Abstract
Anthropogenic disturbance is a relevant and widespread facilitator of environmental change and there is clear evidence that it impacts natural populations. While population-level responses to major anthropogenic changes have been well studied, individual physiological responses to mild disturbance can be equally critical to the long-term survival of a species, yet they remain largely unexamined. The current study investigated the impact of seemingly low-level anthropogenic disturbance (ecotourism) on stress responsiveness and specific fitness-related immune measures in different breeding stages of the marine iguana (Amblyrhynchus cristatus). Specifically, we found stress-induced elevations in plasma corticosterone among tourist-exposed populations relative to undisturbed populations. We also found changes in multiple immunological responses associated with stress-related effects of human disturbance, including bacterial killing ability, cutaneous wound healing, and hemolytic complement activity, and the responses varied according to reproductive state. By identifying health-related consequences of human disturbance, this study provides critical insight into the conservation of a well-known species that has a very distinct ecology. The study also broadens the foundation of knowledge needed to understand the global significance of various levels of human disturbance.
Keywords: Corticosterone, reproduction, immunity, tourism
Introduction
Anthropogenic disturbance is a relevant and widespread facilitator of environmental change, from local (e.g., tourism, pollution, habitat modification) to global (e.g., climate change) scales (Acevedo-Whitehouse and Duffus, 2009). Many of these changes have the capacity to affect the long-term persistence of natural populations. However, the impact of human disturbances on individual physiological responses and the resulting effects on long-term health, survival, and fitness of a species remains largely unexamined (Wikelski and Cooke, 2006). Understanding such effects is a critical component for effectively assessing a population’s risk of decline.
Recent studies demonstrate that physiological responses to tourism are pronounced in a number of species (Ellenberg et al., 2007; Romero and Wikelski, 2002; Walker et al., 2005), but it is unclear what effects these responses have on health and survival in natural populations. The direction and temporal nature of the response also varies depending on the species, age class, or even population (Mullner et al., 2004; Walker et al., 2005; Walker et al., 2006). While the vertebrate stress response is a key physiological response allowing organisms to cope with environmental change (Wingfield et al., 1998), there is no consensus as to the direction of the response or the downstream effects of an altered stress response in natural populations. Further, circulating glucocorticoid concentrations and stress responses are not static and instead vary according to duration of the stressor, sex, season, reproductive state, and body condition making interpretation of glucocorticoid results complicated (Breuner et al., 1999; Ilmonen et al., 2003; Moore and Jessop, 2003; Romero, 2002). These context-dependent modifications of the stress response in populations continuously exposed to human disturbances likely have subsequent physiological and behavioral effects. Therefore, to fully understand the consequences of human-induced alterations of endocrine responses on an individual, it is important to concomitantly measure related and relevant physiological systems, such as the immune system, and to examine these responses under different individual and environmental contexts (e.g., season, reproductive state, sex).
Investigating immune system function of individuals within given populations is highly relevant to understanding the dynamics of the population in question, especially considering the introduction of novel pathogens in many areas including the Galapagos (Dobson and Foufopoulos, 2001; Harvell et al., 2002; Wikelski et al., 2004). Additionally, connections between stress, glucocorticoids, and immunity are well established; glucocorticoid receptors are present on lymphatic tissues and leukocytes throughout the body, and stress induces modifications of the immune system (Cidlowski et al., 1996; Leonard and Song, 2002; Weyts et al., 1998; Wiegers et al., 1993). The specific relationships between stress and immunity, however, vary considerably with context. For example, a large number of studies have demonstrated immunosuppression under chronic stress conditions, while others have reported that acute stress can actually enhance immune responses (Dhabhar, 1998; Dhabhar, 2000; Dhabhar and McEwen, 1997). Thus, the notion of stress-induced immunosuppression is overly simplistic and the exact effects vary extensively depending on the type of stressor, the duration of the stress, the specific immune response measured, and the energetic and/or reproductive condition of the individuals (Dhabhar, 2000; Dhabhar and McEwen, 1997; French and Moore, 2008).
The current study investigated the impact of ecotourism, a seemingly low-level human disturbance, on stress responsiveness and specific fitness-related immune measures in the Galapagos marine iguana (Amblyrhynchus cristatus). Marine iguanas are an ideal model to examine anthropogenic change because the geography provides adjacent iguana populations which have varying levels of human disturbance. Tourist sites are regularly visited by hundreds of people daily; in contrast non-tourist sites are highly protected Federal Reserve lands that are rarely visited by people. Tourist and non-tourist iguana populations of similar size, density, and composition can be located along the same coastline less than a kilometer from each other. Previously, Romero and Wikelski investigated the effects of tourism on stress responsiveness of marine iguanas under extreme environmental conditions of increased water temperatures and low food availability (El Niño); however, it is unknown how individuals are affected by eco-tourism under non-extreme conditions (Romero and Wikelski, 2002). More importantly, it is unclear what these endocrine changes mean for individual health and survival.
In the current study we tested whether human disturbance results in modifications to immune responses critical to health in marine iguanas and whether these modifications were related to baseline or stress-induced levels of plasma corticosterone. The populations examined came from two pairs of sites. Populations of iguanas were chosen because they were of similar size and density. Each pair consisted of a site heavily exposed to tourists (up to hundreds of people daily) and a corresponding non-tourist, protected site ~1–2km away (rarely exposed to people). We assessed stress-induced changes in plasma corticosterone among populations to understand the impacts of human exposure. Then, to assess stress-related effects on the health of individuals in different populations, we measured a set of holistic immunological responses, including bacterial killing ability, cutaneous wound healing, and hemolytic complement activity. A myriad of individual and environmental factors can alter endocrine and immunological responses. In particular reproductive investment and hormones mediating reproduction and resource allocation, including testosterone and glucocorticoids, can often exacerbate stress-induced effects on an organism’s physiology, including immune function (Bentley et al., 1998; French et al., 2007a; French et al., 2007b; Nelson, 2004; Zapata et al., 1992; Zuk and Johnsen, 1998). Assessing an individual’s immunity in any given reproductive state and season may not accurately characterize their overall immunocompetence. To test the effects of season and reproductive state on endocrine and immune responses to human disturbance we sampled animals in two different seasons, non-breeding and breeding.
Materials and Methods
Animals and study sites
Reproduction in this species is highly seasonal (Wikelski et al., 1996; Wikelski et al., 2005). Thus we studied adult marine iguanas in both July 2008 (non-breeding season) and December 2008 (breeding season). Animals were studied at 2 pairs of sites on the island of Santa Cruz, at Estacion Charles Darwin (CDF) (Santa Cruz; 90º17′ W, 0º46′ S) and Tortuga Bay (TB) (Santa Cruz; 90º17′ W, 0º46′ S). Each pair consisted of a heavily touristed site and a site which did not allow access by tourists. Sites within a pair were approximately 1.5 – 2 kilometers apart and otherwise experience similar environmental conditions. In July 2008 we sampled only males (10 males at each of the four sites), and in December 2008 we sampled both males and females at each of the same 4 sites (CDF tourist = 7M, 11F; CDF undisturbed = 8M, 8F; TB tourist = 8M, 8F; TB undisturbed = 9M, 6F)
We selected animals of a similar body size (snout vent length (SVL) = 30.87 ± 0.39 cm; mean ± s.e.) and body mass (1.80 ± 0.07 kg; mean ± s.e.) to study. Selection of adults was random within the given size class of animals being studied. Individuals were caught by hand and marked with paint to allow observation and recapture. Paint markings have not altered behavior in previous studies (Audet and Wikelski, unpublished data). After stress series and measurements (see below) all animals were released at the site of capture. The same animals were used at both sampling times. This work was reviewed and approved by the Institutional Animal Care and Use Committee at Indiana University under protocol # 08-007.
