Abstract
Whole-brain irradiation (WBI) therapy produces progressive learning and memory deficits in patients with primary or secondary brain tumors. Exercise enhances memory and adult hippocampal neurogenesis in the intact brain, so we hypothesized that exercise may be an effective treatment to alleviate consequences of WBI. Previous studies using animal models to address this issue have yielded mixed results and have not examined potential molecular mechanisms. We investigated the short- and long-term effects of WBI on spatial learning and memory retention, and determined whether voluntary running after WBI aids recovery of brain and cognitive function. Forty adult female C57Bl/6 mice given a single dose of 5 Gy or sham WBI were trained 2.5 weeks and up to four months after WBI in a Barnes maze. Half of the mice received daily voluntary wheel access starting one month after sham- or WBI. Daily running following WBI prevented the marked decline in spatial memory retention observed months after irradiation. Bromodeoxyuridine (BrdU) immunolabeling and ELISA indicated that this behavioral rescue was accompanied by a partial restoration of newborn BrdU+/NeuN+ neurons in the dentate gyrus and increased hippocampal expression of brain-derived vascular endothelial growth factor and insulin-like growth factor, and occurred despite irradiation-induced elevations in hippocampal pro-inflammatory cytokines. WBI in adult mice produced a progressive memory decline consistent with what has been reported in cancer patients receiving WBI therapy. Our findings show that running can abrogate this memory decline and aid recovery of adult hippocampal plasticity, thus highlighting exercise as a potential therapeutic intervention.
Keywords: adult neurogenesis, insulin-like growth factor, memory, mouse, vascular endothelial growth factor
Introduction
Whole-brain irradiation (WBI) therapy is a treatment mainstay for patients with primary or metastatic brain tumors, and is also given as a prophylactic treatment for advanced solid tumors. However, WBI causes progressive and persistent learning and memory deficits that impair quality of life and predict survival independent of tumor progression (1–4). A lack of gross structural neurological change and/or peripheral tissue damage following WBI suggests more subtle neural dysfunction (2). For example, adult hippocampal neurogenesis, which has been implicated in aspects of learning and memory (5–8), is severely diminished weeks to months following WBI in rodents (9–11) and in post-mortem analyses of patients who underwent brain irradiation (12). Given the devastating consequences, interventions to mitigate or prevent radiation-induced deficits in neural and cognitive function are needed.
Exercise (voluntary wheel running) improves learning and memory in animal models of aging (13), stroke (14), and Alzheimer’s disease (15). In humans, aerobic exercise is associated with reduced risk for Alzheimer’s disease, dementia, and cognitive decline in elderly individuals (16–18). These benefits may be mediated in part by enhanced hippocampal neurogenesis. Exercise increases cell proliferation and neuronal differentiation in the dentate gyrus of healthy adult and aged rodents and those with compromised plasticity (13, 14, 19, 20), and these neuroplastic changes have been associated with improved memory (13, 14, 19).
Few studies have examined the effects of exercise on cognitive and neurogenic function following cranial irradiation in adulthood. Moreover, the results have been inconsistent, with exercise either rescuing (21, 22) or having no effect (8, 23) on hippocampal neurogenesis after irradiation. The behavioral tasks used in these studies (8, 21, 23) may not have been sensitive to detecting irradiation-induced deficits. Therefore, it remains unclear whether exercise improves memory after irradiation, and no studies to date have examined molecular mechanisms of exercise in this context.
We investigated the effects of voluntary wheel running on spatial learning and memory (recent and remote) using a Barnes maze task, and hippocampal neurogenesis in an adult mouse model of WBI. We also examined whether alterations in hippocampal growth factor expression potentially contribute to exercise-induced neurogenesis and protection against cognitive decline following irradiation.
Materials and Methods
Animals
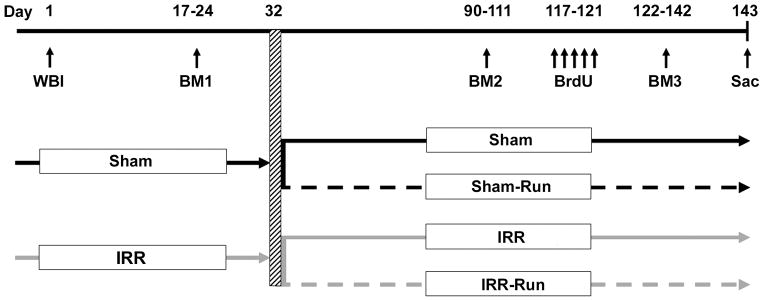
Forty 8-week-old female C57BL/6 mice (Harlan Laboratories, Dublin, VA) socially housed 5/cage in ventilated shoebox cages with standard chow and water ad libitum served as subjects. We chose female mice because female patients experience more adverse cognitive symptoms than males following cranial radiation (24, 25). All procedures were approved by the Institutional Animal Care and Use Committee of Duke University. At 12 weeks of age, all mice were anesthetized with 100 mg/kg ketamine plus 10 mg/kg xylazine and given sham (Sham; n = 20) or bilateral cranial irradiation (IRR; n = 20), using a 350 kV orthovoltage radiator while the body was shielded by a lead plate. IRR mice received a single dose of 5 gray (Gy) X-ray irradiation at a dose rate of 258 cGy/min; this dose has been shown to produce reliable deficits on hippocampal-dependent tasks and reduce neurogenesis in mice (9, 26). Figure 1 presents the timeline of all experimental procedures.
Figure 1.
Experimental design and timeline of procedures. Adult female mice were given sham-(Sham) or whole-brain irradiation (WBI; IRR) and allowed to recover. Mice were then tested in the Barnes maze (BM 1). Approximately 1 month after sham- or WBI, mice either remained socially housed (Sham or IRR) or were given daily access to an individual running wheel for 8–12 h per day during the dark phase of their light-dark cycle (Sham-Run or IRR-Run) throughout the duration of the experiment. At 90 days after sham- or WBI, mice were retested on the Barnes maze (BM 2). Mice were then given 5 daily injections of BrdU, and tested once more on the Barnes maze (BM 3) prior to being sacrificed (21 days after the last BrdU injection) at ~5 mo after sham- or WBI.
Mice were given a tail suspension test (TST) at 2 weeks and 2.5 mo after irradiation to assess depressive-like behavior (27) (see Supplementary Methods). Mice were also trained on the Barnes maze at 2.5 weeks (BM1), 3 mo (BM2), and 4 mo (BM3) after irradiation to assess short- and long-term cognitive effects of WBI. All behavioral testing occurred during the dark phase of the 12/12-h light-dark cycle.
