
However, the exact mechanism of the relatively lower liver uptake and faster liver clearance of 99mTcN-MPO compared to 99mTc-Sestamibi is not clear at this stage. It seems that the liver clearance of 99mTcN-MPO is at least partially caused by the over-expression of multidrug resistance P-glycoproteins (particularly MDR1 Pgp) and multi-drug resistance-associated proteins (MRPs) in the liver [26–29]. This explanation is supported by the fact that 99mTcNMPO has significantly (p<0.01) higher liver uptake and slower liver clearance kinetics in SD rats pre-treated with excess amount of cyclosporin-A, a well-known wide-spectrum MDR modulator [30], than in those without cyclosporin-A pre-treatment [18]. Further investigations may be necessary to elucidate whether Pgp and MRPs have different impacts on the uptake and retention of 99mTcNMPO and 99mTc-sestamibi.
Our SPECT MPI results showed that left ventricular wall and perfusion defects were clearly visible as early as 30 min post-injection of 99mTcN-MPO. The perfusion defects remained well-defined over more than 120 min p.i. (Fig. 7). These promising results demonstrate that 99mTcNMPO is able to detect perfusion defects and the sizes of infarctions within an optimal imaging window of between 30 and 120 min p.i. The early imaging window suggests that 99mTcN-MPO may shorten the duration of imaging protocols. It is better suited for acutely infarcted patients than 99mTc-Sestamibi, which has an optimal imaging window of 60-120 min p.i. The fact that the perfusion defect is clearly seen without administration of a stress agent provides further support for the validity of this canine infarction model.
The study had some limitations. There is a prolonged retention of 99mTcN-MPO in the gallbladder due to its hepatobiliary excretion. Considering the location of gallbladder in humans, i.e., not overlapping the heart in an AP projection, this shortcoming may not be a significant issue.
Moreover, various maneuvers (drinking whole milk or eating other fatty food) can help alleviate the problem by causing partial emptying of the gall bladder before repeating imaging [31]. It is also worth mentioning that 99mTcN-MPO had a decreased myocardial retention over the 2 h study period and its myocardial radioactivity accumulation was slightly lower than that of 99mTc-Sestamibi. Thus, early acquisition may be required to achieve a total myocardial count density and compensate for myocardial washout. Our future work will include the determination of the precise kinetics parameters, as well as first pass extraction and its potential to differentiate between viable and ischemic myocardium under both stress and rest conditions. Despite these limitations, 99mTcN-MPO offers compensating advantages that make it an excellent alternative to 99mTc-Sestamibi for myocardial perfusion imaging.
Conclusions
The fast blood clearance kinetics, reasonable heart uptake with acceptable myocardial retention, low lung radioactivity, rapid hepatobiliary excretion, and consequently high heart/liver and heart/lung contrast make 99mTcN-MPO a potentially better perfusion imaging radiotracer than 99mTc-Sestamibi. The short waiting time between radiotracer injection and the first available myocardial image is of particular interest for the diagnosis of emergency patients. These advantages warrant its translation to human trials.
Acknowledgments
This work was supported, in part, by the following research grants: 2009DFB30040 from the international cooperation projects of the Chinese Ministry of Science and Technology, 20070420165 from the National Science Foundation for Postdoctoral Scientists of China, 2007AA3CS085 from the Science and Technology Tackle Key Problem Plan Foundation of Harbin. The authors wish to thank Drs. Fan Wang and Bing Jia from Peking University for technical support.