Blood samples, stress series, and size measures
Glucocorticoids increase in response to capture in many species (Romero et al., 1997; Wingfield et al., 1995) including reptiles (Moore et al., 2000; Moore et al., 1991). Blood samples (1ml total) were collected from all animals within three minutes (0.5ml) of capture and again 30 minute post-capture (0.5ml), between 0800hr and 1200hr. Upon capture, we placed each iguana in an opaque cloth bag and a heparinized 1 ml syringe with a 25 gauge needle was used to collect a blood sample from the caudal vein. We used restraint as a stressor because it is known to elicit large increases in circulating corticosterone levels in reptiles, including marine iguanas (French et al., 2006; Romero and Wikelski, 2002). Following the initial bleed (< 3 minutes after initial disturbance), iguanas remained in cloth bags until the end of the 30 minute restraint period. After the restraint period, animals were re-bled to measure stress-related changes in corticosterone levels, the predominant glucocorticoid present in reptiles (reviewed in: Moore and Jessop, 2003). Samples were stored on ice until returning to the field camp (less than 6 hours). After collecting blood samples, ecto-parasite load (tick prevalence), body mass (g), and SVL (cm) were assessed for each animal. Ectoparasites were done by a careful visual scan (via the same observer). Total number of ectoparasite present (of all species) on an animal at the time of capture was recorded (with 97% repeatability of measurement). To measure body mass animals were placed in opaque cloth bags which were attached to a pesola scale. SVL was measured using a ruler placed along the ventral surface of the animal extending from the tip of the snout to the cloacal vent.
Hormone assays
Plasma was separated from the cells via centrifugation and stored at −20 ºC until assayed. Samples from the study were analyzed within two radioimmunoassays. Plasma samples were assayed in duplicate for testosterone (antibody #WLI-T3003-01916) and corticosterone (antibody from Fitzgerald #20-CR45) using a previously described and established protocol (Moore, 1986). For each sample we used an aliquot of the resuspended fractions to measure individual recoveries following extraction and chromatography. These recoveries were used to adjust the final sample concentration values to account for any losses during these procedures. The inter-assay and intra-assay variation for the separate hormones were 6.4%, 5.4% (assay 1) and 7.1% (assay 2) for testosterone, and 6.5%, 6.9% (assay 1) and 6.2% (assay 2) for corticosterone. Minimum detectable values were 0.4ng/ml for testosterone and 0.3ng/ml for corticosterone. All assayed samples fell within the standard curve for the assay (i.e., were detectable values).
Cutaneous wound healing
After stress challenge, all iguanas received a standardized cutaneous wound to assess wound healing capability, a generalized measure of immune function (French et al., 2006), following (French and Moore, 2008). The only modifications were that animals were given a 0.05 ml subcutaneous injection of lidocaine, rather than general anesthesia, and digital images were captured using a Canon Power Shot SX10. Wound size was determined using image analysis software (ImageJ, U. S. National Institutes of Health, Bethesda, Maryland, USA, http://rsb.info.nih.gov/ij/, 1997–2009). Percent change from initial wound area was calculated and used to assess healing over time.
Hemolytic complement activity assays
The complement pathway is part of the innate immune response and consists of a series of proteins present in the plasma (Janeway et al., 2005). Activation of the complement system initiates an enzymatic cascade, which leads to bacterial cell lysis, the formation of chemotactic peptides that attract immune cells, an increase in phagocytotic clearance of infected cells, and act as the major effector of the humoral immune response (Janeway et al., 2005; Mayer, 1948). This (CH50) complement assay can be used to qualitatively measure overall pathway integrity, cell lysis, and functional activity (Mayer, 1948).
There was no difference between hemolytic complement activity at baseline and 30 minute sampling times and we therefore only present baseline results here. Complement activity in plasma was measured based on methods previously described (Freedberg et al., 2008; Greives et al., 2006; Sinclair and Lochmiller, 2000). Modifications to the protocol include using duplicate 80 μl samples of both 1:5 and 1:10 dilutions of plasma. Hemolytic-complement activity was expressed as CH50 units/ml plasma, where 1 CH50 unit equals the reciprocal of the dilution of plasma required to lyse 50% of the SRBC in culture (Mayer, 1948). Because values violated the assumption of normality, all values were increased by two (so that all CH50 values were above one) and then normalized via a log transformation. The resulting values, ln(CH50 + 2), were then used in statistical analyses.
Bacterial killing assays
The bacterial killing assay characterizes a functionally relevant immune response that involves that action of phagocytes (macrophages, heterophils, and thrombocytes), opsonizing proteins (complement and acute phase proteins) and natural antibodies (predominantly IgM and IgA). Because this assay assesses the ability to eliminate an actual pathogen, it provides a functionally relevant assessment of host immune function. Bacterial killing assay were used to measure a functional response by the animal’s innate immune system against a relevant pathogen, Escherichia coli (Irene Tieleman et al., 2005). Working under a sterile laminar flow hood, we performed assays on both baseline and stress plasma samples, following Zysling et al (2009), using E. coli (EpowerTM Microorganisms #0483E7, ATCC 8739, MicroBioLogics, St. Cloud, MN) and a 1:5 dilution of plasma samples. Bactericidal capacity was calculated as the mean number of colonies for each sample which were run in duplicate, divided by the mean of colonies for the positive controls (three plates containing only media and bacterial solution), and multiplied by 100 (i.e., % bacteria killed relative to the positive control).
Statistical approaches
Breeding and non-breeding season animals were analyzed separately. Female data is not available for the non-breeding season. For the breeding season sexes were pooled for all analyses except testosterone, due to no obvious significant differences between males and females. Differences among all dependent measures and body mass and SVL were determined using separate two-way factorial (site X tourist) analyses of variance (ANOVA) (JMP 8.0.1 SAS Institute Inc., Cary, NC, USA). We used one-way ANOVAs to assess differences between the breeding and non-breeding seasons at individual sites. When significant interactions were present, separate one-way ANOVAs were used to test main effects. The changes in corticosterone concentrations and bactericidal ability over time (30 minute stress trial) were compared using two-way repeated measures ANOVAs (time X tourist), respectively, but, when significant interactions were present, separate one-way repeated measures ANOVAs were used to test main effects. We also preformed regression analysis of body mass against SVL to obtain residuals equivalent to “body condition”, to probe season, site, and tourist effects. Within subject comparisons that violated assumptions of sphericity were therefore Greenhouse-Geisser corrected. Post hoc comparisons between pairwise means were conducted using Fisher’s LSD tests when the overall ANOVAs were statistically significant. To meet the assumptions of normality for parametric statistics, all corticosterone and testosterone values were log-transformed prior to analysis. In all cases, differences between group means were considered statistically significant if p ≤ 0.05.