Following BM1 (~1 mo post-WBI), Sham and IRR mice remained socially-housed in their home cage (Sham and IRR) or were socially-housed and given individual daily access to a running wheel (Sham-Run and IRR-Run) for the remainder of the experiment. There were no significant within-group differences for Sham vs. Sham-Run or IRR vs. IRR-Run mice on any behavioral measure collected at BM1. Sham-Run and IRR-Run mice were removed from their home cage during their dark phase and placed individually into a new cage with food and water ad libitum, and a running wheel (10.9 cm in diameter) equipped with a wireless device that recorded the number of wheel revolutions/day for each mouse (Wheel Manager, Med Associates, St. Albans, VT). Wheel access varied between 8 and 12 h from each day, but total wheel access on a given day was identical for all mice. After 6 weeks, all four groups were retested on the TST and Barnes maze (BM2 and BM3).
Immediately prior to BM3, all mice were injected intraperitoneally with bromodeoxyuridine (BrdU; 150 mg/kg; Sigma, St. Louis, MO) for 5 consecutive days to label newborn cells in the hippocampus, and were sacrificed 3 weeks later to assess cell survival and differentiation.
Barnes maze
Mice were trained on a circular Barnes maze to assess spatial learning and memory (28). In brief, mice were trained to locate a hole (of 20, which were equally spaced around the perimeter of the circular platform that was divided into 4 quadrants with 5 holes/quadrant), which led to an escape box. Mice received 3 training trials/day in a well-lit room with salient extramaze cues for 3 days (90 s trial maximum; ~10 min inter-trial interval), followed by one 30 s probe trial (with no escape box) at 1 hr and 4 d (and 18 d for BM2 and BM3) after the last training trial. A single training trial with the escape box in place was given immediately after each probe trial in order to prevent extinction of prior learning. At the end of BM1 testing, mice were given one training trial with a curtain around the maze to assess the use of extramaze cues. Hole location was in a different maze quadrant for BM2 and BM3 sessions. Latency to locate the escape hole (learning) and total time spent in each quadrant on probe trials (memory) were recorded with a computerized video tracker (HVS Image, UK) for statistical analyses. While analyses of hole approaches (errors) revealed a similar pattern of findings, we found time was the most reliable measure.
Histology
Three weeks after the last BrdU injection, mice were anesthetized with ketamine (i.p., 80 mg/kg) and xylazine (i.p., 10 mg/kg), and transcardially perfused with ice-cold saline. Brains were rapidly harvested and midsagitally sectioned. The hippocampus from one-half brain was dissected immediately for protein analyses and stored at −80°C until assayed. The other half-brain was immediately post-fixed in 4% paraformaldehyde in 0.1M PB for 48 hrs, then cryoprotected in 30% sucrose in 0.1M PB for 24 hrs. These half-brains were sectioned coronally at 60 μm on a freezing microtome through the extent of the hippocampus and every fifth section was collected in 0.1% sodium azide in 0.1M PB.
Peroxidase BrdU immunolabeling procedures were performed on free-floating sections as previously described (29). After denaturing, sections were first incubated in a mouse-on-mouse blocking reagent (Vector Laboratories, Burlingame, CA) for 1 h before being incubated with the mouse anti-BrdU primary antibody (1:400; Roche, Indianapolis, IN). For triple immunofluorescent labeling of BrdU, neuronal nuclei (NeuN), and glial fibrillary acidic protein (GFAP), staining was performed as previously described (29). Sections were incubated for 3 d in a primary antibody cocktail that included polyclonal sheep anti-BrdU (1:100, Abcam, Cambridge, MA), monoclonal mouse anti-NeuN (1:50, Millipore, Billerica, MA), and polyclonal rabbit anti-GFAP (1:500, Abcam). The fluorescent secondary antibodies used were biotinylated anti-sheep (1:200; Jackson Immuno, West Grove, PA) and streptavidin-conjugated Alexa Fluor 555 antibody to detect BrdU (1:500; Molecular Probes, Carlsbad, CA), Alexa Fluor 488 anti-mouse (1:200; Molecular Probes), and Cy5 anti-rabbit (1:200; Jackson Immuno).
Quantification of BrdU+ and BrdU co-labeled cells
For peroxidase stained tissue, every fifth section through the extent of the dentate gyrus was counted for the total number of BrdU+ cells in 8 sections/mouse. Contours were drawn around a region that encompassed the suprapyramidal and infrapyramidal granule cell blades and subgranular zone (SGZ). We used a modified fractionator principle using StereoInvestigator (Microbrightfield Inc., Williston, VT) to move exhaustively throughout each region, using an optical dissector height of 20 μm with a 4-μm guard zone, and counted stained cells at 40x on a Nikon light microscope. To generate estimates of BrdU+ cells/brain, counts were multiplied by 5 and then by 2 to account for both hemispheres. Dentate gyrus volume estimates were generated using the optical fractionator and according to Cavalleri’s principle (30) and also multiplied by 2. For phenotypic analysis of BrdU+ cells co-labeled with NeuN or GFAP, at least 25 BrdU+ cells per subject (n = 5 per group) were analyzed using a Zeiss Axio Observer inverted confocal laser-scanning microscope equipped with LSM 510 software. BrdU+ cells were individually examined for the co-expression of NeuN or GFAP using z-sectioning at 1 μm intervals at a 40x objective.
ELISAs
Hippocampal tissue was thawed and lysed using T-PER® Tissue Protein Extraction Reagent (Thermo Scientific, Rockford, IL) as described by the manufacturer. Collected protein was measured using Quantikine® colorimetric sandwich ELISAs (R&D Systems, Minneapolis, MN) for brain-derived neurotrophic factor (BDNF), vascular endothelial growth factor (VEGF), insulin-like growth factor-1 (IGF-1), tumor necrosis factor-alpha (TNF-α), interferon-γ (INF-γ), and interleukin-6 (IL-6), as per manufacturer’s instructions.
Statistical analysis
All data are presented as means ± SEM. Using an alpha level of 0.05, data were analyzed using ANOVA and a priori comparisons where appropriate.
Results
WBI slows spatial learning 2.5 weeks after treatment
Both Sham and IRR mice exhibited decreasing escape latencies from day 1 to day 3 of training (main effect of Day: F2, 76 = 51.92, p < 0.001). On day 2, Sham mice achieved asymptotic performance and had faster mean escape latencies than IRR mice (p < 0.05), whereas IRR mice required an additional day of training to reach performance levels equivalent to Sham mice (Fig. 2A), indicating slower spatial learning. There were no group differences in short- or long-term retention of the escape hole location at 1 h and 4 d following training with both groups spending significantly more time in the target quadrant than all other quadrants (all ps < 0.05; Fig. 2B). After extramaze cues were obscured, latency to find the escape hole increased for both Sham and IRR mice, indicating that all mice used extramaze cues for navigation, which relies on the hippocampus (31) (Fig. 2C). WBI did not significantly alter total immobility time on the TST; modest effects were detected only during the initiation of the task (Supplementary Fig. 1).
Figure 2.