Footnotes
Lihong Bu and Renfei Li contributed equally to this work
References
- 1.Beller GA, Zaret BL. Contributions of nuclear cardiology to diagnosis and prognosis of patients with coronary artery disease. Circulation. 2000;101:1465–1478. doi: 10.1161/01.cir.101.12.1465. [DOI] [PubMed] [Google Scholar]
- 2.Jain D. Technetium-99 m labeled myocardial perfusion imaging agents. Semin Nucl Med. 1999;29:221–236. doi: 10.1016/s0001-2998(99)80012-9. [DOI] [PubMed] [Google Scholar]
- 3.Llaurado JG. The quest for the perfect myocardial perfusion indicator...still a long way to go. J Nucl Med. 2001;42:282–284. [PubMed] [Google Scholar]
- 4.Kailasnath P, Sinusas AJ. Comparison of Tl-201 with Tc-99 m-labeled myocardial perfusion agents: technical, physiologic, and clinical issues. J Nucl Cardiol. 2001;8:482–498. doi: 10.1067/mnc.2001.115078. [DOI] [PubMed] [Google Scholar]
- 5.Banerjee S, Pillai MR, Ramamoorthy N. Evolution of Tc-99 m in diagnostic radiopharmaceuticals. Semin Nucl Med. 2001;31:260–277. doi: 10.1053/snuc.2001.26205. [DOI] [PubMed] [Google Scholar]
- 6.Liu S. Ether and crown ether-containing cationic 99mTc complexes useful as radiopharmaceuticals for heart imaging. Dalton Trans. 2007;12:1183–1193. doi: 10.1039/b618406e. [DOI] [PubMed] [Google Scholar]
- 7.Lisic EC, Heeg MJ, Deutsch E. 99mTc(L-L)3+ complexes containing ether analogs of DMPE. Nucl Med Biol. 1999;26:563–571. doi: 10.1016/s0969-8051(99)00016-5. [DOI] [PubMed] [Google Scholar]
- 8.Tisato F, Maina T, Shao LR, et al. Cationic [99mTcIII (DIARS)2(SR)2]+ complexes as potential myocardial perfusion imaging agents (DIARS=o-phenylenebis(dimethylarsine); SR=thiolate). J Med Chem. 1996;39:1253–1261. doi: 10.1021/jm9507789. [DOI] [PubMed] [Google Scholar]
- 9.Boschi A, Bolzati C, Uccelli L, et al. A class of asymmetrical nitrido 99mTc heterocomplexes as heart imaging agents with improved biological properties. Nucl Med Commun. 2002;23:689–693. doi: 10.1097/00006231-200207000-00014. [DOI] [PubMed] [Google Scholar]
- 10.Hatada K, Riou LM, Ruiz M, et al. 99mTc-N-DBODC5, a new myocardial perfusion imaging agent with rapid liver clearance: comparison with 99mTc-sestamibi and 99mTc-tetrofosmin in rats. J Nucl Med. 2004;45:2095–2101. [PubMed] [Google Scholar]
- 11.Hatada K, Ruiz M, Riou LM, et al. Organ biodistribution and myocardial uptake, washout, and redistribution kinetics of Tc-99 mN-DBODC5 when injected during vasodilator stress in canine models of coronary stenoses. J Nucl Cardiol. 2006;13:779–790. doi: 10.1016/j.nuclcard.2006.08.016. [DOI] [PubMed] [Google Scholar]
- 12.Kim YS, He Z, Hsieh WY, et al. A novel ternary ligand system useful for preparation of cationic 99mTc-diazenido complexes and 99mTc-labeling of small biomolecules. Bioconjug Chem. 2006;17:473–484. doi: 10.1021/bc0502715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liu S, He Z, Hsieh WY, et al. Evaluation of novel cationic 99mTc-nitrido complexes as radiopharmaceuticals for heart imaging: improving liver clearance with crown ether groups. Nucl Med Biol. 2006;33:419–432. doi: 10.1016/j.nucmedbio.2006.01.005. [DOI] [PubMed] [Google Scholar]
- 14.He Z, Hsieh WY, Kim YS, et al. Evaluation of novel cationic 99mTc(I)-tricarbonyl complexes as potential radiotracers for myocardial perfusion imaging. Nucl Med Biol. 2006;33:1045–1053. doi: 10.1016/j.nucmedbio.2006.08.009. [DOI] [PubMed] [Google Scholar]
- 15.Kim YS, He Z, Hsieh WY, et al. Impact of bidentate chelators on lipophilicity, stability, and biodistribution characteristics of cationic 99mTc-nitrido complexes. Bioconjug Chem. 2007;18:929–936. doi: 10.1021/bc0603182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cittanti C, Uccelli L, Pasquali M, et al. Whole-body biodistribution and radiation dosimetry of the new cardiac tracer 99mTc-N-DBODC. J Nucl Med. 2008;49:1299–1304. doi: 10.2967/jnumed.108.053132. [DOI] [PubMed] [Google Scholar]