Results
Stress response and corticosterone
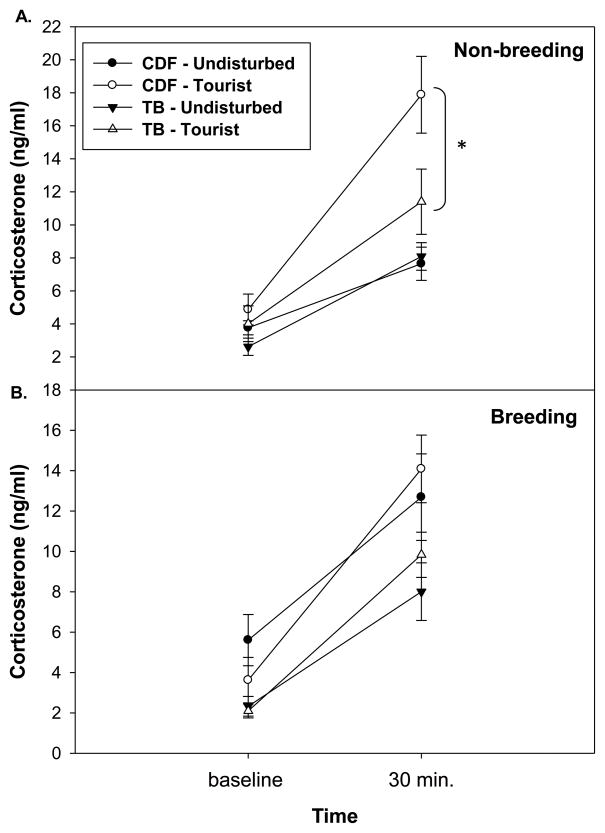
The repeated measures two-way ANOVA for corticosterone in non-breeding season animals (i.e., baseline, stress induced) showed that corticosterone concentrations were significantly elevated in animals at tourist sites relative to undisturbed sites during the non-breeding season (F = 7.40, df = 1, 36, P < 0.01; Figure 1a). There was also a significant effect of time (F = 12.17, df = 1, 36, P < 0.01; Figure 1a), where all animals showed increased corticosterone in response to restraint and handling stress. Lastly, there was a time by tourist effect interaction (F = 7.31, df = 1, 36, P = 0.01; Figure 1a), where animals at tourist sites showed a greater corticosterone response to stress than animals at undisturbed sites. There was no effect of site (CDF versus TB) or interactions according to site (all F < 2.69, all P > 0.11). Separate one-way ANOVAs revealed that effects of tourism on corticosterone levels were driven by stress-induced levels of corticosterone being significantly elevated at tourist sites (F = 12.29, df = 1, 39, P < 0.01), and that there were no statistical differences among sites for baseline levels of corticosterone (F = 1.60, df = 1, 39, P = 0.21).
Figure 1. Circulating corticosterone concentrations.
There is no difference between populations at baseline, but corticosterone is significantly elevated at the 30 minute stress sample in all animals. (a) In non-breeding animals corticosterone responses are significantly greater at tourist sites relative to undisturbed sites, (b) but not in breeding animals. Asterisks denote groups that differ significantly in their corticosterone response over time (α = 0.05 level). Error bars represent ± 1 standard error.
During the breeding season, corticosterone response to stress was again significant in all animals and different between tourist-exposed and undisturbed animals (all F > 6.90, all P < 0.01). However there was no significant overall effect of tourism on corticosterone levels over time (F = 1.23, df = 1, 59, P = 0.27; Figure 1b); instead, there was a significant effect of site (CDF versus TB) (F = 10.50, df = 1, 59, P < 0.01). Separate one-way ANOVAs revealed that TB sites had lower baseline and stress-induced levels of corticosterone than CDF sites (all F > 4.72, all P < 0.03). There was also no significant effect of tourism on either baseline or stress-induced corticosterone (one-way ANOVA; all F < 2.69, all P > 0.11).
Testosterone
There are inter-sex differences in circulating concentrations of testosterone in most species, and our results verified this (baseline testosterone: male mean = 13.96 ± 2.55ng/ml, female mean = 2.18 ± 1.35ng/ml; F = 155.75, df = 1, 62, P < 0.01). Thus, we analyzed male and female testosterone concentrations separately. Further, there was no significant change in circulating testosterone according to restraint stress (baseline mean both sexes = 9.27 ± 1.72ng/ml) versus stress levels (mean = 8.26 ± 1.57ng/ml; t = −0.79, df = 1, 102, P = 0.43) and therefore all reported statistics represent baseline concentrations of testosterone (there is no difference according to stress for either sex when analyzed together or separately). For females during the breeding season there were no significant differences among animals according to site, tourism, or interaction between effects (mean = 2.54 ± 1.63ng/ml; all F < 0.88, all P > 0.35; Table 1).
Table 1. Testosterone concentrations (ng/ml).
Baseline circulating testosterone concentrations in male marine iguanas from different populations in both non-breeding and breeding seasons, ± one standard error of the mean.
| Site | N | Non-breeding (ng/ml) | Breeding (ng/ml) |
|---|---|---|---|
| CDF - undisturbed | 17 | 1.39 ± 0.73 | 9.73 ± 2.38* |
| CDF - tourist | 16 | 0.37 ± 0.08 | 29.96 ± 4.94 |
| TBN - undisturbed | 15 | 2.41 ± 1.59 | 43.10 ± 13.26 |
| TBT - tourist | 14 | 0.70 ± 0.23 | 34.70 ± 5.84 |
Asterisks denote significant differences among treatment groups (α = 0.05).
During the non-breeding season, males showed no significant differences according to site, tourism, or interactions between site and tourism (all F < 1.21, all P > 0.28; Table 1). However, during the breeding season, male testosterone showed a significant interaction between site and tourism (F = 5.59, df = 1, 28, P = 0.03; Table 1). Separate one-way ANOVAs, revealed that testosterone concentrations were significantly higher in animals at the tourist relative to the undisturbed CDF sites (F = 13.66, df = 1, 14 P < 0.01). However, there was no difference in circulating testosterone between tourist and undisturbed sites at TB (F = 0.01, df = 1, 13 P = 0.92). Looking within tourist and undisturbed groups, we saw significant differences between the TB and CDF sites. Specifically, animals at the TB undisturbed site had higher testosterone levels than animals at the CDF undisturbed site (F = 7.78, df = 1, 13 P = 0.02), but there was no difference in testosterone levels between animals at the two tourist sites (CDF tourist versus TB tourist; F = 0.42, df = 1, 14 P = 0.53).
Body size, mass, and ectoparasites
Body mass and SVL did not vary among sites within a season (all F < 0.91, all P > 0.34). While there was no seasonal variation in SVL mass at any site (all F < 3.57, all P > 0.07), there was an increase in body mass from the non-breeding season to the breeding season at both the CDF undisturbed site and the TB tourist site (all F > 4.47, all P < 0.05), but not the CDF tourist or TB undisturbed sites (all F < 3.43, all P > 0.08). These results suggest seasonal changes in body condition. We probed the effects of season, tourist exposure, and site on body condition (residuals of regression of body mass against SVL). We found a significant effect of season with increasing body condition in breeding season (F = 9.88, df = 1, 102, P < 0.01), but did not find a significant effect of site, tourism, nor interaction between site and tourist exposure on body condition (all F < 0.66, all P > 0.20).
During the non-breeding season, there was no effect of site, tourism, or interaction between site and tourism on tick prevalence (all F < 2.74, all P > 0.11; Table 2). However, during the breeding season tick prevalence was significantly affected by tourism (F = 10.19, df = 1, 62, P < 0.01), but not site, and there was no interaction between variables (all F < 0.16, all P > 0.69). Animals at touristed sites had fewer ticks than animals at undisturbed sites.