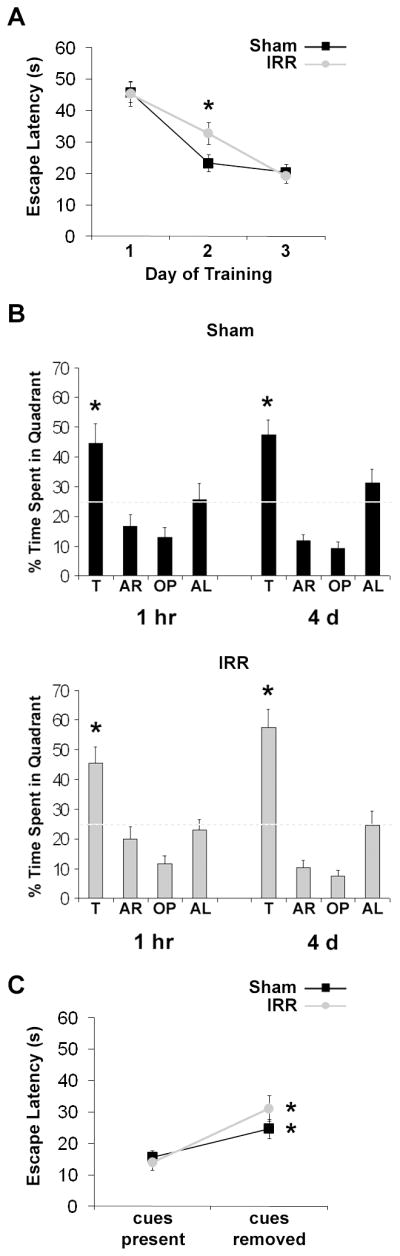
Slower spatial learning was evident shortly after WBI in IRR mice during BM1 testing, but spatial memory remained intact (Sham, n = 20; IRR, n = 20). All data represent group means. Error bars indicate SEM. A, spatial learning performance was analyzed using mean escape latencies for each day of training (across 3 trials/day) for each mouse. * significantly different from Sham mice at p < 0.05. B, probe trial performance at 1 hr and 4 d after BM1 training (during which the escape box was removed) was analyzed by dividing the maze into four quadrants and recording the percentage of time spent in each quadrant. A target quadrant bias was evident if the percent time spent in the target quadrant was significantly greater than all other quadrants. The dashed line represents chance performance. T, target; AR, adjacent right; OP, opposite; AL, adjacent left. * significantly different from all other quadrants at p < 0.05. C, both Sham and IRR mice showed a significant increase in mean escape latency from the last training trial where spatial cues were visible (cues present) to the trial where spatial cues were occluded by a curtain (cues removed). * significantly different from “cues present” at p < 0.05.
Running prevents spatial memory retention decline up to four months after WBI
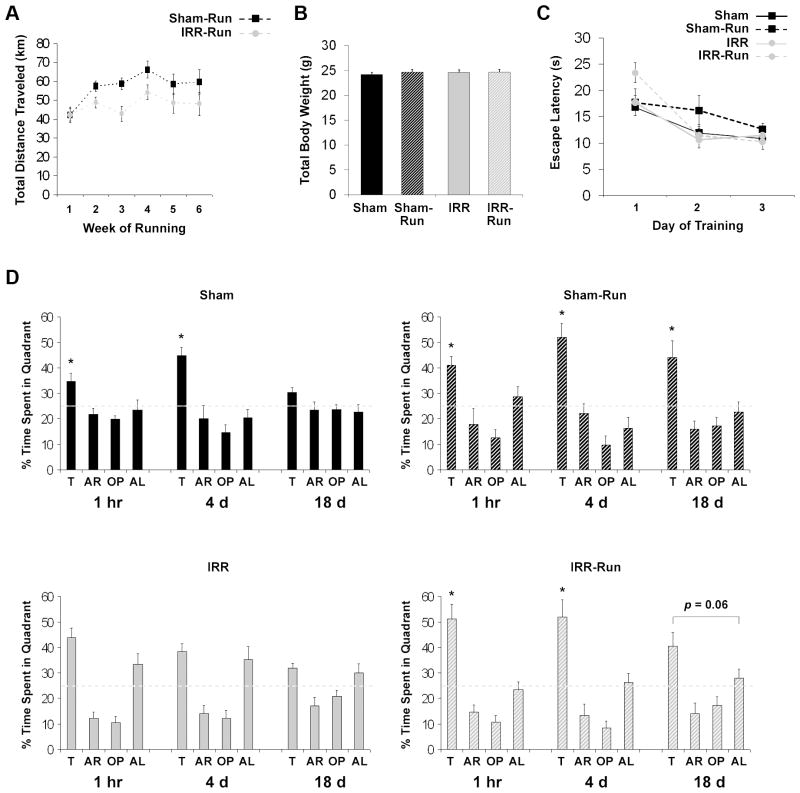
The first 6 weeks of running (initiated ~1 mo after WBI) constituted the longest period during which running was undisturbed by behavioral training. During this period, IRR-Run mice ran significantly less each day (6.92 ± 0.56 km) than Sham-Run mice (8.58 ± 0.52 km; p < 0.05). Although WBI adversely impacted voluntary wheel running behavior (Fig. 3A), both IRR-Run and Sham-Run mice ran substantial distances with 95% of mice averaging over 40 km/week. All mice appeared healthy; body weight did not differ among groups (Fig. 3B).
Figure 3.
Daily running prevented decline in spatial memory observed months after WBI during BM2 testing (Sham, n = 10; Sham-Run, n = 10; IRR, n = 10; IRR-Run, n = 10). All data represent group means. Error bars indicate SEM. A, Sham-Run and IRR-Run mice increased total distance traveled (km) per week from weeks 1 to 2, which subsequently plateaued. Over weeks 2 to 6, IRR-Run mice ran significantly less (242.34 ± 19.66 total km) than Sham-Run mice (300.47 ± 18.35 total km), F1, 18 = 4.72, p < 0.05. B, body weight at the time of perfusion did not significantly differ between groups (F < 1). C, spatial learning performance was analyzed using mean escape latencies for each day of training (across 3 trials/day) for each mouse. D, probe trial performance for each group at 1 hr, 4 d, and 18 d after BM2 training. A target quadrant bias was evident if the percent time spent in the target quadrant was significantly greater than all other quadrants. The dashed line represents chance performance. T, target; AR, adjacent right; OP, opposite; AL, adjacent left. * significantly different from all other quadrants at p < 0.05.