- 17.Kim YS, Wang J, Broisat A, et al. Tc-99 m-N-MPO: novel cationic Tc-99 m radiotracer for myocardial perfusion imaging. J Nucl Cardiol. 2008;15:535–546. doi: 10.1016/j.nuclcard.2008.02.022. [DOI] [PubMed] [Google Scholar]
- 18.Kim YS, Shi J, Zhai S, et al. Mechanism for myocardial localization and rapid liver clearance of Tc-99 m-N-MPO: a new perfusion radiotracer for heart imaging. J Nucl Cardiol. 2009;4:571–579. doi: 10.1007/s12350-009-9068-y. [DOI] [PubMed] [Google Scholar]
- 19.Fang W, Liu Y, Zhu L, et al. Evaluation of 99mTcN-15C5 as a new myocardial perfusion imaging agent in normal dogs and canines with coronary stenosis. Nucl Med Commun. 2008;29:775–781. doi: 10.1097/MNM.0b013e328302ca4a. [DOI] [PubMed] [Google Scholar]
- 20.Krejcy K, Krumpl G, Todt H, et al. Lidocaine has a narrow antiarrhythmic dose range against ventricular arrhythmias induced by programmed electrical stimulation in conscious postinfarction dogs. Naunyn Schmiedebergs Arch Pharmacol. 1992;346:213–218. doi: 10.1007/BF00165304. [DOI] [PubMed] [Google Scholar]
- 21.Dib N, Diethrich EB, Campbell A, et al. A percutaneous swine model of myocardial infarction. J Pharmacol Toxicol Methods. 2006;53:256–263. doi: 10.1016/j.vascn.2005.10.005. [DOI] [PubMed] [Google Scholar]
- 22.Katori R, Yamashita K, Miyazaki T, et al. Beta-adrenergic stimulation induces ST-segment elevation in dogs with healing myocardial infarction. Tohoku J Exp Med. 1995;177:233–248. doi: 10.1620/tjem.177.233. [DOI] [PubMed] [Google Scholar]
- 23.Chua T, Kiat H, Germano G, et al. Rapid back to back adenosine stress/rest technetium-99 m teboroxime myocardial perfusion SPECT using a triple-detector camera. J Nucl Med. 1993;34:1485–1493. [PubMed] [Google Scholar]
- 24.Berman DS, Germano G, Shaw LJ. The role of nuclear cardiology in clinical decision making. Semin Nucl Med. 1999;29:280–297. doi: 10.1016/s0001-2998(99)80017-8. [DOI] [PubMed] [Google Scholar]
- 25.Acampa W, Di Benedetto C, Cuocolo A. An overview of radiotracers in nuclear cardiology. J Nucl Cardiol. 2000;7:701–707. doi: 10.1067/mnc.2000.109969. [DOI] [PubMed] [Google Scholar]
- 26.Lee CH, Bradley G, Zhang JT, et al. Differential expression of P-glycoprotein genes in primary rat hepatocyte culture. J Cell Physiol. 1993;157:392–402. doi: 10.1002/jcp.1041570223. [DOI] [PubMed] [Google Scholar]
- 27.Mayer R, Kartenbeck J, Buchler M, et al. Expression of the MRP gene-encoded conjugate export pump in liver and its selective absence from the canalicular membrane in transport-deficient mutant hepatocytes. J Cell Biol. 1995;131:137–150. doi: 10.1083/jcb.131.1.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Agrawal M, Abraham J, Balis FM, et al. Increased 99mTc-sestamibi accumulation in normal liver and drug-resistant tumors after the administration of the glycoprotein inhibitor, XR9576. Clin Cancer Res. 2003;9:650–656. [PubMed] [Google Scholar]
- 29.Gatmaitan ZC, Arias IM. Structure and function of P-glycoprotein in normal liver and small intestine. Adv Pharmacol. 1993;24:77–97. doi: 10.1016/s1054-3589(08)60934-5. [DOI] [PubMed] [Google Scholar]
- 30.Qadir M, O'Loughlin KL, Fricke SM, et al. Cyclosporin A is a broad-spectrum multidrug resistance modulator. Clin Cancer Res. 2005;11:2320–2326. doi: 10.1158/1078-0432.CCR-04-1725. [DOI] [PubMed] [Google Scholar]
- 31.van Dongen AJ, van Rijk PP. Minimizing liver, bowel, and gastric activity in myocardial perfusion SPECT. J Nucl Med. 2001;41:1315–1317. [PubMed] [Google Scholar]