Abstract
Obesity and the metabolic syndrome represent serious health threats affecting increasing numbers of individuals, with females being more affected than males and with growing incidence among children and adolescents. In the present study, we used the OLETF rat model of early-onset obesity to examine the influence of different timing of food restriction on long term obesity levels in females. Food restriction took place at different time windows: from weaning until postnatal day (PND) 45 (early); from weaning until PND90 (chronic); or from PND45 until PND70 (late). Follow-up continued until PND90. During and after the termination of the diet-restriction period, we focused on peripheral adiposity-related measures such as fat pad weight (brown, retroperitoneal & inguinal); inguinal adipocyte size and number; and leptin, oxytocin & glucose levels. We also examined body weight, feeding efficiency, spontaneous intake after release from diet-restriction, and plasma creatinine levels and estrous cycle characteristics as a result of the chronic diet. The results suggest that while food restriction produced significant weight and adiposity loss, OLETF females presented poor weight loss retention after the early and late short term diets. The estrous cycle structure and time of first estrous of the OLETF rats were normalized by chronic food restriction. Females responded to early food restriction in a different manner than males did in previous studies, further emphasizing the importance of sex-appropriate approaches in the investigation and treatment of the pathologies related to obesity and the metabolic syndrome.
Key terms: pair feeding, obesity, animal models, early onset obesity
Introduction
Obesity has developed into a global epidemic, and its prevalence among women has been reported as being consistently higher than in men (Hedley et al., 2004; Geary, 2008). The dissimilar profiles between sexes show that women are not only more affected by obesity but are also more resistant to weight loss (Legato, 1997). The treatments and interventions currently in use have failed to provide successful long term solutions, leading to an increasing number of obese individuals, with consequent health-related complications.
Sex-specific responses to fat and weight loss during periods of energy imbalance have been frequently reported (Bjorntorp, 1989), with a sexually dimorphic response in feeding and appetite-associated hormonal responses to food restriction and energy challenges. Females seem to deal more successfully with energy deficits because they defend their body weight in a more efficient manner than males (Nance et al., 1977; Cortright et al., 1997; Gayle et al., 2006). Gayle et al. reported differential outcomes between the sexes after a 12 hour fast, showing a more marked ‘rebound eating’ in females than in males when given renewed ad libitum food access; this was accompanied by higher fasted plasma ghrelin levels and reduced plasma leptin (Gayle et al., 2006). Similar results were also reported by others in human studies (Barkan et al., 2003; Ostergard et al., 2003; Espelund et al., 2005). Hence, drastic dietary regimens are unlikely to achieve successful weight loss retention in adult females, because their orexigenic responses will likely lead to compensatory hyperphagia.
Whether early life food restriction may have more success in preventing weight regain in females hasn’t been evaluated. According to the Barker hypothesis, early interventions may be more successful than late interventions in re-programming genetic predispositions, leading to an improved outcome (Barker, 1998, 2002; Plagemann, 2006; Aggoun, 2007; Miller & Silverstein, 2007; Taylor & Poston, 2007). Thus, interventions directed towards specific time windows, before or during critical obesity-related developmental changes, may be more effective in programming a different, more moderate adiposity target and thus, in reducing obesity levels than when the obesity is already established (Taylor & Poston, 2007).
The Otsuka Long Evans Tokushima Fatty (OLETF) rat is a model of non-insulin dependent diabetes mellitus (NIDDM) (Kawano et al., 1992; Kawano et al., 1994) and early-onset hyperphagia- induced obesity (Moran & Bi, 2006; Schroeder et al., 2006; Schroeder et al., 2007a; Schroeder et al., 2007b; Schroeder et al., 2009a; Schroeder et al., 2009b), that has a congenital defect in the expression of the cholecystokinin-1 (CCK1) receptor gene (Nakamura et al., 1998). Since CCK is a critical brain-gut peptide that acts as a peripheral short-term satiety molecule (Smith, 2006; Weller, 2006), its absence causes chronic hyperphagia and eventually induces OLETF males and females to become obese and hyperleptinemic (Moran & Bi, 2006; Schroeder et al., 2009b). OLETF pups are already born heavier probably as a consequence of the "obese" pregnancy (Schroeder et al., 2006; Schroeder et al., 2009a) and are hyperphagic during lactation (Blumberg et al., 2006; Schroeder et al., 2007a). In addition, OLETF females present a higher estimated number of adipocytes than OLETF males as early as PND7 (Schroeder et al., 2009a) but this difference disappears later in life (Schroeder et al., 2009b). After weaning, OLETF females develop obesity in a relatively gradual manner, and while hyper-adiposity is evident soon after birth, there is a slow increase in obesity levels toward adulthood, when they become explicitly obese (around PND90). At that point, their fat percentages, leptin levels and adipocyte size are twice as high as in the LETO (Long Evans Tokushima Otsuka) controls, and by PND120, adiposity and related parameters are three times higher than in controls (Schroeder et al., 2009b).