For BM2 testing (3 mo post-WBI), mean escape latencies from day 1 to 3 of training were decreased in all groups (main effect of Day: F2,72 = 23.61, p < 0.01; Fig 3C). IRR-Run mice had significantly longer escape latencies than all other groups on day 1 (p < 0.05), but all mice had equivalent mean escape latencies by day 3 (Fig. 3C). Sham and Sham-Run mice showed a significant target quadrant bias during the 1 h and 4 d probe (all ps < 0.05), but only Sham-Run mice exhibited a significant target quadrant bias (ps < 0.05) at the longest (18 d) retention interval (Fig. 3D), indicating that running enhanced spatial memory retention in Sham mice. In contrast, IRR mice did not show a significant target quadrant preference during the 1 h and 4 d probe trials, but spent a similar amount of time searching both the target and adjacent left quadrants (Fig. 3D). This behavioral pattern in IRR mice was not initially detected at 2.5 weeks post-WBI (Fig. 2B), revealing that spatial memory retention at short and intermediate delays in IRR mice declined substantially over time. In contrast, IRR-Run mice performed comparably to Sham mice, spending significantly more time in the target quadrant than all other quadrants during both the 1 h and 4 d probe (ps < 0.05), and showing a tendency toward a target quadrant bias during the 18 d probe (Fig. 3D). Results of BM3 testing (4 mo post-WBI) were consistent with BM2 testing in that IRR mice continued to showed poor memory, which was rescued in IRR-Run mice (Supplementary Fig. 2). WBI and running also had no effect on total immobility time in the second TST; again, modest effects were detected only during the initiation of the task (Supplementary Fig. 1).
Running partially restores adult hippocampal neurogenesis and increases growth factor expression after WBI
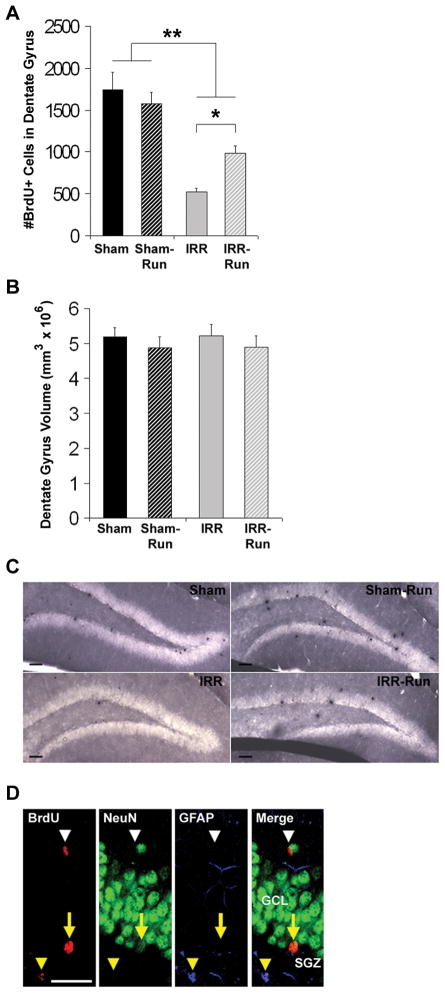
Three weeks after the last BrdU injection, IRR mice had 70% fewer BrdU+ cells in the dentate gyrus compared to Sham mice (main effect of WBI: F1, 34 = 42.65, p < 0.01; Figs. 4A and 4C). Running had no effect on the number of BrdU+ cells in Sham mice, but significantly increased the number of BrdU+ cells in IRR mice by 87%, (WBI × Running interaction: F1, 34 = 5.04, p < 0.05; Figs. 4A and 4C). There were no significant group differences in dentate gyrus volume (Fig. 4B). BrdU+ cells were examined for co-expression of the mature neuronal marker NeuN or the astrocyte marker GFAP (n = 5 per group; Fig. 4D). Overall, WBI decreased the proportion of BrdU+/NeuN+ cells, F1, 15 = 43.47, p < 0.001, reducing neurogenesis in IRR mice by more than half compared to Sham mice (p < 0.01), whereas running significantly enhanced the proportion of BrdU+/NeuN+ cells in both Sham and IRR mice, F1, 15 = 29.99, p < 0.001 (Table 1). Notably, IRR-Run mice had an increase in the percentage of BrdU+/NeuN+ neurons that was comparable to that which was observed in Sham mice (Table 1); these percentages are consistent with those reported in mice 3 to 4 weeks following BrdU administration (13, 32, 33). There were no effects of WBI or running on BrdU+/GFAP+ cells, but WBI led to an overall increase in the proportion of BrdU+ cells that expressed neither NeuN nor GFAP, F1, 15 = 17.12, p < 0.01, while running effectively decreased this proportion, F1, 15 = 4.95, p < 0.05. These data suggest that running may have altered the rate of neuronal differentiation, neuronal maturation, and/or rate of newborn activated microglia in the dentate gyrus following irradiation (34), which we did not quantify in the current study. However, hippocampal levels of pro-inflammatory cytokines TNF-α, INF-γ, and IL-6 (secreted by activated microglia) were similarly elevated in both IRR and IRR-Run mice (TNF-α, F1, 35 = 500.07, p < 0.001; INF-γ, F1, 35 = 1102.29, p < 0.001; IL-6, F1, 35 = 443.90, p < 0.001) compared to negligible levels in Sham and Sham-Run mice (Fig. 5).
Figure 4.
Daily running partially rescued adult hippocampal neurogenesis months after WBI (Sham, n = 10; Sham-Run, n = 10; IRR, n = 10; IRR-Run, n = 9). All data represent group means. Error bars indicate SEM. A, number of BrdU+ cells in the dentate gyrus at 3 weeks after 5 daily injections of BrdU prior to BM3 training. * p < 0.05; ** main effect of WBI at p < 0.05. B, dentate gyrus volume estimated using the optical fractionator and according to Cavalleri’s principle. C, newly divided cells (peroxidase-stained with anti-BrdU) in the dentate gyrus of Sham, Sham-Run, IRR, and IRR-Run mice. Scale bars indicate 50 μm. Photomicrographs taken at a 10x. D, confocal images of BrdU+ cells in the dentate gyrus that co-expressed NeuN (yellow arrow), GFAP (yellow arrowhead), or neither (white arrow head). Scale bars indicate 25 μm. Confocal images taken at 40x. GCL, granule cell layer. SGZ, subgranular zone.
Table 1. Phenotype of BrdU+ Cells in the Dentate Gyrus.
Mice in each group received 5 daily injections (150 mg/kg per day) of BrdU and were sacrificed 21 days later. Percentages of BrdU+ cells double labeled for NeuN (mature neurons), GFAP (mature astrocytes), or neither (Other) were analyzed (n = 5 per group). Data are presented as group means (± SEM).
| % NeuN+ | %GFAP+ | %Other | |
|---|---|---|---|
| Sham | 45.64 (6.97) | 21.24 (3.39) | 33.11 (7.71) |
| Sham-Run | 64.00 (2.56)* | 14.97 (2.88) | 21.03 (4.22) |
| IRR | 20.33 (3.22)* | 22.24 (4.51) | 57.43 (5.81)* |
| IRR-Run | 41.60 (2.73)** | 14.34 (5.04) | 44.05 (2.77) |
significantly different from Sham group at p < 0.01
significantly different from IRR group at p < 0.01.
Figure 5.
