Conspectus
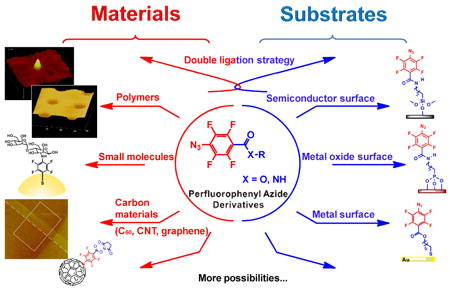
A major challenge in materials science is the ongoing search for coupling agents that are readily synthesized, capable of versatile chemistry, able to easily functionalize materials and surfaces, and efficient in covalently linking organic and inorganic entities. A decade ago, we began a research program investigating perfluorophenylazides (PFPAs) as the coupling agents in surface functionalization and nanomaterial synthesis. The p-substituted PFPAs are attractive heterobifunctional coupling agents because of their two distinct and synthetically distinguishable reactive centers: (i) the fluorinated phenylazide, which is capable of forming stable covalent adducts, and (ii) the functional group R, which can be tailored through synthesis.
Two approaches have been undertaken for material synthesis and surface functionalization. The first method involves synthesizing PFPA bearing the first molecule or material with a functional linker R, and then attaching the resulting PFPA to the second material by activating the azido group. In the second approach, the material surface is first functionalized with PFPA via functional center R, and coupling of the second molecule or material is achieved with the surface azido groups. In this Account, we review the design and protocols of the two approaches, providing examples in which PFPA derivatives were successfully used in material surface functionalization, ligand conjugation, and the synthesis of hybrid nanomaterials.
The methods developed have proved to be general and versatile, and they are applicable to a wide range of materials (especially those that lack reactive functional groups or are difficult to derivatize) and to various substrates of polymers, oxides, carbon materials, and metal films. The coupling chemistry can be initiated by light, heat, and electrons. Patterned structures can be generated by selectively activating the areas of interest. Furthermore, the process is easy to perform, and light activation occurs in minutes, greatly facilitating the efficiency of the reaction. PFPAs indeed demonstrate many benefits as versatile surface coupling agents and offer opportunities for further exploration.
1. Introduction
Phenylazide and derivatives were first introduced by Fleet and coworkers as photoaffinity labeling (PAL) agents to probe the binding site structure of biological receptors.1 A PAL agent, consisting of a ligand derivatized with a photosensitive moiety, binds to the receptor site bringing along the photoactive group (Figure 1).2-15 Upon activation by light, the photoprobe forms covalent linkages with the biomolecule at its binding site. The labeled biomolecule is then isolated, characterized, and the binding site structure can thus be determined. Commonly used photoaffinity labels include benzophenones,9,10 aryldiazirines,11-13 and arylazides.14,15 These reagents, upon photoactivation, yield reactive intermediates of biradical, carbene, or nitrene, which subsequently undergo H abstraction (radical) or insertion reactions (carbene and nitrene) with the neighboring biomolecules to form stable covalent adducts.
Figure 1.

Schematic illustration of the photoaffinity labeling technique.
Phenylazides are among the most popular PAL agents due to their high reaction efficiencies, fast kinetics, excellent storage stability, and ease of preparation. Phenylazide has complex photochemistry; a few relevant reactions are shown in Figure 2. Upon light activation, it decomposes by releasing N2 to give the singlet phenylnitrene, a highly reactive intermediate which can undergo numerous non-selective reactions leading to a wide range of products. 15-21 Three main processes of phenylnitrene reactions are of relevance to photoaffinity labeling: I) rearrangement to the corresponding seven-membered ketenimine which reacts with amines to give azepinamines, or produces polymer tars in the absence of a nucleophile; II) CH or NH insertion, and C=C addition reactions which are the key contributions for the covalent bond formation with the target molecules; and III) relaxation via intersystem crossing (ISC) to the triplet phenylnitrene which undergoes H-abstraction reactions to form primarily aniline-type products, or bimolecular reactions to yield the corresponding azo compound. The singlet phenylnitrene is the key intermediate dictating whether stable covalent adducts can be formed via pathway II. The partitioning between the singlet and triplet states is temperature-dependent. Higher temperature favors the formation of ketenimine, whereas ISC, a barrier-less process, is preferred at low temperatures and can be catalyzed by heavy atoms or alcohols.22 An important finding in the photochemistry of phenylazide is that the introduction of halogen atoms (F or Cl) on the aromatic ring greatly suppresses the ring expansion reaction and thus increases the yields of the insertion/addition reactions.17,20,23 Platz and coworkers have conducted a series of laser flash photolysis experiments and found that the halogen atoms, either per-halogenated or 2,6-disubstituted and ortho to the azido group, raised the energy barrier of the ring-expansion reaction and significantly increased the lifetime of the corresponding halogenated singlet phenylnitrenes.17,24-26 The longer lifetime offers the singlet nitrene increased opportunity to react with neighboring molecules. The pathway for the covalent adduct formation is thus promoted and the insertion reaction yield is greatly enhanced.
Figure 2.
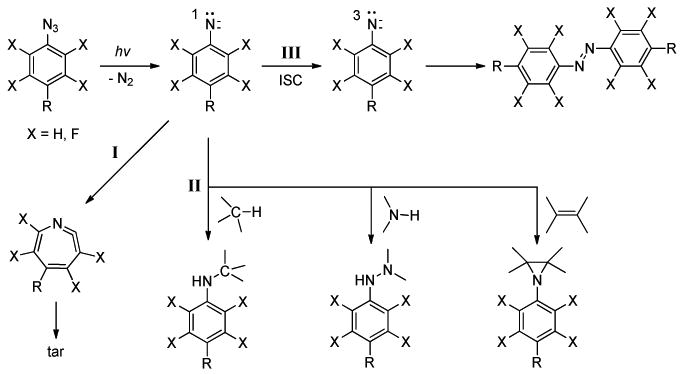
Simplified description of phenylazide photochemistry: ring expansion (I), insertion and addition reactions (II), and ISC (III).
The heterobifunctional nature of PAL agents makes them excellent candidates as coupling agents for materials synthesis and surface functionalization. In this Account, we focus our discussions on PFPAs, although examples using benzophenone27-30 and 3-(trifluoromethyl)-3-phenyldiazirine31-34 have also been reported. The differential reactivity of the two functional groups, PFPA and R, allows the coupling reaction to be carried out selectively and sequentially, bringing together molecules or materials of varying natures. Light offers a highly chemoselective means where only the photosensitive moieties are activated and other structural entities are unaffected. The activation is accomplished under mild conditions without damaging its surrounding components. In addition, because PFPA reacts with CH, NH or C=C bonds, the coupling chemistry is applicable to a wide range of molecules and materials, and is therefore highly general and versatile.