Table 2. Ectoparasite counts.
Number of ectoparasites present on male and female male marine iguanas from different populations in breeding season and males only in the non-breeding season, ± one standard error of the mean.
| Site | N | Non-breeding | Breeding |
|---|---|---|---|
| CDF - undisturbed | 17 | 16.40 ± 2.29 | 17.50 ± 5.03 |
| CDF - tourist | 16 | 14.30 ± 3.61 | 4.50 ± 1.22* |
| TBN - undisturbed | 15 | 24.40 ± 2.76 | 18.73 ± 2.84 |
| TBT - tourist | 14 | 23.30 ± 4.57 | 8.13 ± 1.18* |
Asterisks denote significant differences among treatment groups (α = 0.05).
Cutaneous wound healing
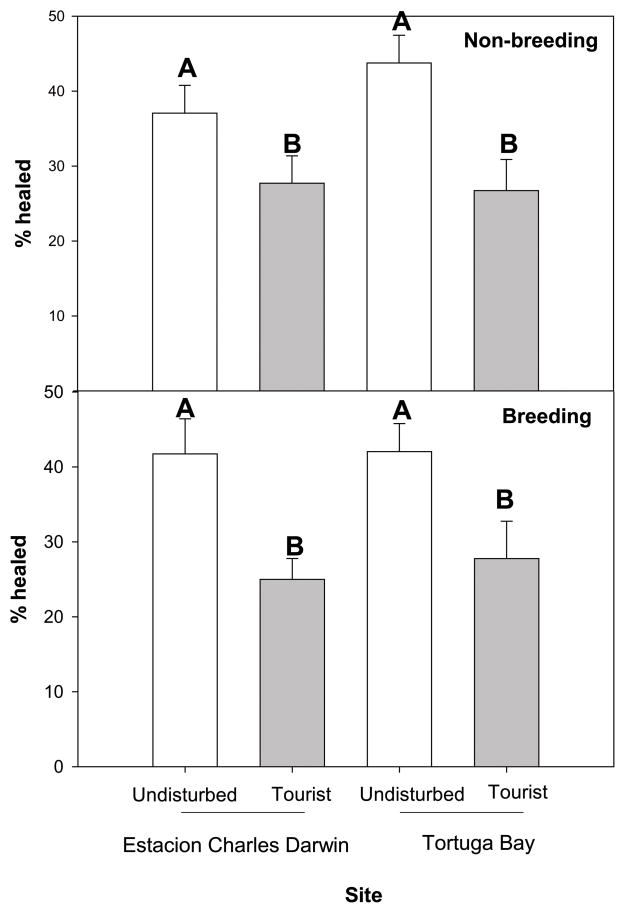
Separate two-way ANOVAs for non-breeding and breeding season animals revealed that there was a significant suppression of wound healing at tourist sites (all F > 7.79, all P < 0.01; Figure 2a, 2b), but no difference in healing rate between sites (CDF versus TB) nor a site by tourism interactions (all F < 1.90, all P > 0.18).
Figure 2. Cutaneous wound healing.
Cutaneous wound healing is significantly suppressed at tourist sites compared to undisturbed sites, in both (a) non-breeding and (b) breeding seasons. Differing letters denote groups that differ significantly (α = 0.05 level). Error bars represent ± 1 standard error.
Hemolytic complement activity
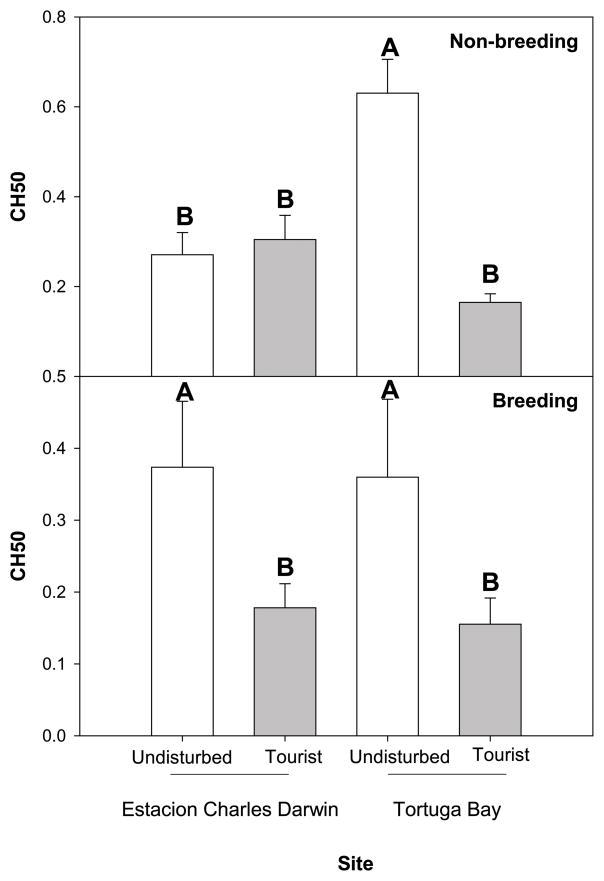
The results for complement activity (measured via CH50, see methods) show that, during the non-breeding season, the animals at the two sites, CDF versus TB, responded differently to tourism (F = 21.95, df = 1, 39, P < 0.01; Figure 3a). Separate one-way ANOVAs, revealed that CH50 was significantly lower at the TB tourist site relative to the TB undisturbed site (F = 36.08, df = 1, 19, P < 0.01), but there was no significant difference between the tourist and undisturbed CDF sites (F = 0.21, df = 1,19 P = 0.65). Further, looking within tourist and undisturbed groups, we saw significant differences according to site, explaining the given interaction. Specifically, the animals at the TB undisturbed site had greater CH50 than those at the CDF undisturbed site (F = 15.99, df = 1, 19 P < 0.01), and CDF tourist site animals had greater CH50 than TB tourist site animals (F = 5.97, df = 1, 19 P = 0.03).
Figure 3. Total hemolytic complement activity.
CH50 = Total hemolytic complement activity is significantly suppressed at tourist sites compared to undisturbed sites, in both (a) non-breeding and (b) breeding seasons, except for non-breeding CDF (Estacion Charles Darwin) populations. Differing letters denote groups that differ significantly (α = 0.05 level). Error bars represent ± 1 standard error.
During the breeding season, there was a significant effect of tourism on CH50, such that animals from tourism sites had significantly reduced complement activity relative to animals from undisturbed sites (F = 3.83, df = 1, 63, P = 0.05; Figure 3b). There was no significant difference between CDF and TB sites or interaction between site and tourism (all F < 0.02, all P > 0.90).