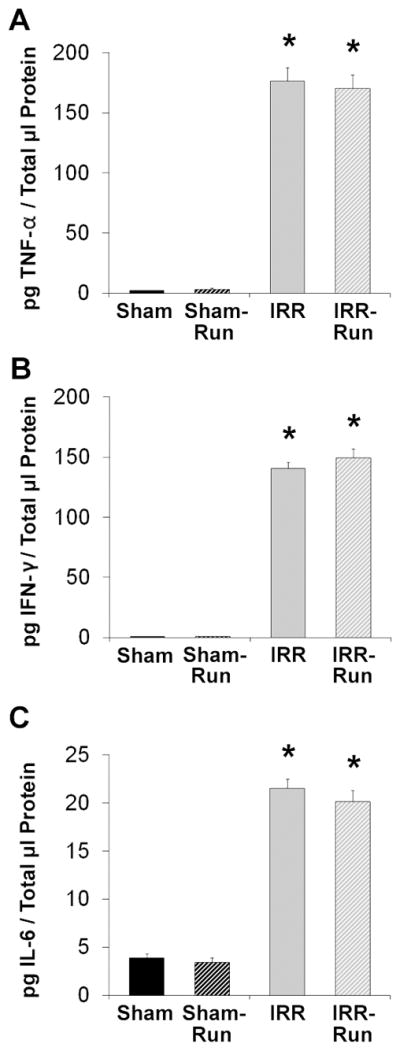
Elevated cytokine expression in the adult hippocampus at ~5 months after WBI (Sham, n = 10; Sham-Run, n = 10; IRR, n = 10; IRR-Run, n = 9). All data are expressed as pg of cytokine protein per μl of total protein, and represent group means. Error bars indicate SEM. A, hippocampal TNF-α expression at ~5 mo after sham- or WBI. * significantly different from Sham and Sham-Run groups at p < 0.001. B, hippocampal INF-γ expression at ~5 mo after sham- or WBI. IFN-γ protein levels in Sham and Sham-Run mice were < 1 pg/μl. * significantly different from Sham and Sham-Run groups at p < 0.001. C, hippocampal IL-6 expression at ~5 mo after sham- or WBI. * significantly different from Sham and Sham-Run groups at p < 0.001.
WBI decreased hippocampal BDNF by 33%, F1, 35 = 20.85, p < 0.01, and VEGF by 38%, F1, 35 = 17.31, p < 0.01, and increased IGF-1 by 194%, F1, 35 = 165.56, p < 0.001 (Fig. 6). Running did not alter BDNF or VEGF in Sham mice, but partially restored levels of VEGF in IRR mice (p < 0.05; Fig. 6B). Running also had an overall effect on IGF-1 protein in both Sham and IRR mice, F1, 34 = 16.78, p < 0.001. While IGF-1 was increased ~2-fold in IRR mice compared to Sham mice, running further augmented this effect to ~3-fold (WBI × Running interaction: F1, 35 = 6.70, p < 0.05; Fig. 6C).
Figure 6.
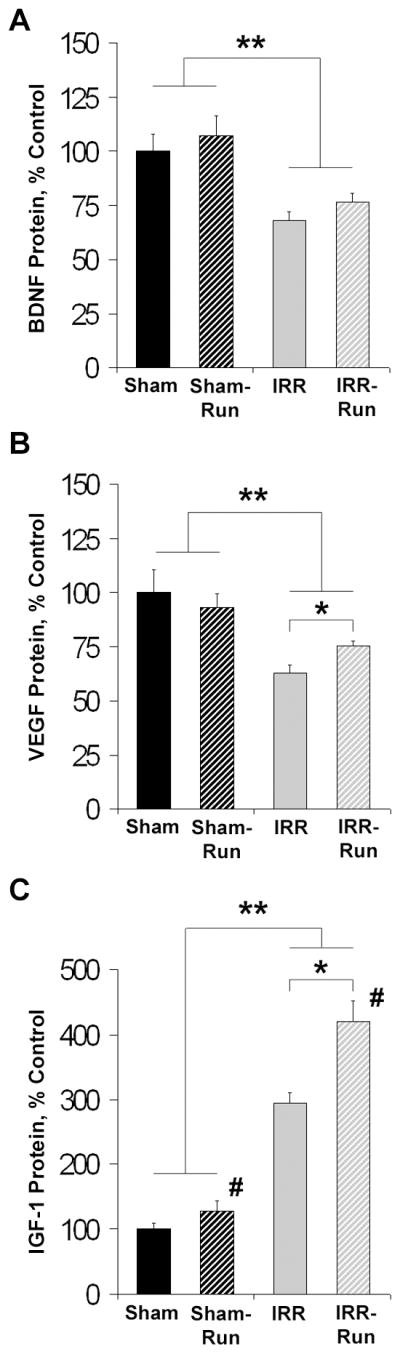
WBI and running alter hippocampal neurotrophic/growth factor expression at ~5 mo after treatment (Sham, n = 10; Sham-Run, n = 10; IRR, n = 10; IRR-Run, n = 9). All data expressed as percent of control (Sham) levels and represent group means. Error bars indicate SEM. A, hippocampal BDNF expression at ~5 mo after sham- or WBI. ** main effect of WBI at p < 0.05. B, hippocampal VEGF expression at ~5 mo after sham- or WBI. * p < 0.05; ** main effect of WBI at p < 0.05. C, hippocampal IGF-1 expression at ~5 mo after sham- or WBI. * p < 0.05; ** main effect of WBI at p < 0.05; # main effect of running at p < 0.05.
Discussion
We report that WBI-induced memory decline is prevented by daily voluntary running initiated one month after irradiation. This behavioral rescue was accompanied by a partial restoration of neurogenesis in the dentate gyrus and increased expression of VEGF and IGF-1 in the hippocampus. These protective effects appeared to be independent of persistent hippocampal neuroinflammation, although we cannot rule out the possibility that exercise altered rates of dentate-specific microglia proliferation following radiation. Our findings suggest that exercise may aid recovery of hippocampal function, neurogenesis and growth factor expression following cranial irradiation.
Our findings are consistent with prior work demonstrating hippocampal memory deficits several weeks to months following cranial irradiation (6, 11, 26, 35), but to our knowledge, our study is the first to reveal a progressive deterioration of memory and protection by exercise after cranial irradiation. Our irradiated mice showed intact spatial memory shortly after WBI, but then developed memory dysfunction over time, perhaps due to deterioration of memory consolidation and/or increased interference from prior learning. These data are remarkably similar to that reported in patients receiving WBI therapy and show progressive cognitive decline months to years after treatment (1–4), suggesting our WBI treatment to adult mice is a clinically relevant model.