We used p-substituted tetrafluorophenylazide as the coupling agents because of the convenience and simplicity in their syntheses. The preparation starts with commercially available pentafluorobenzene derivatives that can be readily converted to the corresponding p-azidotetrafluorobenzene derivatives via a facile nucleophilic substitution reaction using NaN3 (Figure 3). PFPAs bearing acid chloride, carboxylic acid or its active ester can be prepared in gram quantities and stored under ambient conditions in dark until use. These precursors can be further derivatized using, for example, coupling reactions with amines or alcohols.
Figure 3.
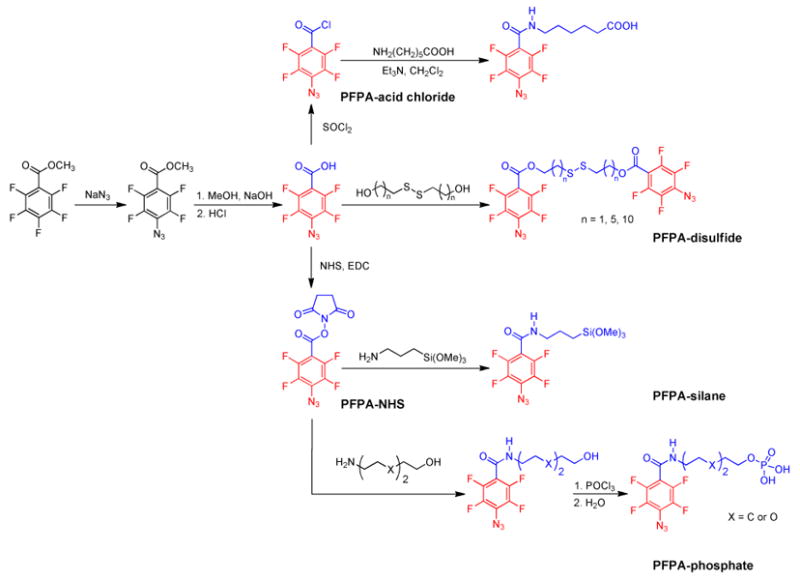
Synthetic schemes of selected PFPA derivatives.
2. Surface Engineering via PFPA Chemistry
Functionalized PFPAs serve as heterobifunctional coupling agents by bringing together molecules and materials via the two reactive centers, i.e., a chemoselective functional group R, and the light-activatable azido group. Two main approaches can be conceived for material synthesis and surface functionalization. In the first approach, PFPA is derivatized with the molecule of interest, and the resulting PFPA is then coupled to a material or substrate surface via the insertion/addition reactions of PFPA (Approach 1, Table 1). This strategy applies to materials and substrates that possess CH, NH, C=C bonds including organic materials, polymers, biomolecules, and carbon materials. In the second approach, a material or substrate is first functionalized with PFPA, attaching the azido group to the surface. The second molecule or material is then coupled to the material or substrate by activating the surface azido groups (Approach 2, Table 1).
Table 1.
Two approaches applying PFPAs in surface functionalization and materials synthesis.
| Approach 1 | Approach 2 | |
|---|---|---|
| Strategy |  |
 |
| Substrates | Organic surfaces | Oxides (SiO2, FexOy, TiO2) |
| Polymers (synthetic, natural) | Metals (Au, Ag, Al) | |
| Carbon materials (fullerenes, carbon nanotubes, graphene) | Minerals (mica, clay, calcites) | |
2.1 Approach 1: Functionalization of organic and carbon materials
This approach takes advantage of the reactivity of the azido group towards organic and carbon materials. In an early example, polymer films were functionalized with PFPA-NHS (Figure 3) by irradiating the film in the presence of a spin-coated layer of PFPA-NHS.35 Amine-containing organic molecules and proteins were then conjugated to the polymer films via the surface NHS groups.36 When a photomask is used during the photoactivation, spatially-selective functionalization was possible resulting in patterned protein structures (Figure 4).35
Figure 4.
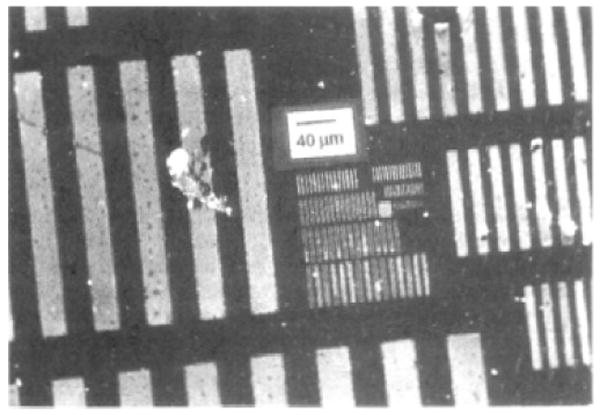
Fluorescence image of patterned protein structures. The sample was prepared by treating polystyrene film with PFPA-NHS followed by amino-biotin and then fluorescein-labeled avidin. Adapted with permission from ref 35, Copyright © 1993 American Chemical Society.
PFPAs have also been used to functionalize carbon materials of fullerenes,37 carbon nanotubes (CNTs),38 and graphene.39,40 Photochemical reaction of C60 with PFPA-NHS gave exclusively the monoadduct azamethanofullerene (Figure 5).37 The reaction took place via the addition of the perfluorophenylnitrene to a 6,6 double bond in C60. The NHS active ester group on the resulting compound serves as the reactive site for further conjugation of other molecules to C60.37 Fréchet and co-workers functionalized CNT forests using PFPAs bearing hydroxyl and fluoroalkyl groups to render the resulting CNTs hydrophilic or hydrophobic.38 The authors furthermore grafted poly(N-isopropylacrylamide) on CNTs by a surface-initiated polymerization using PFPA derivatized with 2-bromoisobutyrate, and were able to fabricate superhydrophilic patterns on a superhydrophobic background. Recently, we have successfully functionalized solvent-exfoliated graphene flakes with alkyl-, perfluoroalkyl-, and ethylene oxide-functionalized PFPAs, rendering them soluble in organic solvents as well as water.41
Figure 5.