Bactericidal ability
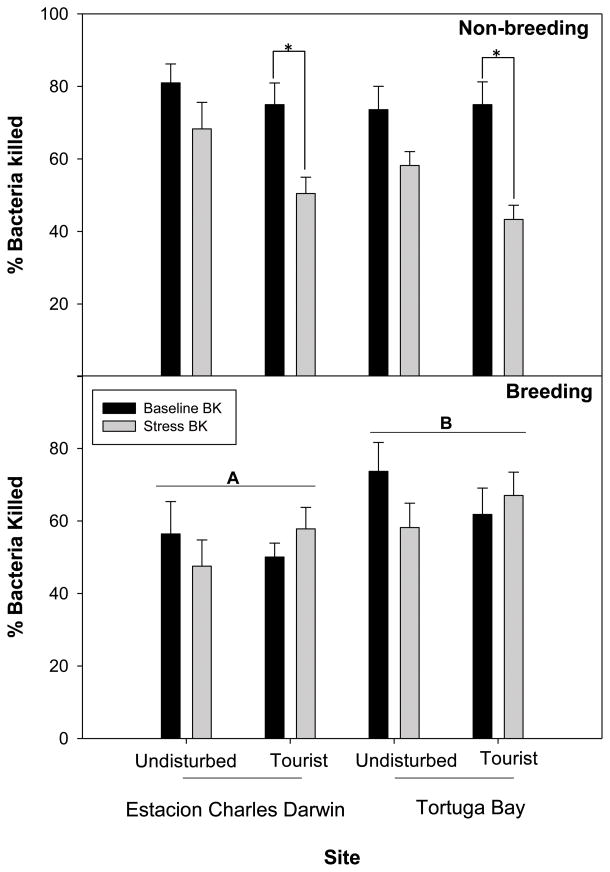
There were significant tourism by time interactions for both non-breeding and breeding season analyses (two-way repeated measures ANOVA), suggesting that bacterial killing response over time differed in animals from tourist sites relative to animals from undisturbed sites (all F > 4.41, all P < 0.04). We therefore performed separate one-way repeated measures ANOVAs to test main effects in both cases.
During the non-breeding season, there was a significant effect of time and significant time by tourist interaction (all F > 4.41, all P < 0.04). Separate two-way ANOVAs revealed that there was no significant difference according to site, tourism, or interaction for baseline bacterial killing ability (all F < 0.68, all P > 0.41). However, the observed repeated measures ANOVA effects seem to be driven by significant stress-induced suppression of bacterial killing at tourist sites relative to undisturbed sites (F = 5.57, df = 1, 36, P = 0.02; Figure 4a). There was no effect of site (CDF versus TB) and no interaction between sites and tourism (all F < 2.55, all P > 0.12).
Figure 4. Bactericidal capacity.
(a) In non-breeding animals there was no significant difference in baseline bactericidal ability among populations; however killing ability was significantly suppressed during stress at tourist sites. Asterisks denote a significant decrease in killing at 30 minute stress sample (α = 0.05 level). (b) In breeding animals bacterial killing ability was significantly greater at TB sites than at CDF (Estacion Charles Darwin) sites. Differing letters denote groups that differ significantly (α = 0.05 level). Error bars represent ± 1 standard error.
During the breeding season, we saw a similar bacterial killing response, where there was a significant effect of time and significant time by tourist interaction (all F > 10.34, all P < 0.01), such that bacterial killing ability changed over time, and that this change in bactericidal ability varied according to tourist exposure. Separate two-way ANOVAs revealed that there was an effect of site on baseline killing ability, with both TB sites having greater bacterial killing ability than both CDF sites (F = 4.86, df = 1, 61, P = 0.03; Figure 4b), but there was no effect of tourism or site by tourism interaction for bacterial killing ability (all F < 0.79, all P > 0.38), such that all animals bactericidal response was initially similar. After stress, there was no significant effect of site, tourism, or site by tourism interaction for bacterial killing ability (all F < 1.84, all P > 0.18).
Discussion
The current study presents one of the most comprehensive examinations of human-induced effects on stress and immunological responses in natural populations to date, demonstrating that ecotourism alters basic physiological functions likely to affect consequences on survival and fitness in many species. Environmental perturbations can cause stress-induced increases in plasma corticosterone, which have a suite of effects on organismal physiology, including alterations of immunological responses. We examined multiple endocrine and immune responses to human disturbance at two separate pairs of sites, tested the effects of reproductive state on these responses, and found that tourism alters basic physiological functions that likely affect survival and fitness. While we have not yet looked at individual or population survival, the data clearly demonstrate a strong negative effect of mild human disturbance on both stress-induced changes in circulating hormone levels and multiple ecologically relevant immune responses. Further, these responses varied according to reproductive season, revealing the potential to miss critical effects of disturbance if not examined under multiple individual and seasonal contexts.
Stress response
We found significant alterations in corticosterone responses to restraint stress in animals at touristed sites. Non-breeding animals from tourist sites responded more strongly to restraint stress than did animals from undisturbed sites. Stress-induced levels of corticosterone were significantly elevated in animals at tourist sites, but there was no difference in baseline levels of corticosterone among sites. In breeding season animals a similar pattern persisted but was not significant. Because the only observable difference between populations was exposure to people, the results likely indicate common physiological responses to different environmental contexts. Further, it is also possible that tourist activity differed between the two sampling dates at the sites. We also cannot rule out the possibility that there may be physiological adaptations to human disturbance at tourist sites. However, the negative downstream consequences on immunity make adaptations less likely. Supporting the former, a previous study also found altered stress response to tourism (Romero and Wikelski, 2002), but the response was in the opposite direction from what was previously shown in marine iguanas and expected for a continually exposed population. This suggests that endocrine responses to human disturbance in this species are plastic. Romero and Wikelski found that marine iguanas in a different population (Punta Espinosa, Fernandina Island) regularly exposed to tourism had lower stress-induced corticosterone elevations in comparison to iguanas from undisturbed populations (2002).
Additionally, individuals from the current study and Romero et al. 2002 study were from different populations on different islands and these populations may likely experience different environmental conditions, including tourist load, leading to differences in the ability to respond to stressors. It is also significant that the previous report was during an extreme El Niño environmental event (Romero and Wikelski, 2002). Under El Niño conditions animals may have maximized their ability to increase in corticosterone and are unable to respond further (i.e., in response to tourism). Further, corticosterone levels were predicative of survival during El Niño events (Romero and Wikelski, 2001). Combined, these previous studies and the current one strongly suggest that tourism leads to large alterations of a system that needs to remain in homeostatic balance.
Conflicting stress data in natural populations are commonplace in the literature, even within species. For example, in magellanic penguins (Spheniscus magellanicus) juveniles show elevated responses to stress at tourist sites whereas adults show suppressed responses to stress at the same sites (Walker et al., 2005; Walker et al., 2006). It is important to clarify that altered stress responsiveness in iguanas and other species may reflect either an altered response capability or an altered perception of a potential stressor (e.g., habituation). Future studies investigating ability to respond to negative feedback (e.g. DEX) or the capacity of the adrenals to respond to ACTH may help to elucidate the underlying mechanisms responsible for observed differences. While acclimatization clearly alters the stress response, its overall effects on organismal health and susceptibility are unknown. Thus, by examining the effect of the stress response on other physiological parameters such as the immune response, results better elucidate the impact that changes in stress have on organism susceptibility to abiotic and biotic factors and thus on survival and ultimately population persistence.
Immunological responses
In the present study, we found clear and pronounced effects of tourism on multiple immunological responses during both the non-breeding and breeding seasons. In general, animals exposed to tourism had suppressed responses relative to animals at undisturbed sites. The general trend towards immunosuppression at tourism sites holds across seasons, although it is not surprising that some inter-season variation does exist. First, wound healing was suppressed at tourist sites relative to undisturbed sites across seasons and breeding stages. Healing is acknowledged as a stress-sensitive and integrative measure of immunity, where corticosterone concentrations are inversely related to healing rate in chronically stressed tree lizards (French et al., 2006). Further, stress can increase susceptibility to bacterial infection during wound healing in mice (Rojas et al., 2000). Wound healing is particularly important to marine iguanas, as abrasions, open wounds, scars, and missing digits are commonplace in individuals across populations, where ~43% of animals sampled had a scarring from previous injury (personal observations, 2008). These injuries are likely related to their natural history, in particular, their foraging mode entails grasping slick and jagged lava rocks in intertidal zones (females) and subtidal zones (males) with strong currents and waves.