Our findings that WBI caused a progressive loss of memory that can be rescued by exercise is consistent with a previous study demonstrating protection against spatial memory deficits by environmental enrichment that included shared access to a running wheel (36). However, our findings appear contrary with other work in this field. These discrepancies may be partially explained by several contributing factors. Unlike our study, previous reports only assessed memory function at a single time point several weeks after irradiation and prior studies in mice utilized behavioral tasks that did not capture radiation-induced memory impairments (8, 21), with one study showing that radiation inhibited the memory enhancement caused by running (21). There may also be species differences in cognitive vulnerability to cranial irradiation: contextual fear conditioning after irradiation remains intact in adult mice (8, 21, 32), but is compromised and not rescued by running in adult rats (23, 32). It also appears that some, but not all, types of hippocampal-dependent tasks are sensitive to cranial irradiation (6, 32, 35, 37). Long-term memory, for example, appears to be particularly vulnerable (6, 7) while spatial learning is resilient (6–8, 11, 35). Moreover, cranial irradiation in mice impairs memory examined using a Barnes maze, but not the Morris water maze (11). Memory retention as assessed using the Barnes maze may thus be a particularly sensitive measure for identifying cognitive deficits in mice and revealing the beneficial effects of exercise following cranial irradiation.
Several studies have shown that exercise reliably increases adult hippocampal neurogenesis and improves cognitive performance (13, 19, 20), while neurogenesis and hippocampal-dependent memory are reduced by various ablation methods (5–8, 11, 26, 32), strongly supporting a relationship between adult hippocampal neurogenesis and memory function. In our study, WBI diminished neurogenesis, did not eliminate spatial learning, but progressively impaired spatial memory retention. This delayed cognitive impairment after WBI may be explained the loss of new neurons or by alterations in newborn granule cell functionality. Indeed, dentate granule cell immediate-early gene Arc expression is decreased at 2 months, but not 1 week after WBI (38). Studies that did not report radiation-induced cognitive deficits have also observed running-induced increases in hippocampal neurogenesis after cranial irradiation in adult (21) and juvenile mice (22), but see (8, 23). Here, we observed that post-WBI exercise caused a partial recovery of neurogenesis and protected against spatial memory loss.
Interestingly, running increased neurogenesis despite radiation-induced elevations in hippocampal pro-inflammatory cytokines, which inhibit adult hippocampal neurogenesis (34), suggesting that exercise may trigger molecular signals that override this inhibitory effect. Indeed, we discovered that post-WBI running increased expression of hippocampal VEGF and IGF-1—growth factors known to mediate exercise-induced enhancements in neurogenesis and spatial memory (39–41). Little is known, however, about the hippocampal neurotrophic milieu following cranial irradiation, with one study reporting decreased VEGF and no change in BDNF expression at ~3 weeks post-irradiation (42). Thus, we provide new evidence for decreased hippocampal BDNF and VEGF expression and a striking increase in IGF-1 months after WBI, suggesting that the neurogenic consequences of post-irradiation fluctuations in hippocampal growth factor expression may be specific for each factor. IGF-1, in particular, may play a primary role in restoring neurogenesis following irradiation because it has been shown to antagonize inflammatory microglia activation and protect hippocampal function (43). Future studies are needed to further investigate this question.
We did not detect a significant recovery in hippocampal BDNF following ~2 months of exercise, but the contribution of BDNF cannot be completely disregarded. BDNF signaling is one central mechanism underlying improved memory with exercise (44, 45). It is possible that there was a rapid initial recovery of BDNF following radiation that was not detected. We measured running-induced changes in local growth factor expression, but these growth factors are also produced peripherally (44). It was not possible to measure growth factor content peripherally in our mice because they were saline-perfused at sacrifice. Thus, exercise may be stimulating both CNS- and peripherally-derived growth factors that trigger a host of signaling cascades, which lead to improved hippocampal neuroplasticity and cognitive outcomes (44).
A surprising finding was the lack of effect of running on the number of BrdU+ cells in our Sham mice, which is contrary to previous reports (8, 13, 39–41). It is possible that the effect of running on cell proliferation waned with time (20, 46), or that neurogenesis in Sham mice was at ceiling because mice received considerable spatial learning and physical activity on the Barnes maze (47). However, running did increase the percentage of new neurons and improved memory in Sham mice, which is consistent with previous findings (13, 21). Alternately, the observed benefits of exercise in both Sham and irradiated mice may also be due to enhancements in other features of adult hippocampal plasticity (19, 48, 49).
Our findings indicate that exercise may be a promising adjunct therapeutic strategy to facilitate recovery of hippocampal function and neurogenesis following WBI. To this end, there are several points to consider when interpreting our data. First, we chose to study young adult female mice because female patients experience more adverse cognitive symptoms than males following cranial radiation (24, 25). Studies are needed to examine whether exercise-induced improvements in neurogenesis are influenced by sex, estrogen status, and/or age. Second, radiation dose is also an important consideration. Intriguingly, we found a marked decline in memory function with a relatively low-dose of WBI. Patients, in general, receive much higher doses of radiation, suggesting that the incidence of cognitive dysfunction is likely much higher than currently suspected. Prospective studies utilizing more comprehensive neuropsychological tests in combination with novel imaging platforms to assess the incidence and pathogenesis of injury in patients receiving WBI are needed. Finally, in this study mice ran, on average, 40 km/week and the comparable amount of exercise required to produce equivalent protective properties in humans is not known. However, our group and others have demonstrated that structured exercise training is a feasible and well-tolerated therapy associated with significant and potentially important improvements in a range of biopsychosocial outcomes in patients undergoing chemoradiation for early and advanced cancer (for review, 50). We contend that our findings provide ‘proof of concept’ evidence to aid design of clinical trials to investigate the efficacy of exercise in cancer patients undergoing WBI. In summary, our findings show that running can prevent memory decline and aid recovery of adult hippocampal plasticity following WBI, thus highlighting exercise as a potential therapeutic intervention.
Supplementary Material
Acknowledgments
This work was supported by AG09525 to C.L.W., by CA143254, CA138634, CA133895, CA125458 to L.W.J., and funds from the Susan and George Beischer Foundation.
Footnotes
No potential conflicts of interest are disclosed.