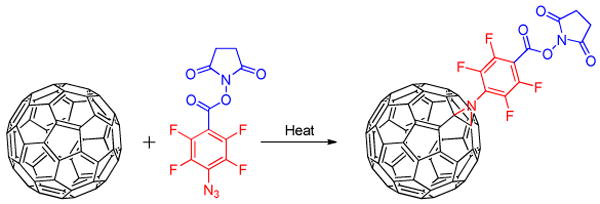
Functionalization of C60 with PFPA-NHS.
2.2 Approach 2: PFPA-surface as a general platform for the immobilization of molecules and materials
In this approach, the substrate material is first functionalized with PFPA, and a second molecule or material is then attached to the substrate by way of the azide coupling chemistry (Table 1). Because PFPA reacts with a wide range of molecules and materials, this method serves as a general platform bringing together two molecules or materials. When both are biomolecules, a ligase results. In the work of Ting and coworkers,42 the active site of an enzyme was engineered to accept PFPA, which was subsequently used to covalently conjugate a recognition peptide in a highly sequence-specific fashion.
We employed this strategy for material surface functionalization and the synthesis of hybrid nanomaterials. The key step in this approach is the preparation of PFPA-functionalized surfaces. Depending on the substrate material, a PFPA bearing a substrate-reactive functional group is used. PFPAs derivatized with silane, phosphate, and disulfide were synthesized (Figure 3) and were utilized to functionalize substrate materials including silicon oxide, metal films, and metal oxides. In the sections below, we discuss how this approach can be used to attach polymers, small molecules and carbon materials to various substrates for material surface functionalization and for the synthesis of organic-inorganic hybrid nanomaterials. We show that the surface and interface chemistry can be fine-tuned to control the surface composition, topography, density as well as binding affinity.
2.2.1 Polymers
Polymers are excellent materials for this photocoupling chemistry. The high molecular weight offers a large number of insertable bonds increasing the probability of their reactions with PFPA. Polymers are readily processable in solution and can be coated on substrates and materials of various shapes and sizes by spin-coating, dip-coating, or spraying. The ability of polymer chains to entangle in comparison to small molecules and the high solution viscosity allow polymers to form uniform films in conformal contact with the substrate, greatly enhancing the insertion yield of the PFPA coupling reaction.
We tested the effectiveness of this method using polymers that lack reactive functional groups. The substrate, silicon wafer for example, was first treated with PFPA-silane (Figure 3), thus introducing azido groups to the substrate surface. A solution of polystyrene (PS) or poly(2-ethyl-2-oxazoline) (PEOX) was spin-coated on the substrate followed by light activation for 5 min with a medium pressure Hg lamp using a 280-nm long-pass optical filter.43,44 Because the reaction occurs at the interface of surface azido groups and the coated polymer, only a monolayer of polymer remained after the excess polymer was removed by solvent.
We have since employed this method to immobilize a wide range of polymers including poly(allyl amine), poly(acrylic acid), poly(4-vinylpyridine), poly(4-vinylphenol), polyvinylpyrrolidone, poly(ethylene oxide) (PEO), poly(ethylene glycol) (PEG), and polypropylene. The results demonstrate that the azido group can be specifically activated to react with solid materials to produce stable covalent adducts. Efficient insertion reaction in the solid state requires the molecules to be in close contact with the surface azido groups. For polymers that can easily crystallize upon deposition, for example, low molecular weight PEG and isotactic polypropylene (iPP), the immobilization was less efficient, and sometimes, no polymer film was obtained after light activation. In these cases, thermal treatment was applied. Heat itself can be used in place of light to initiate the insertion reactions. For example, PS films were immobilized on PFPA-functionalized wafer by heating at 140 °C for 20 min.45 Using this protocol, we have successfully immobilized uniform thin films of iPP, which was otherwise impossible by light activation.46 In this case, iPP was heated at a temperature (140 °C) above its glass transition temperature (Tg, ∼100 °C) where the polymer became softened and amorphous. This effectively enhances the contact between iPP and the surface, resulting in the efficient reaction between azido groups and the polymer. Alternatively, a combination of heat and photoactivation can be applied where the thermal treatment improves the contact of the polymer with the substrate and the light initiates the insertion reaction. This strategy was applied to immobilize low molecular weight PEG where the polymer was heated to 70 °C while irradiating to yield covalently attached PEG films.47
The thickness of the immobilized polymer film is governed by the nature and the molecular weight of the polymer,48 and can be further controlled by the irradiation dose,49 and the density of surface PFPA.44 In principle, only one attachment point is necessary to tether the entire polymer to the surface. Depending on the size, ie, the molecular weight of the polymer, the concentration of the surface azido groups can be drastically reduced while still ensuring the covalent attachment of the polymer. This photocoupling process is therefore highly defect-tolerant. In fact, uniform polymer films were obtained on surfaces treated with PFPA-silane at concentrations of a few μM or when more than 100 times of a non-photoactive silane was added.44
2.2.2 Small molecules
Small organic molecules that lack reactive functional groups or are difficult to chemically derivatize are another class of compounds that are well-suited for this photocoupling chemistry. Since the probability of the insertion/addition reactions decreases with the size of the molecule or the number of PFPA-reactive bonds in the molecule, small molecules require higher surface density of PFPA than polymers to be covalently attached. In addition, while polymer chains can entangle to form uniform films, small molecules may not form highly packed monolayer structures.
We have successfully immobilized furanone, an Australian red marine alga Delisea Pulchra that possesses antibacterial properties.50 The covalently attached molecules were characterized by X-ray photoelectron spectroscopy (XPS) and time-of-flight secondary ion mass spectroscopy. The grafting density was controlled by adjusting the concentration of surface azido groups.
Carbohydrates are another class of compounds that are well-suited for this photocoupling chemistry. Carbohydrate immobilization remains a challenge, especially for complex carbohydrate structures, the syntheses of which are often complicated due to the stereochemistry control and multiple protection/deprotection steps involved in the site-specific glycosylation and derivatization reactions. Current methods for conjugating carbohydrates generally require the use of derivatized carbohydrates, amenable to coupling to the chosen nanomaterials. Underivatized carbohydrate structures present a considerable challenge, and only few strategies were reported in the literature.51-54 Using the PFPA photocoupling chemistry, Joester and coworkers immobilized hyaluronan on PS beads.55 In the process, amine-modified PS beads were treated with PFPA-NHS to introduce PFPA to the bead surface. Direct irradiation of the functionalized beads in hyaluronan solution did not yield any immobilized hyaluronan. To enhance the immobilization yield, lanthanide cations were added to precipitate hyaluronan to increase the local concentration of hyaluronan on the bead surface. We have successfully attached carbohydrates to various substrates including gold films,56 gold57,58 and iron oxide nanoparticles (NPs).59,60 The chemistry applies to mono-, oligo- and poly-saccharides with high coupling yield and efficiency. In the case of Au nanoparticles, a one-pot process was developed whereby the synthesized nanoparticles were functionalized in situ with the thiol-functionalized PFPA. To couple carbohydrates to the NPs, a solution of PFPA-NPs mixed with the carbohydrate ligand was irradiated with 280 nm UV light for 5 min to yield glyconanoparticles that were well-dispersed and readily soluble in water.