We observed a similar tourist-associated suppression in total hemolytic complement activity (CH50). This measure demonstrates a functional response of the primary complement pathway to induce hemolysis of foreign erythrocytes. The complement system is critical for both innate and humoral immune responses, whereby complement activity is necessary for the destruction (i.e., lysis, phagocytosis) of foreign bacteria and viruses and also plays a role in the initiation of humoral antibody responses to antigens (Carroll, 1998; Carroll, 2000; Johnston et al., 1969). Similar to our findings, studies in rats have found changes in complement activity (CH50) in response to chronic mild stress and elevated corticosterone (Ayensu et al., 1995).
The individual’s ability to kill foreign bacteria, showed a disparate pattern from other immune responses. Bacterial killing ability was not different among populations at baseline sampling. However there was a stress-induced suppression of killing ability at tourist sites (in non-breeding season), suggesting that this immunosuppression was mediated via activation of the hypothalamic-pituitary-adrenal (HPA) axis. In vitro killing ability of plasma measures a functionally relevant and integrative immune response, which includes phagocytic activity (macrophages, heterophils, and thrombocytes), opsonizing proteins (complement and acute phase proteins) and natural antibodies (i.e., IgM and IgY). Killing of the bacteria (E. coli) utilized in the current study requires a complement-dependent response, but, unlike the measuring of total hemolytic complement activity, killing of E. coli also relies on the presence of natural antibodies and phagocytes. This functional difference in the immune response could account for some of the variability in the results between the two different responses measured (bacterial killing ability vs. total hemolytic complement activity). Therefore, based on these results, animals at tourist sites may not be immunocompromised under non-stressful conditions, but their innate immune defenses may be compromised if/when they are exposed to acute or chronic stressors.
It is evident that all immunological responses measured are, under some circumstances, sensitive to tourist exposure. The proximate mechanisms regulating this relationship, however, are not yet known. For example, HPA activity and immunological responses are both affected by human exposure but the data do not support whether there is a causal relationship or whether they are both responding to the same stimulus. Further, changes in season also appear important for both endocrine and immune responses, but it is unclear whether the seasonality of the responses are related to reproduction (e.g., seasonal changes in sex steroids, reproduction-induced changes in social interactions, high energetic costs of reproduction), related to energy resources (e.g., seasonal changes in food availability), or a function of both. For example, breeding song sparrows (Melospiza melodia) display suppressed sickness behavior, lower body fat, and higher corticosterone relative to non-breeding (i.e., winter) individuals (Owen-Ashley and Wingfield, 2006). Therefore, known seasonal changes in marine iguana behavior (Wikelski et al., 1996; Wikelski et al., 2005) and energy expenditure likely contribute to the observed differences in immunity observed across seasons.
Given the complex nature of immunological responses, it is not surprising that we observed variations in the measured immune responses according to reproductive season, however a general trend of lower innate immune defenses in response to tourism was clearly observed. Evidence to date suggests a multifaceted interaction among various physiological processes impacts and defines an immune response. For example, reproduction especially is known to greatly influence immunocompetence (Abell, 2000; Grassman and Crews, 1990; Jessop, 2001; Tanriverdi et al., 2003; Wingfield and Sapolsky, 2003; Woodley and Moore, 2002). Resource availability and reproductive state are highly indicative of immunocompetence in male and female tree lizards, Urosuarus ornatus (French et al., 2007a; French and Moore, 2008). Further, testosterone and food supplementation interacted to increase innate immunity in sagebrush lizards, Sceloporus graciosus (Ruiz et al., 2009). Differences in reproductive strategy, including energy expenditure and hormone concentrations can result in differences in immune function, such as male marine iguanas employing different strategies (Berger et al., 2005), where during reproduction glucocorticoids suppressed cell-mediated immunity in territorial but not alternative strategy male marine iguanas (Berger et al., 2005). Given the prolific evidence for context-dependent effects of both individual state and stress on immunity, differences in the stress of ecotourism are likely a contributing factor to the observed differences in immunity in the current study, rendering certain animals more susceptible to human-induced change.
Conclusions
Tourism significantly alters physiological parameters known to affect fitness and survival including the stress response, multiple important immunological responses, and potentially plasma testosterone concentrations. Future work should strive to link these parameters more directly to individual and population-level survival and fitness. Additionally, following populations under differing environmental conditions (e.g. El Niño, La Niña) will help clarify the relationship between ecotourism, the environment and fitness-related physiological parameters. Understanding these interactions will provide novel insights into the effects of anthropogenic disturbance, and will also provide much needed biological knowledge regarding unusual and/or threatened species. Future studies will provide a more comprehensive examination of additional populations, including specific reproductive stages, reproductive strategies, and coupled behavioral effects and direct affects on survival between populations. We will hopefully shed light on novel approaches to mitigate the deleterious effects of human activities on natural populations.
Research Highlights.
Anthropogenic disturbance (ecotourism) alters stress responsiveness and specific fitness-related immune measures in marine iguanas.
Exposure to tourism increases stress-induced plasma corticosterone levels.
Animals exposed to tourism regularly, show suppressed immunity.
The effects of tourist exposure vary according to season and animal breeding condition.