References
- 1.Meyers CA, Smith JA, Bezjak A, Mehta MP, Liebmann J, Illidge T, et al. Neurocognitive function and progression in patients with brain metastases treated with whole-brain radiation and motexafin gadolinium: results of a randomized phase III trial. J Clin Oncol. 2004;22:157–65. doi: 10.1200/JCO.2004.05.128. [DOI] [PubMed] [Google Scholar]
- 2.Monje M. Cranial radiation therapy and damage to hippocampal neurogenesis. Dev Disabil Res Rev. 2008;14:238–42. doi: 10.1002/ddrr.26. [DOI] [PubMed] [Google Scholar]
- 3.Douw L, Klein M, Fagel SS, van den Heuvel J, Taphoorn MJ, Aaronson NK, et al. Cognitive and radiological effects of radiotherapy in patients with low-grade glioma: long-term follow-up. Lancet Neurol. 2009;8:810–8. doi: 10.1016/S1474-4422(09)70204-2. [DOI] [PubMed] [Google Scholar]
- 4.Marko NF, Weil RJ. Radiotherapy: Neurocognitive considerations in the treatment of brain metastases. Nat Rev Clin Oncol. 2010;7:185–6. doi: 10.1038/nrclinonc.2010.30. [DOI] [PubMed] [Google Scholar]
- 5.Shors TJ, Townsend DA, Zhao M, Kozorovitskiy Y, Gould E. Neurogenesis may relate to some but not all types of hippocampal-dependent learning. Hippocampus. 2002;12:578–84. doi: 10.1002/hipo.10103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Snyder JS, Hong NS, McDonald RJ, Wojtowicz JM. A role for adult neurogenesis in spatial long-term memory. Neuroscience. 2005;130:843–52. doi: 10.1016/j.neuroscience.2004.10.009. [DOI] [PubMed] [Google Scholar]
- 7.Deng W, Saxe MD, Gallina IS, Gage FH. Adult-born hippocampal dentate granule cells undergoing maturation modulate learning and memory in the brain. J Neurosci. 2009;29:13532–42. doi: 10.1523/JNEUROSCI.3362-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kitamura T, Saitoh Y, Takashima N, Murayama A, Niibori Y, Ageta H, et al. Adult neurogenesis modulates the hippocampus-dependent period of associative fear memory. Cell. 2009;139:814–27. doi: 10.1016/j.cell.2009.10.020. [DOI] [PubMed] [Google Scholar]
- 9.Mizumatsu S, Monje ML, Morhardt DR, Rola R, Palmer TD, Fike JR. Extreme sensitivity of adult neurogenesis to low doses of X-irradiation. Cancer Res. 2003;63:4021–7. [PubMed] [Google Scholar]
- 10.Monje ML, Palmer T. Radiation injury and neurogenesis. Curr Opin Neurol. 2003;16:129–34. doi: 10.1097/01.wco.0000063772.81810.b7. [DOI] [PubMed] [Google Scholar]
- 11.Raber J, Rola R, LeFevour A, Morhardt D, Curley J, Mizumatsu S, et al. Radiation-induced cognitive impairments are associated with changes in indicators of hippocampal neurogenesis. Radiat Res. 2004;162:39–47. doi: 10.1667/rr3206. [DOI] [PubMed] [Google Scholar]
- 12.Monje ML, Vogel H, Masek M, Ligon KL, Fisher PG, Palmer TD. Impaired human hippocampal neurogenesis after treatment for central nervous system malignancies. Ann Neurol. 2007;62:515–20. doi: 10.1002/ana.21214. [DOI] [PubMed] [Google Scholar]
- 13.van Praag H, Shubert T, Zhao C, Gage FH. Exercise enhances learning and hippocampal neurogenesis in aged mice. J Neurosci. 2005;25:8680–5. doi: 10.1523/JNEUROSCI.1731-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Luo CX, Jiang J, Zhou QG, Zhu XJ, Wang W, Zhang ZJ, et al. Voluntary exercise-induced neurogenesis in the postischemic dentate gyrus is associated with spatial memory recovery from stroke. J Neurosci Res. 2007;85:1637–46. doi: 10.1002/jnr.21317. [DOI] [PubMed] [Google Scholar]
- 15.Adlard PA, Perreau VM, Pop V, Cotman CW. Voluntary exercise decreases amyloid load in a transgenic model of Alzheimer’s disease. J Neurosci. 2005;25:4217–21. doi: 10.1523/JNEUROSCI.0496-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yaffe K, Barnes D, Nevitt M, Lui LY, Covinsky K. A prospective study of physical activity and cognitive decline in elderly women: women who walk. Arch Intern Med. 2001;161:1703–8. doi: 10.1001/archinte.161.14.1703. [DOI] [PubMed] [Google Scholar]
- 17.Laurin D, Verreault R, Lindsay J, MacPherson K, Rockwood K. Physical activity and risk of cognitive impairment and dementia in elderly persons. Arch Neurol. 2001;58:498–504. doi: 10.1001/archneur.58.3.498. [DOI] [PubMed] [Google Scholar]
- 18.Podewils LJ, Guallar E, Kuller LH, Fried LP, Lopez OL, Carlson M, et al. Physical activity, APOE genotype, and dementia risk: findings from the Cardiovascular Health Cognition Study. Am J Epidemiol. 2005;161:639–51. doi: 10.1093/aje/kwi092. [DOI] [PubMed] [Google Scholar]
- 19.van Praag H, Christie BR, Sejnowski TJ, Gage FH. Running enhances neurogenesis, learning, and long-term potentiation in mice. Proc Natl Acad Sci U S A. 1999;96:13427–31. doi: 10.1073/pnas.96.23.13427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kronenberg G, Bick-Sander A, Bunk E, Wolf C, Ehninger D, Kempermann G. Physical exercise prevents age-related decline in precursor cell activity in the mouse dentate gyrus. Neurobiol Aging. 2006;27:1505–13. doi: 10.1016/j.neurobiolaging.2005.09.016. [DOI] [PubMed] [Google Scholar]
- 21.Clark PJ, Brzezinska WJ, Thomas MW, Ryzhenko NA, Toshkov SA, Rhodes JS. Intact neurogenesis is required for benefits of exercise on spatial memory but not motor performance or contextual fear conditioning in C57BL/6J mice. Neuroscience. 2008;155:1048–58. doi: 10.1016/j.neuroscience.2008.06.051. [DOI] [PubMed] [Google Scholar]
- 22.Naylor AS, Bull C, Nilsson MK, Zhu C, Bjork-Eriksson T, Eriksson PS, et al. Voluntary running rescues adult hippocampal neurogenesis after irradiation of the young mouse brain. Proc Natl Acad Sci U S A. 2008;105:14632–7. doi: 10.1073/pnas.0711128105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wojtowicz JM, Askew ML, Winocur G. The effects of running and of inhibiting adult neurogenesis on learning and memory in rats. Eur J Neurosci. 2008;27:1494–502. doi: 10.1111/j.1460-9568.2008.06128.x. [DOI] [PubMed] [Google Scholar]
- 24.Kadan-Lottick NS, Zeltzer LK, Liu Q, Yasui Y, Ellenberg L, Gioia G, et al. Neurocognitive functioning in adult survivors of childhood non-central nervous system cancers. J Natl Cancer Inst. 2010;102:881–93. doi: 10.1093/jnci/djq156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Raber J. Unintended effects of cranial irradiation on cognitive function. Toxicol Pathol. 2010;38:198–202. doi: 10.1177/0192623309352003. [DOI] [PubMed] [Google Scholar]