We showed that the surface-bound carbohydrate ligands retained their binding affinity and selectivity.56-58 Furthermore, the carbohydrate ligands, when bound to nanoparticles, exhibited binding affinities up to five orders of magnitude higher than the corresponding free ligands in solution, demonstrating that nanoparticles served as an excellent scaffold promoting the cooperative interactions of multiple ligands leading to greatly enhanced affinity with their binding partners.58 The fact that nanomaterials amplify the affinity of carbohydrate ligands makes them highly useful in applications where carbohydrate recognitions are applied. Figure 6 showed that d-mannose-functionalized iron oxide nanoparticles bound Concanavalin A (Con A) and bacteria E. coli strain ORN178.59 Potential application of this strategy include the detection of carbohydrate-binding proteins and bacteria, and the de-contamination of pathogens taking advantage of the magnetic properties of iron oxide nanoparticles.
Figure 6.
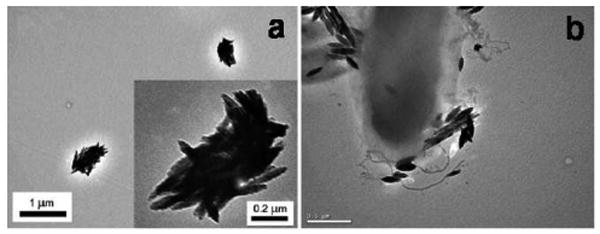
d-Mannose-functionalized iron oxide nanoparticles binding with Con A (a), and E. coli strain ORN178 (b). Adapted with permission from ref 59, Copyright © 2009 American Chemical Society.
2.2.3 Carbon materials
Graphene with well-defined and controllable surface and interface properties are important for both fundamental studies and practical applications. Using the photocoupling chemistry, we immobilized mechanically-exfoliated graphene on PFPA-functionalized wafers (Figure 7a).39 The attached graphene sheets were highly stable, withstanding extensive solvent treatment and repetitive sonication. Using solution-produced graphene flakes, we fabricated patterned graphene structures where the feature sizes could be conveniently controlled (Figure 7b).40 Both methods are applicable to various substrates, and we have generated graphene sheets and patterned structures on silicon wafers, gold films, and glass slides.
Figure 7.
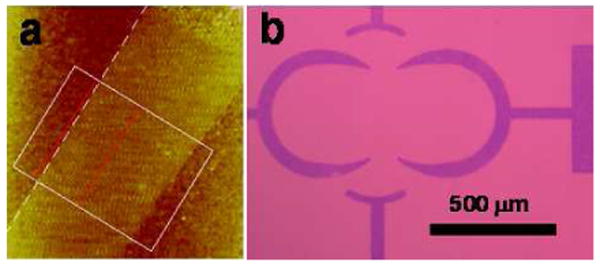
Covalently immobilized single-layer graphene sheet (a) and patterned graphene structures (b) on silicon wafer. Adapted with permission from refs 39 and 40, Copyright © 2009 American Chemical Society and Copyright © 2010 Royal Society of Chemistry.
The covalent bond formation between the graphene flakes and the PFPA-functionalized wafer was clearly demonstrated by XPS. The N 1s spectra before and after reaction with graphene was consistent with the conversion of Ar-N3 to Ar-N upon light activation (Figure 8, b and e). After the graphene was attached to the surface, the percentage of C-C (285.0 eV) increased due to the added graphene layer (Figure 8, c and f). In addition, the increase in C-N (286.4 eV) relative to C-F (288.1 eV) was attributed to the formation of additional C-N bonds (aziridine) upon reaction of PFPA with graphene.39,40,61
Figure 8.
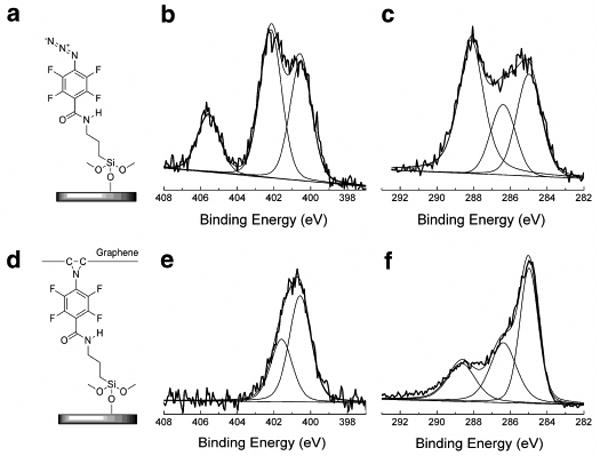
PFPA-decorated wafer (a) and the corresponding high-resolution XPS N 1s and C 1s core level spectra (b and c); Covalently attached graphene (d) and the corresponding high-resolution XPS N 1s and C 1s core level spectra (e and f). Peak assignments are 400.5 eV (CONH), 402.1 eV (Ar-N=N+=N−), 405.6 eV (Ar-N=N+=N−), 285.0 eV (C-C), 286.4 eV (C-N), and 288.1 eV (C-F), respectively. Adapted with permission from ref 40, Copyright © 2010 Royal Society of Chemistry.
3 Applications
3.1 Synthesis of hybrid nanomaterials
Organic-inorganic hybrid nanomaterials are attracting considerable attention due to its improved structural and functional properties. One route to the synthesis of hybrid nanomaterials is to covalently attach organic entities to the inorganic nanomaterials. Our photocoupling chemistry can be readily applied to synthesize hybrid nanomaterials. For example, we have successfully attached polymers to silica nanoparticles.62 A one-pot process was developed to simultaneously synthesize and functionalize silica nanoparticles with PFPA-silane. Polymer was subsequently immobilized by photoactivation. The process is fast, efficient, and is applicable to various polymer structures and of different molecular weights. No chemical derivatization is necessary on the polymer, and the method can be extended to other nanomaterials simply by using the corresponding PFPA.