Acknowledgments
We thank Martin Wikelski for help with logistics and permits. We thank the Charles Darwin Foundation and Parque National de Galapagos. We also thank Christian Pilamunga, Dianne DeNardo, Dominick DeNardo, and Derek DeNardo for help in the field, and Mayte Ruiz and David Kabelik for help with radioimmunoassays. We thank Susan Durham for statistical consultations regarding the analyses. Lastly, we thank Trevor Brown for help with all parts of this project from conception to manuscripts. Funding for this project was provided by the American Philosophical Society, Franklin Research Grant and the Common Themes in Reproductive Diversity Training grant, NIH no. HD 049336-04. This publication is contribution number 2022 of the Charles Darwin Foundation for the Galapagos Islands.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abell AJ. Costs of reproduction in male lizards, Sceloporus virgatus. Oikos. 2000;88:630–640. [Google Scholar]
- Acevedo-Whitehouse K, Duffus ALJ. Effects of environmental change on wildlife health. Philosophical Transactions of the Royal Society B: Biological Sciences. 2009;364:3429–3438. doi: 10.1098/rstb.2009.0128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ayensu WK, Pucilowski O, Mason GA, Overstreet DH, Rezvani AH, Janowsky DS. Effects of chronic mild stress on serum complement activity, saccharin preference, and corticosterone levels in Flinders lines of rats. Physiology and Behavior. 1995;57:165–9. doi: 10.1016/0031-9384(94)00204-i. [DOI] [PubMed] [Google Scholar]
- Bentley GE, Demas GE, Nelson RJ, Ball GF. Melatonin, immunity and cost of reproductive state in male European starlings. Proceedings of the Royal Society of London Series B-Biological Sciences. 1998;265:1191–1195. doi: 10.1098/rspb.1998.0418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berger S, Martin LB, Wikelski M, Romero LM, Kalko EKV, Vitousek MN, Rodl T. Corticosterone suppresses immune activity in territorial Galapagos marine iguanas during reproduction. Hormones and Behavior. 2005;47:419–429. doi: 10.1016/j.yhbeh.2004.11.011. [DOI] [PubMed] [Google Scholar]
- Breuner CW, Wingfield JC, Romero LM. Diel rhythms of basal and stress-induced corticosterone in a wild, seasonal vertebrate, Gambel's white-crowned sparrow. Journal of Experimental Zoology. 1999;284:334–342. [PubMed] [Google Scholar]
- Carroll MC. The role of complement and complement receptors in induction and regulation of immunity. Annual Review of Immunology. 1998;16:545–568. doi: 10.1146/annurev.immunol.16.1.545. [DOI] [PubMed] [Google Scholar]
- Carroll MC. The role of complement in B cell activation and tolerance. Advances in Immunology. 2000;74:61–88. doi: 10.1016/s0065-2776(08)60908-6. [DOI] [PubMed] [Google Scholar]
- Cidlowski JA, King KL, EvansStorms RB, Montague JW, Bortner CD, Hughes FM. The biochemistry and molecular biology of glucocorticoid-induced apoptosis in the immune system. Recent Progress in Hormone Research. 1996;51:457–491. [PubMed] [Google Scholar]
- Dhabhar FS. Stress-induced enhancement of cell-mediated immunity. Neuroimmunomodulation. 1998:359–372. doi: 10.1111/j.1749-6632.1998.tb09575.x. [DOI] [PubMed] [Google Scholar]
- Dhabhar FS. Acute stress enhances while chronic stress suppresses skin immunity - The role of stress hormones and leukocyte trafficking. Neuroimmunomodulation. 2000:876–893. doi: 10.1111/j.1749-6632.2000.tb05454.x. [DOI] [PubMed] [Google Scholar]
- Dhabhar FS, McEwen BS. Acute stress enhances while chronic stress suppresses cell-mediated immunity in vivo: A potential role for leukocyte trafficking. Brain Behavior and Immunity. 1997;11:286–306. doi: 10.1006/brbi.1997.0508. [DOI] [PubMed] [Google Scholar]
- Dobson A, Foufopoulos J. Emerging infectious pathogens of wildlife. Philosophical Transactions of the Royal Society of London. Series B: Biological Sciences. 2001;356:1001–12. doi: 10.1098/rstb.2001.0900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellenberg U, Setiawan AN, Cree A, Houston DM, Seddon PJ. Elevated hormonal stress response and reduced reproductive output in Yellow-eyed penguins exposed to unregulated tourism. General and Comparative Endocrinology. 2007;152:54–63. doi: 10.1016/j.ygcen.2007.02.022. [DOI] [PubMed] [Google Scholar]
- Freedberg S, Greives TJ, Ewert MA, Demas GE, Beecher N, Nelson CE. Incubation environment affects immune system development in a turtle with environmental sex determination. Journal of Herpetology. 2008;42:536–541. doi: 10.1670/07-133.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- French SS, DeNardo DF, Moore MC. Trade-offs between the reproductive and immune systems: Facultative responses to resources or obligate responses to reproduction? American Naturalist. 2007a;170:79–89. doi: 10.1086/518569. [DOI] [PubMed] [Google Scholar]
- French SS, Matt KS, Moore MC. The effects of stress on wound healing in male tree lizards (Urosaurus ornatus) General and Comparative Endocrinology. 2006;145:128–132. doi: 10.1016/j.ygcen.2005.08.005. [DOI] [PubMed] [Google Scholar]
- French SS, McLemore R, Vernon B, Johnston GIH, Moore MC. Corticosterone modulation of reproductive and immune systems trade-offs in female tree lizards: long-term corticosterone manipulations via injectable gelling material. Journal of Experimental Biology. 2007b;210:2859–2865. doi: 10.1242/jeb.005348. [DOI] [PubMed] [Google Scholar]
- French SS, Moore MC. Immune function varies with reproductive stage and context in female and male tree lizards, Urosaurus ornatus. General and Comparative Endocrinology. 2008;155:148–156. doi: 10.1016/j.ygcen.2007.04.007. [DOI] [PubMed] [Google Scholar]
- Grassman M, Crews D. Ovarian and adrenal-function in the parthenogenetic whiptail lizard Cnemidophorus uniparensin the field and laboratory. General and Comparative Endocrinology. 1990;76:444–450. doi: 10.1016/0016-6480(89)90141-x. [DOI] [PubMed] [Google Scholar]
- Greives TJ, McGlothlin JW, Jawor JM, Demas GE, Ketterson ED. Testosterone and innate immune function inversely covary in a wild population of breeding dark-eyed juncos (Junco hyemalis) Functional Ecology. 2006;20:812–818. [Google Scholar]
- Harvell CD, Mitchell CE, Ward JR, Altizer S, Dobson AP, Ostfeld RS, Samuel MD. Climate Warming and Disease Risks for Terrestrial and Marine Biota. Science. 2002;296:2158–2162. doi: 10.1126/science.1063699. [DOI] [PubMed] [Google Scholar]
- Ilmonen P, Hasselquist D, Langefors A, Wiehn J. Stress, immunocompetence and leukocyte profiles of pied flycatchers in relation to brood size manipulation. Oecologia. 2003;136:148–154. doi: 10.1007/s00442-003-1243-2. [DOI] [PubMed] [Google Scholar]
- Irene Tieleman B, Williams JB, Ricklefs RE, Klasing KC. Constitutive innate immunity is a component of the pace-of-life syndrome in tropical birds. Proc R Soc B-Biol Sci. 2005;272:1715–1720. doi: 10.1098/rspb.2005.3155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janeway C, Travers P, Walport M, Shlomchik M. Immunobiology: The Immune System in Health and Disease. 6. Garland Science; New York: 2005. [Google Scholar]
- Jessop TS. Modulation of the adrenocortical stress response in marine turtles (Cheloniidae): evidence for a hormonal tactic maximizing maternal reproductive investment. Journal of Zoology. 2001;254:57–65. [Google Scholar]