- 26.Rola R, Raber J, Rizk A, Otsuka S, VandenBerg SR, Morhardt DR, et al. Radiation-induced impairment of hippocampal neurogenesis is associated with cognitive deficits in young mice. Exp Neurol. 2004;188:316–30. doi: 10.1016/j.expneurol.2004.05.005. [DOI] [PubMed] [Google Scholar]
- 27.Cryan JF, Mombereau C, Vassout A. The tail suspension test as a model for assessing antidepressant activity: review of pharmacological and genetic studies in mice. Neurosci Biobehav Rev. 2005;29:571–625. doi: 10.1016/j.neubiorev.2005.03.009. [DOI] [PubMed] [Google Scholar]
- 28.Koopmans G, Blokland A, van Nieuwenhuijzen P, Prickaerts J. Assessment of spatial learning abilities of mice in a new circular maze. Physiol Behav. 2003;79:683–93. doi: 10.1016/s0031-9384(03)00171-9. [DOI] [PubMed] [Google Scholar]
- 29.Wong-Goodrich SJ, Glenn MJ, Mellott TJ, Liu YB, Blusztajn JK, Williams CL. Water maze experience and prenatal choline supplementation differentially promote long-term hippocampal recovery from seizures in adulthood. Hippocampus. 2010 doi: 10.1002/hipo20783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mouton P. Principles and practices of unbiased stereology. Baltimore: The Johns Hopkins University Press; 2002. [Google Scholar]
- 31.Morris RG, Garrud P, Rawlins JN, O’Keefe J. Place navigation impaired in rats with hippocampal lesions. Nature. 1982;297:681–3. doi: 10.1038/297681a0. [DOI] [PubMed] [Google Scholar]
- 32.Snyder JS, Choe JS, Clifford MA, Jeurling SI, Hurley P, Brown A, et al. Adult-born hippocampal neurons are more numerous, faster maturing, and more involved in behavior in rats than in mice. J Neurosci. 2009;29:14484–95. doi: 10.1523/JNEUROSCI.1768-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Steiner B, Kronenberg G, Jessberger S, Brandt MD, Reuter K, Kempermann G. Differential regulation of gliogenesis in the context of adult hippocampal neurogenesis in mice. Glia. 2004;46:41–52. doi: 10.1002/glia.10337. [DOI] [PubMed] [Google Scholar]
- 34.Monje ML, Toda H, Palmer TD. Inflammatory blockade restores adult hippocampal neurogenesis. Science. 2003;302:1760–5. doi: 10.1126/science.1088417. [DOI] [PubMed] [Google Scholar]
- 35.Winocur G, Wojtowicz JM, Sekeres M, Snyder JS, Wang S. Inhibition of neurogenesis interferes with hippocampus-dependent memory function. Hippocampus. 2006;16:296–304. doi: 10.1002/hipo.20163. [DOI] [PubMed] [Google Scholar]
- 36.Fan Y, Liu Z, Weinstein PR, Fike JR, Liu J. Environmental enrichment enhances neurogenesis and improves functional outcome after cranial irradiation. Eur J Neurosci. 2007;25:38–46. doi: 10.1111/j.1460-9568.2006.05269.x. [DOI] [PubMed] [Google Scholar]
- 37.Madsen TM, Kristjansen PE, Bolwig TG, Wortwein G. Arrested neuronal proliferation and impaired hippocampal function following fractionated brain irradiation in the adult rat. Neuroscience. 2003;119:635–42. doi: 10.1016/s0306-4522(03)00199-4. [DOI] [PubMed] [Google Scholar]
- 38.Rosi S, Andres-Mach M, Fishman KM, Levy W, Ferguson RA, Fike JR. Cranial irradiation alters the behaviorally induced immediate-early gene arc (activity-regulated cytoskeleton-associated protein) Cancer Res. 2008;68:9763–70. doi: 10.1158/0008-5472.CAN-08-1861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Trejo JL, Carro E, Torres-Aleman I. Circulating insulin-like growth factor I mediates exercise-induced increases in the number of new neurons in the adult hippocampus. J Neurosci. 2001;21:1628–34. doi: 10.1523/JNEUROSCI.21-05-01628.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Trejo JL, Llorens-Martin MV, Torres-Aleman I. The effects of exercise on spatial learning and anxiety-like behavior are mediated by an IGF-I-dependent mechanism related to hippocampal neurogenesis. Mol Cell Neurosci. 2008;37:402–11. doi: 10.1016/j.mcn.2007.10.016. [DOI] [PubMed] [Google Scholar]
- 41.Fabel K, Fabel K, Tam B, Kaufer D, Baiker A, Simmons N, et al. VEGF is necessary for exercise-induced adult hippocampal neurogenesis. Eur J Neurosci. 2003;18:2803–12. doi: 10.1111/j.1460-9568.2003.03041.x. [DOI] [PubMed] [Google Scholar]
- 42.Warner-Schmidt JL, Madsen TM, Duman RS. Electroconvulsive seizure restores neurogenesis and hippocampus-dependent fear memory after disruption by irradiation. Eur J Neurosci. 2008;27:1485–93. doi: 10.1111/j.1460-9568.2008.06118.x. [DOI] [PubMed] [Google Scholar]
- 43.Maher FO, Clarke RM, Kelly A, Nally RE, Lynch MA. Interaction between interferon gamma and insulin-like growth factor-1 in hippocampus impacts on the ability of rats to sustain long-term potentiation. J Neurochem. 2006;96:1560–71. doi: 10.1111/j.1471-4159.2006.03664.x. [DOI] [PubMed] [Google Scholar]
- 44.Cotman CW, Berchtold NC, Christie LA. Exercise builds brain health: key roles of growth factor cascades and inflammation. Trends Neurosci. 2007;30:464–72. doi: 10.1016/j.tins.2007.06.011. [DOI] [PubMed] [Google Scholar]
- 45.Vaynman S, Ying Z, Gomez-Pinilla F. Hippocampal BDNF mediates the efficacy of exercise on synaptic plasticity and cognition. Eur J Neurosci. 2004;20:2580–90. doi: 10.1111/j.1460-9568.2004.03720.x. [DOI] [PubMed] [Google Scholar]
- 46.Naylor AS, Persson AI, Eriksson PS, Jonsdottir IH, Thorlin T. Extended voluntary running inhibits exercise-induced adult hippocampal progenitor proliferation in the spontaneously hypertensive rat. J Neurophysiol. 2005;93:2406–14. doi: 10.1152/jn.01085.2004. [DOI] [PubMed] [Google Scholar]
- 47.Gould E, Beylin A, Tanapat P, Reeves A, Shors TJ. Learning enhances adult neurogenesis in the hippocampal formation. Nat Neurosci. 1999;2:260–5. doi: 10.1038/6365. [DOI] [PubMed] [Google Scholar]
- 48.Redila VA, Christie BR. Exercise-induced changes in dendritic structure and complexity in the adult hippocampal dentate gyrus. Neuroscience. 2006;137:1299–307. doi: 10.1016/j.neuroscience.2005.10.050. [DOI] [PubMed] [Google Scholar]
- 49.Farmer J, Zhao X, van Praag H, Wodtke K, Gage FH, Christie BR. Effects of voluntary exercise on synaptic plasticity and gene expression in the dentate gyrus of adult male Sprague-Dawley rats in vivo. Neuroscience. 2004;124:71–9. doi: 10.1016/j.neuroscience.2003.09.029. [DOI] [PubMed] [Google Scholar]
- 50.Jones LW, Peppercorn J, Scott JM, Battaglini C. Exercise Therapy in the Management of Solid Tumors. Curr Treat Options Oncol. 2010 doi: 10.1007/s11864-010-0132-2. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.