3.2 Single molecule immobilization
Our photocoupling chemistry offers a convenient means to immobilize single molecules by the general and versatile insertion reactions of PFPAs. This is achieved by diluting the surface azido group until the attached molecules are no longer densely packed. The density of the surface azido group can be controlled by varying the concentration of PFPA or by the addition of a non-photoactive agent to PFPA when treating the substrate. For example, when silicon wafers were treated with low concentration of PFPA-silane (5 × 10-5 mg/mL), or with a mixture of PFPA-silane and n-propyltrimethoxysilane at the mole ratio of 1:2000, discrete polystyrene molecules were observed (Figure 9).44,48,63
Figure 9.
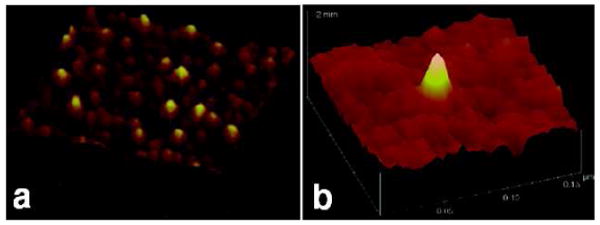
PS single molecules on silicon wafer. Wafers were treated with PFPA-silane and PTMS at the mole ratio of 1:2000 before PS (M̅w 223,200 g/mol) was spin-coated and irradiated. The scan area is 200 nm × 200 nm, and the Z-scale is 10 nm. Adapted with permission from refs 44 and 48, Copyright © 2006 American Chemical Society and Copyright © 2006 WILEY-VCH Verlag GmbH & Co. KGaA.
In principle, this method can be used to prepare single molecules of any sizes. The probability of the molecule to be attached increases with the number of nitrene-reactive bonds; the more bonds are available, the less surface azido groups are needed. Indeed, we found that the higher the molecular weight of the polymer, the lower the concentration of PFPA-silane was used to achieve single molecule immobilization.48
3.3 Patterned structures and microwell arrays
The photochemical process allows us to fabricate patterned structures and microarrays with controls over both spatial and topographical features. The spatial control is achieved by microfabrication; the feature size is defined by the lateral resolution of the fabrication technique. For example, initiating the photocoupling reaction in the presence of a photomask generated patterned polymer structures (Figure 10a).43 Microstructures can also be fabricated by printing solutions on PFPA-functionalized substrates using a robotic printer followed by light activation. We have successfully created carbohydrate microarrays using this approach.56 Besides photons, PFPAs can be activated by electrons where nano-sized polymer patterns can be generated by rastering the surface with an electron beam.64
Figure 10.
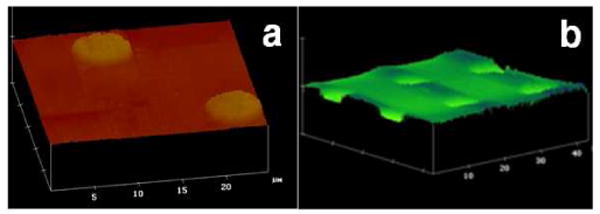
a) Patterned PEOX films on PFPA-functionalized wafer by activating the PFPA in the presence of a photomask; b) a microwell array created by spin coating PS film on the sample shown in a) followed by irradiation and solvent extraction. Adapted with permission from ref 43, Copyright © 2001 WILEY-VCH Verlag GmbH & Co. KGaA.
We have employed this method to create microarrays from covalently immobilized polymer thin films. The strategy is based on the fact that after the first polymer pattern is created, un-reacted azido groups are still present in the unexposed areas. When a second polymer is coated and activated, it would be covalently attached in these areas. Depending on the thickness of each polymer film, microarrays of different topography can be generated. Figure 10b is an example of a polymer microwell array fabricated from PEOX and PS, where PEOX was thinner forming the bottom of the wells whereas the surrounding was covered by PS which was thicker. This method of creating microwells is simple and general. By using different polymeric materials, the chemical property of the bottom and top of the wells can be controlled.43
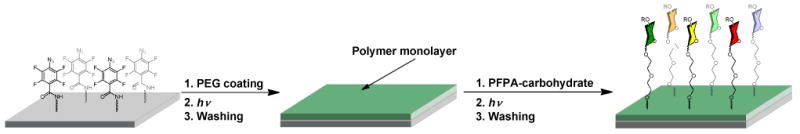
3.4 Double ligation strategy
The two approaches described in Table 1 can be utilized in a single process to construct multifunctional materials. We demonstrated this double ligation strategy in the fabrication of carbohydrate microarrays (Figure 11).56,65 Using Approach 2, PEO film was first covalently immobilized on the PFPA-functionalized glass slide to produce a protein-resistant surface. Carbohydrates derivatized with PFPAs were then printed on the surface using a robotic printer and were subsequently attached to the PEO surface by light activation (Approach 1). The molecular recognition property of immobilized carbohydrates were studied by applying them to either a microarray system56 or a flow-through quartz crystal microbalance (QCM) biosensor.65 Both microarray and QCM results confirmed that the photochemically immobilized carbohydrates bound to the corresponding lectins as expected. The microarray studies also revealed additional lectin binding patterns, which can be further employed for screening unknown carbohydrate-binding proteins.
Figure 11.
Carbohydrate microarray generated by double surface ligation. Adapted with permission from ref 56, Copyright © 2007 WILEY-VCH Verlag GmbH & Co. KGaA.
4. Conclusions and Perspectives
In this Account, we summarize the design and applications of PFPAs in coupling polymers, small molecules, and carbon materials to the substrate of organic, oxides, metal films, and nanoparticles. A key feature of this method is its versatility, where a wide range of molecules and materials can be attached to various substrate materials. The process is simple, efficient, and highly reproducible. The coupling reaction can be initiated by a variety of energy sources including heat, photons, electrons, and X-ray, which can be selected depending on the type and configuration of the substrate material. For example, for substrates that have areas that are inaccessible by light, heat can be applied instead. Controlled activation of PFPAs is also possible by focusing the light or energetic beams on the areas of interest. In this case, selective functionalization is achieved, generating patterned structures with controls over spatial and topographical features. By adjusting the concentration of the surface azido group, the density of the immobilized molecules can be controlled from monolayer to discrete single molecules.