- Johnston RB, Jr, Klemperer MR, Alper CA, Rosen FS. The enhancement of bacterial phagocytosis by serum: The role of complement components and two cofactors. Journal of Experimental Medicine. 1969;129:1275–1290. doi: 10.1084/jem.129.6.1275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leonard BE, Song C. Changes in the immune system in rodent models of depression. International Journal of Neuropsychopharmacology. 2002;5:345–356. doi: 10.1017/S1461145702003140. [DOI] [PubMed] [Google Scholar]
- Mayer MM. Complement and complement fixation. In: Kabat EA, Mayer MM, editors. Experimental Immunochemistry. Charles C. Thomas; Springfield, Ill: 1948. [Google Scholar]
- Moore IT, Jessop TS. Stress, reproduction, and adrenocortical modulation in amphibians and reptiles. Hormones and Behavior. 2003;43:39–47. doi: 10.1016/s0018-506x(02)00038-7. [DOI] [PubMed] [Google Scholar]
- Moore IT, Lemaster MP, Mason RT. Behavioural and hormonal responses to capture stress in the male red-sided garter snake, Thamnophis sirtalis parietalis. Animal Behaviour. 2000;59:529–534. doi: 10.1006/anbe.1999.1344. [DOI] [PubMed] [Google Scholar]
- Moore MC. Elevated testosterone levels during nonbreeding-season territoriality in a fall-breeding lizard, Sceloporus jarrovi. Journal of Comparative Physiology a-Sensory Neural and Behavioral Physiology. 1986;158:159–163. doi: 10.1007/BF01338559. [DOI] [PubMed] [Google Scholar]
- Moore MC, Thompson CW, Marler CA. Reciprocal changes in corticosterone and testosterone levels following acute and chronic handling stress in the tree lizard, Urosaurus ornatus. General and Comparative Endocrinology. 1991;81:217–226. doi: 10.1016/0016-6480(91)90006-r. [DOI] [PubMed] [Google Scholar]
- Mullner A, Linsenmair KE, Wikelski M. Exposure to ecotourism reduces survival and affects stress response in hoatzin chicks (Opisthocomus hoazin. )Biological Conservation. 2004;118:549–558. [Google Scholar]
- Nelson RJ. Seasonal immune function and sickness responses. Trends in Immunology. 2004;25:187–192. doi: 10.1016/j.it.2004.02.001. [DOI] [PubMed] [Google Scholar]
- Owen-Ashley NT, Wingfield JC. Seasonal modulation of sickness behavior in free-living northwestern song sparrows (Melospiza melodia morphna) Journal of Experimental Biology. 2006;209:3062–3070. doi: 10.1242/jeb.02371. [DOI] [PubMed] [Google Scholar]
- Rojas IG, Padgett DA, Sheridan JF, Marucha PT. Effects of restraint stress on susceptibility of bacterial infection during cutaneous wound healing. Journal of Dental Research. 2000;79:393–393. [Google Scholar]
- Romero LM. Seasonal changes in plasma glucocorticoid concentrations in free-living vertebrates. General and Comparative Endocrinology. 2002;128:1–24. doi: 10.1016/s0016-6480(02)00064-3. [DOI] [PubMed] [Google Scholar]
- Romero LM, Ramenofsky M, Wingfield JC. Season and Migration Alters the Corticosterone Response to Capture and Handling in an Arctic Migrant, the White-Crowned Sparrow (Zonotrichia leucophrys gambelii) Comparative Biochemistry and Physiology Part C: Pharmacology, Toxicology and Endocrinology. 1997;116:171–177. doi: 10.1016/s0742-8413(96)00208-3. [DOI] [PubMed] [Google Scholar]
- Romero LM, Wikelski M. Corticosterone levels predict survival probabilities of Galapagos marine iguanas during El Nino events. Proceedings of the National Academy of Sciences of the United States of America. 2001;98:7366–7370. doi: 10.1073/pnas.131091498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romero LM, Wikelski M. Exposure to tourism reduces stress-induced corticosterone levels in Galapagos marine iguanas. Biological Conservation. 2002;108:371–374. [Google Scholar]
- Ruiz M, French SS, Demas GE, Martins EP. Food supplementation and testosterone interact to influence reproductive behavior and immune function in Sceloporus graciosus. Hormones and Behavior. 2009 doi: 10.1016/j.yhbeh.2009.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinclair JA, Lochmiller RL. The winter immunoenhancement hypothesis: associations among immunity, density, and survival in prairie vole (Microtus ochrogaster) populations. Can J Zool. 2000;78:254–264. [Google Scholar]
- Tanriverdi F, Silveira LFG, MacColl GS, Bouloux PMG. The hypothalamic-pituitary-gonadal axis: immune function and autoimmunity. Journal of Endocrinology. 2003;176:293–304. doi: 10.1677/joe.0.1760293. [DOI] [PubMed] [Google Scholar]
- Walker BG, Boersma PD, Wingfield JC. Physiological and behavioral differences in Magellanic Penguin chicks in undisturbed and tourist-visited locations of a colony. Conservation Biology. 2005;19:1571–1577. [Google Scholar]
- Walker BG, Boersma PD, Wingfield JC. Habituation of adult magellanic penguins to human visitation as expressed through behavior and corticosterone secretion. Conservation Biology. 2006;20:146–154. doi: 10.1111/j.1523-1739.2005.00271.x. [DOI] [PubMed] [Google Scholar]
- Weyts FAA, Verburg-van Kemenade BML, Flik G. Characterisation of glucocorticoid receptors in peripheral blood leukocytes of carp, Cyprinus carpio L. General and Comparative Endocrinology. 1998;111:1–8. doi: 10.1006/gcen.1998.7080. [DOI] [PubMed] [Google Scholar]
- Wiegers GJ, Croiset G, Reul J, Holsboer F, Dekloet ER. Differential- Effects of Corticosteroids on Rat Peripheral-Blood T-Lymphocyte Mitogenesis in-Vivo and in-Vitro. American Journal of Physiology. 1993;265:E825–E830. doi: 10.1152/ajpendo.1993.265.6.E825. [DOI] [PubMed] [Google Scholar]
- Wikelski M, Carbone C, Trillmich F. Lekking in marine iguanas: Female grouping and male reproductive strategies. Animal Behaviour. 1996;52:581–596. [Google Scholar]
- Wikelski M, Cooke SJ. Conservation physiology. Trends in Ecology & Evolution. 2006;21:38–46. doi: 10.1016/j.tree.2005.10.018. [DOI] [PubMed] [Google Scholar]
- Wikelski M, Foufopoulos J, Vargas H, Snell H. Galapagos birds and diseases: Invasive pathogens as threats for island species. Ecology and Society. 2004;9 [Google Scholar]
- Wikelski M, Steiger SS, Gall B, Nelson KN. Sex, drugs and mating role: testosterone-induced phenotype-switching in Galapagos marine iguanas. Behavioral Ecology. 2005;16:260–268. [Google Scholar]
- Wingfield JC, Maney DL, Breuner CW, Jacobs JD, Lynn S, Ramenofsky M, Richardson RD. Ecological Bases of Hormone--Behavior Interactions: The “Emergency Life History Stage”. Amer Zool. 1998;38:191–206. [Google Scholar]
- Wingfield JC, O'Reilly KM, Astheimer LB. Modulation of the Adrenocortical Responses to Acute Stress in Arctic Birds: A Possible Ecological Basis. Amer Zool. 1995;35:285–294. [Google Scholar]
- Wingfield JC, Sapolsky RM. Reproduction and resistance to stress: When and how. Journal of Neuroendocrinology. 2003;15:711–724. doi: 10.1046/j.1365-2826.2003.01033.x. [DOI] [PubMed] [Google Scholar]
- Woodley SK, Moore MC. Plasma corticosterone response to an acute stressor varies according to reproductive condition in female tree lizards (Urosaurus ornatus) General and Comparative Endocrinology. 2002;128:143–148. doi: 10.1016/s0016-6480(02)00068-0. [DOI] [PubMed] [Google Scholar]
- Zapata AG, Varas A, Torroba M. Seasonal variations in the immune system of lower vertebrates. Immunology Today. 1992;13:142–147. doi: 10.1016/0167-5699(92)90112-K. [DOI] [PubMed] [Google Scholar]
- Zuk M, Johnsen TS. Seasonal changes in the relationship between ornamentation and immune response in red jungle fowl. Proceedings of the Royal Society of London Series B-Biological Sciences. 1998;265:1631–1635. [Google Scholar]
- Zysling DA, Garst AD, Demas GE. Photoperiod and food restriction differentially affect reproductive and immune responses in Siberian hamsters Phodopus sungorus. Functional Ecology. 2009;9999 [Google Scholar]