Like every technique, the PFPA coupling chemistry is not without shortcomings. Because the coupling reaction applies to CH, NH, C=C bonds, the method therefore lacks functional-group specificity. Another challenge is the precise control over the orientation of the immobilized molecule; pre-orientation is necessary prior to the photocoupling reaction. Nevertheless, the PFPA coupling chemistry can be applied to situations where the technique can offer unique advantages. One such case is molecules or materials that lack reactive functional groups or are difficult to derivatize, such as polyolefins and carbon materials where very limited methods are available for their modification to achieve precise control over the type and the density of functional groups. As demonstrated by the many examples discussed in this Account, the technique has already shown potential in the synthesis of functional nanomaterials and fabrication of functional surfaces. New opportunities are waiting to be explored.
Acknowledgments
This work was supported by NIH (R15GM066279, R01GM080295), donors of the Petroleum Research Fund, administered by the American Chemical Society (ACS PRF #43469-AC7), and Oregon Nanoscience and Microtechnologies Institute (ONAMI) and ONR under the contract N00014-08-1-1237.
Biographies
Li-Hong Liu obtained his Ph. D degree from Xiamen University, China in 2005. He is currently a postdoctoral research fellow with Prof. Mingdi Yan at the Department of Chemistry, Portland State University. Before joining the group, he was a postdoctoral research fellow at the Laboratoire de Photophysique et Photochimie Supramoléculaires et Macromoléculaires (PPSM), École Normale Supérieure de Cachan, France. His research interests include functional surfaces and interfaces, supramolecular photophysics and photochemistry, and optical functional materials.
Mingdi Yan obtained her B.S. degree in Polymer Physics from the University of Science and Technology of China (USTC) in 1988, and Ph.D. in Organic Chemistry from the University of Oregon in 1994. She is Professor of Chemistry at Portland State University, and a member of the Oregon Nanoscience and Microtechnology Institute (ONAMI). Her research interests include surfaces and interfaces, nanomaterials functionalization, polymer thin films and nanostructures, and biosensors.
References
- 1.Fleet GWJ, Porter RR, Knowles JR. Affinity Labelling of Antibodies with Aryl Nitrene as Reactive Group. Nature. 1969;224:511–512. [Google Scholar]
- 2.Knowles JR. Photogenerated Reagents for Biological Receptor-Site Labeling. Acc Chem Res. 1972;5:155–160. [Google Scholar]
- 3.Bayley H, Knowles JR. Photoaffinity Labeling. Methods Enzymol. 1977;46:69–114. doi: 10.1016/s0076-6879(77)46012-9. [DOI] [PubMed] [Google Scholar]
- 4.Bayley H. Photogenerated Reagents in Biochemistry and Molecular Biology. Vol. 12 Elsevier Science Publishers B. V.; 1983. [Google Scholar]
- 5.Brunner J. New Photolabeling and Cross-Linking Methods. Annu Rev Biochem. 1993;62:483–514. doi: 10.1146/annurev.bi.62.070193.002411. [DOI] [PubMed] [Google Scholar]
- 6.Fleming SA. Chemical Reagents in Photoaffinity-Labeling. Tetrahedron. 1995;51:12479–12520. [Google Scholar]
- 7.Vodovozova EL. Photoaffinity Labeling and its Application in Structural Biology. Biochemistry (Moscow) 2007;72:1–20. doi: 10.1134/s0006297907010014. [DOI] [PubMed] [Google Scholar]
- 8.Tanaka Y, Bond MR, Kohler JJ. Photocrosslinkers Illuminate Interactions in Living Cells. Mol BioSyst. 2008;4:473–480. doi: 10.1039/b803218a. [DOI] [PubMed] [Google Scholar]
- 9.Dorman G, Prestwich GD. Benzophenone Photophores in Biochemistry. Biochemistry. 1994;33:5661–5673. doi: 10.1021/bi00185a001. [DOI] [PubMed] [Google Scholar]
- 10.Hino N, Okazaki Y, Kobayashi T, Hayashi A, Sakamoto K, Yokoyama S. Protein Photo-Cross-Linking in Mammalian Cells by Site-Specific Incorporation of a Photoreactive Amino Acid. Nat Methods. 2005;2:201–206. doi: 10.1038/nmeth739. [DOI] [PubMed] [Google Scholar]
- 11.Blencowe A, Hayes W. Development and Application of Diazirines in Biological and Synthetic Macromolecular Systems. Soft Matter. 2005;1:178–205. doi: 10.1039/b501989c. [DOI] [PubMed] [Google Scholar]
- 12.Nakashima H, Hashimoto M, Sadakane Y, Tomohiro T, Hatanaka Y. Simple and Versatile Method for Tagging Penyldiazirine Photophores. J Am Chem Soc. 2006;128:15092–15093. doi: 10.1021/ja066479y. [DOI] [PubMed] [Google Scholar]
- 13.Hashimoto M, Hatanaka Y. Recent Progress in Diazirine-Based Photoaffinity Labeling. Eur J Org Chem. 2008:2513–2523. [Google Scholar]
- 14.Bayley H, Knowles JR. Photogenerated Reagents for Membrane Labeling. 1. Phenylnitrene formed within Lipid Bilayer. Biochemistry. 1978;17:2414–2419. doi: 10.1021/bi00605a025. [DOI] [PubMed] [Google Scholar]
- 15.Schnapp KA, Poe R, Leyva E, Soundararajan N, Platz MS. Exploratory Photochemistry of Fluorinated Aryl Azides - Implications for the Design of Photoaffinity-Labeling Reagents. Bioconjugate Chem. 1993;4:172–177. doi: 10.1021/bc00020a010. [DOI] [PubMed] [Google Scholar]
- 16.Poe R, Schnapp K, Young MJT, Grayzar J, Platz MS. Chemistry and Kinetics of Singlet Pentafluorophenylnitrene. J Am Chem Soc. 1992;114:5054–5067. [Google Scholar]
- 17.Platz MS. Comparison of Phenylcarbene and Phenylnitrene. Acc Chem Res. 1995;28:487–492. [Google Scholar]
- 18.Gritsan NP, Platz MS. Kinetics, Spectroscopy, and Computational Chemistry of Arylnitrenes. Chem Rev. 2006;106:3844–3867. doi: 10.1021/cr040055+. [DOI] [PubMed] [Google Scholar]
- 19.Keana JFW, Cai SX. Functionalized Perfluorophenyl Azides - New Reagents for Photoaffinity-Labeling. J Fluorine Chem. 1989;43:151–154. [Google Scholar]
- 20.Keana JFW, Cai SX. New Reagents for Photoaffinity Labeling: Synthesis and Photolysis of Functionalized Perfluorophenyl Azides. J Org Chem. 1990;55:3640–3647. [Google Scholar]
- 21.Cai SX, Glenn DJ, Keana JFW. Toward the Development of Radiolabeled Fluorophenyl Azide-Based Photolabeling Reagents - Synthesis and Photolysis of Iodinated 4-Azidoperfluorobenzoates and 4-Azido-3,5,6-Trifluorobenzoates. J Org Chem. 1992;57:1299–1304. [Google Scholar]
- 22.Poe R, Grayzar J, Young MJT, Leyva E, Schnapp KA, Platz MS. Remarkable Catalysis of Intersystem Crossing of Singlet (Pentafluorophenyl)nitrene. J Am Chem Soc. 1991;113:3209–3211. [Google Scholar]
- 23.Banks RE, Sparkes GR. Studies in Azide Chemistry. Part V. Synthesis of 4-Azido-2,3,5,6-tetrafluoro-, 4-Azido-3-chloro-2,5,6-trifluoro-, and 4-Azido-3,5-dichloro-2,6-difluoro-pyridine, and Some Thermal Reactions of the Tetrafluoro-Compound. J Chem Soc, Perkin Trans 1. 1972:2964–2970. [Google Scholar]
- 24.Soundararajan N, Platz MS. Descriptive Photochemistry of Polyfluorinated Azide Derivatives of Methyl Benzoate. J Org Chem. 1990;55:2034–2044. [Google Scholar]
- 25.Gritsan NP, Platz MS. Kinetics and Spectroscopy of Substituted Phenylnitrenes. Adv Phys Org Chem. 2001;36:255–304. [Google Scholar]
- 26.Leyva E, Platz MS, Persy G, Wirz J. Photochemistry of Phenyl Azide - The Role of Singlet and Triplet Phenylnitrene as Transient Intermediates. J Am Chem Soc. 1986;108:3783–3790. [Google Scholar]
- 27.Delamarche E, Sundarababu G, Biebuyck H, Michel B, Gerber C, Sigrist H, Wolf H, Ringsdorf H, Xanthopoulos N, Mathieu HJ. Immobilization of Antibodies on a Photoactive Self-Assembled Monolayer on Gold. Langmuir. 1996;12:1997–2006. [Google Scholar]
- 28.Prucker O, Naumann CA, Ruhe J, Knoll W, Frank CW. Photochemical Attachment of Polymer Films to Solid Surfaces via Monolayers of Benzophenone Derivatives. J Am Chem Soc. 1999;121:8766–8770. [Google Scholar]
- 29.Griep-Raming N, Karger M, Menzel H. Using Benzophenone-Functionalized Phosphonic Acid to Attach Thin Polymer Films to Titanium Surfaces. Langmuir. 2004;20:11811–11814. doi: 10.1021/la0485327. [DOI] [PubMed] [Google Scholar]
- 30.Jarvholm J, Srinivasarao M, Tolbert LM. Traversing the “Top-Down/Bottom-Up” Divide: Molecular-Scale Lithography of Self-Assembled Ribbons. J Am Chem Soc. 2009;131:398–399. doi: 10.1021/ja805319k. [DOI] [PubMed] [Google Scholar]
- 31.Sigrist H, Gao H, Wegmuller B. Light-Dependent, Covalent Immobilization of Biomolecules on Inert Surfaces. Bio/Technology. 1992;10:1026–1028. doi: 10.1038/nbt0992-1026. [DOI] [PubMed] [Google Scholar]
- 32.Sigrist H, Collioud A, Clemence JF, Gao H, Luginbuhl R, Sanger M, Sundarababu G. Surface Immobilization of Biomolecules by Light. Opt Eng. 1995;34:2339–2348. [Google Scholar]
- 33.Kanoh N, Kumashiro S, Simizu S, Kondoh Y, Hatakeyama S, Tashiro H, Osada H. Immobilization of Natural Products on Glass Slides by using a Photoaffinity Reaction and the Detection of Protein-Small-Molecule Interactions. Angew Chem Int Ed. 2003;42:5584–5587. doi: 10.1002/anie.200352164. [DOI] [PubMed] [Google Scholar]
- 34.Kanoh N, Kyo M, Inamori K, Ando A, Asami A, Nakao A, Osada H. SPR Imaging of Photo-Cross-Linked Small-Molecule Arrays on Gold. Anal Chem. 2006;78:2226–2230. doi: 10.1021/ac051777j. [DOI] [PubMed] [Google Scholar]
- 35.Yan M, Cai SX, Wybourne MN, Keana JFW. Photochemical Functionalization of Polymer Surfaces and the Production of Biomolecule-Carrying Micrometer-Scale Structures by Deep-UV Lithography using 4-Substituted Perfluorophenyl Azides. J Am Chem Soc. 1993;115:814–816. [Google Scholar]
- 36.Yan M, Cai SX, Wybourne MN, Keana JFW. N-Hydroxysuccinimide Ester Functionalized Perfluorophenyl Azides as Novel Photoactive Heterobifunctional Crosslinking Reagents. The Covalent Immobilization of Biomolecules to Polymer Surfaces. Bioconjugate Chem. 1994;5:151–157. doi: 10.1021/bc00026a007. [DOI] [PubMed] [Google Scholar]
- 37.Yan M, Cai SX, Keana JFW. Photochemical and Thermal-Reactions of C-60 with N-Succinimidyl 4-Azido-2,3,5,6-tetrafluorobenzoate - A New Method for Functionalization of C-60. J Org Chem. 1994;59:5951–5954. [Google Scholar]
- 38.Pastine SJ, Okawa D, Kessler B, Rolandi M, Llorente M, Zettl A, Frechet JMJ. A Facile and Patternable Method for the Surface Modification of Carbon Nanotube Forests using Perfluoroarylazides. J Am Chem Soc. 2008;130:4238–4239. doi: 10.1021/ja8003446. [DOI] [PubMed] [Google Scholar]
- 39.Liu LH, Yan M. Simple Method for the Covalent Immobilization of Graphene. Nano Lett. 2009;9:3375–3378. doi: 10.1021/nl901669h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Liu LH, Zorn G, Castner DG, Solanki R, Lerner MM, Yan M. A Simple and Scalable Route to Wafer-Size Patterned Graphene. J Mater Chem. 2010;20:5041–5046. doi: 10.1039/C0JM00509F. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Liu LH, Lerner MM, Yan M. Derivatization of Pristine Graphene with Well-defined Chemical Functionalities. doi: 10.1021/nl1024744. Submitted. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Baruah H, Puthenveetil S, Choi YA, Shah S, Ting AY. An Engineered Aryl Azide Ligase for Site-Specific Mapping of Protein-Protein Interactions through Photo-Cross-Linking. Angew Chem Int Ed. 2008;47:7018–7021. doi: 10.1002/anie.200802088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bartlett MA, Yan M. Fabrication of Polymer Thin Films and Arrays with Spatial and Topographical Controls. Adv Mater. 2001;13:1449–1451. [Google Scholar]
- 44.Liu L, Engelhard MH, Yan M. Surface and Interface Control on Photochemically Initiated Immobilization. J Am Chem Soc. 2006;128:14067–14072. doi: 10.1021/ja062802l. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yan M, Ren J. Covalent Immobilization of Ultrathin Polymer Films by Thermal Activation of Perfluorophenyl Azide. Chem Mater. 2004;16:1627–1632. [Google Scholar]
- 46.Yan M, Ren J. Covalent Immobilization of Polypropylene Thin Films. J Mater Chem. 2005;15:523–527. [Google Scholar]
- 47.Wang H, Ren J, Hlaing A, Yan M. Photochemically and Thermally Immobilized Poly(Ethylene Oxide) and Low Molecular Weight Poly(Ethylene Glycol) doi: 10.1016/j.jcis.2010.10.018. Submitted. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Liu L, Yan M. A General Approach to the Covalent Immobilization of Single Polymers. Angew Chem Int Ed. 2006;45:6207–6210. doi: 10.1002/anie.200602097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Graupner RK, Yan M. Theoretical Model for Photochemical or Thermally Activated Immobilization of Macromolecules. Langmuir. 2004;20:8675–8680. doi: 10.1021/la0494728. [DOI] [PubMed] [Google Scholar]
- 50.Al-Bataineh SA, Luginbuehl R, Textor M, Yan M. Covalent Immobilization of Antibacterial Furanones via Photochemical Activation of Perfluorophenylazide. Langmuir. 2009;25:7432–7437. doi: 10.1021/la900334w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Angeloni S, Ridet JL, Kusy N, Gao H, Crevoisier F, Guinchard S, Kochhar S, Sigrist H, Sprenger N. Glycoprofiling with Micro-Arrays of Glycoconjugates and Lectins. Glycobiology. 2005;15:31–41. doi: 10.1093/glycob/cwh143. [DOI] [PubMed] [Google Scholar]
- 52.Zhi ZL, Powell AK, Turnbull JE. Fabrication of Carbohydrate Microarrays on Gold Surfaces: Direct Attachment of Nonderivatized Oligosaccharides to Hydrazide-Modified Self-Assembled Monolayers. Anal Chem. 2006;78:4786–4793. doi: 10.1021/ac060084f. [DOI] [PubMed] [Google Scholar]
- 53.Carroll GT, Wang DN, Turro NJ, Koberstein JT. Photochemical Micropatterning of Carbohydrates on a Surface. Langmuir. 2006;22:2899–2905. doi: 10.1021/la0531042. [DOI] [PubMed] [Google Scholar]
- 54.Park S, Lee MR, Shin I. Construction of Carbohydrate Microarrays by Using One-Step, Direct Immobilizations of Diverse Unmodified Glycans on Solid Surfaces. Bioconjugate Chem. 2009;20:155–162. doi: 10.1021/bc800442z. [DOI] [PubMed] [Google Scholar]
- 55.Joester D, Klein E, Geiger B, Addadi L. Temperature-Sensitive Micrometer-Thick Layers of Hyaluronan Grafted on Microspheres. J Am Chem Soc. 2006;128:1119–1124. doi: 10.1021/ja0537474. [DOI] [PubMed] [Google Scholar]
- 56.Pei ZC, Yu H, Theurer M, Walden A, Nilsson P, Yan M, Ramström O. Photogenerated Carbohydrate Microarrays. ChemBioChem. 2007;8:166–168. doi: 10.1002/cbic.200600447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wang X, Ramström O, Yan M. A Photochemically Initiated Chemistry for Coupling Underivatized Carbohydrates to Gold Nanoparticles. J Mater Chem. 2009;19:8944–8949. doi: 10.1039/B917900C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wang X, Ramström O, Yan M. Glyconanomaterials: Synthesis, Characterization, and Ligand Presentation. Adv Mater. 2010;22:1946–1953. doi: 10.1002/adma.200903908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Liu LH, Dietsch H, Schurtenberger P, Yan M. Photoinitiated Coupling of Unmodified Monosaccharides to Iron Oxide Nanoparticles for Sensing Proteins and Bacteria. Bioconjugate Chem. 2009;20:1349–1355. doi: 10.1021/bc900110x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wang X, Liu LH, Ramström O, Yan M. Engineering Nanomaterial Surfaces for Biomedical Applications. Exp Biol Med. 2009;234:1128–1139. doi: 10.3181/0904-MR-134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Holzinger M, Abraha J, Whelan P, Graupner R, Ley L, Hennrich F, Kappes M, Hirsch A. Functionalization of Single-Walled Carbon Nanotubes with (R-)Oxycarbonyl Nitrenes. J Am Chem Soc. 2003;125:8566–8580. doi: 10.1021/ja029931w. [DOI] [PubMed] [Google Scholar]
- 62.Gann JP, Yan M. A Versatile Method for Grafting Polymers on Nanoparticles. Langmuir. 2008;24:5319–5323. doi: 10.1021/la7029592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Yan M. Photochemically Initiated Single Polymer Immobilization. Chem Eur J. 2007;13:4138–4144. doi: 10.1002/chem.200700317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wybourne MN, Wu JC, Yan M, Cai SX, Keana JFW. Modification of Polymer Surfaces and the Fabrication of Submicron-Scale Functionalized Structures by Deep-Ultraviolet and Electron-Beam Lithography. J Vac Sci Technol B. 1993;11:2210–2213. [Google Scholar]
- 65.Pei YX, Yu H, Pei ZC, Theurer M, Ammer C, Andre S, Gabius HJ, Yan M, Ramström O. Photoderivatized Polymer Thin Films at Quartz Crystal Microbalance Surfaces: Sensors for Carbohydrate-Protein Interactions. Anal Chem. 2007;79:6897–6902. doi: 10.1021/ac070740r. [DOI] [PMC free article] [PubMed] [Google Scholar]