Previous studies performed on adult OLETF males, revealed that diet-restriction ("pair-feeding" to LETO control levels) induced the normalization of body weight, adiposity, adiposity hormones and central hypothalamic gene expression (Bi et al., 2001); food restriction also prevented NIDDM when performed relatively early during development (Man et al., 2000; Park et al., 2005). When starting at weaning, males responded to chronic food-restriction by normalization of their body weight, fat mass, and leptin and oxytocin levels and by a sharp reduction in adipocyte hypertrophy. A shorter period of diet starting at weaning also achieved long term reductions in body weight, body fat, spontaneous intake and adipocyte hypertrophy (Schroeder et al., 2010).
In the present study we aimed, first, to examine if food restriction can produce similar normalization effects on the females; and second, since weight-reduction in adult females after diet termination is difficult to retain, we examined three different post-weaning periods, where limited periods of food restriction during critical times of development may be more successful in achieving long term effects on body weight and adiposity.
Methods
Subjects
OLETF and LETO females were raised in the specific pathogen free (SPF) facility of the Gonda Brain Research Center at Bar-Ilan University, Ramat-Gan, Israel. The progenitor rats for the colony were received as a generous gift from the Tokushima Research Institute, Japan. OLETF and LETO offspring were housed together with their dams and litters until weaning at PND22-23. During the period of food restriction, females were housed individually, but during the re-feeding periods they were housed in pairs in order to reduce the isolation stress. Polycarbonate cages (18.5 cm height × 26.5 cm width × 43 cm length) were used, with stainless steel wire lids and wood shavings as bedding material. Food (2018S Teklad Global, 5% fat) and water were freely available. The animals were on a 12:12 hr light: dark cycle, with lights on at 06:00. Room temperature was maintained at 22+/−2 °C. Newborn litters were culled to 10 pups (minimum 7), with sex distribution kept as equal as possible in each litter. The research protocol was approved by the Institutional Animal Care and Use Committee, and it adhered to the guidelines of the American Psychological Association and the Society for Neuroscience.
Experimental procedure
Experiment 1: Chronic pair feeding
At the time of weaning, OLETF females were placed in a pair-fed regimen, where they received the daily (at around 12PM) average amount of food consumed by same-age LETO controls during 24 hours. Days of sacrifice included PND22, 38, 65 and 90 to examine the influence of chronic diet on developing females.
Experiment 2: Short term manipulations
Early short-term pair feeding
This pair feeding regimen started at weaning and was continued until PND45. Rats were given renewed access to ad-libitum food from PND 45 until the day of sacrifice, on PND90.
Late short-term pair feeding
This pair feeding regimen started on PND45 and was continued until PND70. Prior to and following the pair feeding period, rats were given ad-libitum access to food. The follow up was designed to allow the same recovery time as in the previous experiment, with sacrifice day on PND120. But given that the animals show a complete recovery by PND90, we realized this time window was not optimal for obesity moderation in these females and decided to sacrifice them already on PND90.
LETO and normal-fed OLETF females were maintained with ad-libitum access to standard chow; they were reared in pairs and served as controls. In addition, a few females were reared alone and their intake and body weight were assessed. No significant differences in body weight or intake were observed between single-housed and pair-housed rats, within strain, in any of the conditions, so their data were pooled.
All experimental and control groups were studied in parallel and were split into 2 different experiments for presentation and comparison purposes. The data of PND90 shown in Experiment 1 are repeated in the figures of Experiment 2 for the same reason.
Body weight and intake
Rats were weighed every fifth day from weaning at PND22-23 until PND90. Intake was assessed daily from pairs of (freely feeding) rats starting at the time of weaning (PND22). Feeding efficiency (FE) was calculated (5 day -body weight gain/ 5 day-intake in grams) in order to control for metabolic deficiencies.
Tissue collection
Experiment 1
Four sacrifice time-points were chosen throughout development in order to examine the influence of chronic dieting on developmental adiposity in the OLETF strain. PND22-23 represented the starting point of the study (weaning day). PND38 is a developmental period previously found to be a transition time-window, where the strains become closer in body weight and adiposity for the only time throughout development. PND65 and 90 represent early and mid-adulthood and are critical points in obesity development both in males and females (Schroeder et al., 2009b).
Experiment 2
The animals in the early and late short-term manipulation groups were sacrificed on PND90. Data of PND90 of both the control groups and the chronic PF group were included in this analysis for comparison.
On the experimental day, rats were weighed and sacrificed between 11:00 AM and 2:00 PM. Interscapular brown adipose tissue (BAT), Retroperitoneal (Retro) and inguinal (IAT) adipose tissues were collected from decapitated animals, weighed and a sample of the inguinal fat pad was immediately frozen on dry ice. Samples were preserved at −80 °C until analyzed. Trunk blood for leptin, oxytocin & creatinine analyses was collected in chilled heparinized vacutainer tubes coated with EDTA. Glucose was assessed immediately after decapitation (from fed animals) from trunk blood by a glucometer (Accu-check active, Roche, Germany). Plasma was stored at −80°C until assayed.
Plasma measurements
Plasma leptin, oxytocin and creatinine levels were assessed using commercial ELISA kits (R&D Systems, Minneapolis, USA for the first two and Cayman, Michigan, USA, respectively) according to the manufacturers' instructions.
Histology
Samples of the inguinal white adipose tissue (IAT) were used to characterize adipocyte cell size. Tissues were sectioned to 8 micrometers by a Cryostat (Leica) at −35°C and mounted on glass slides. Digital photographs were rapidly taken using the ACT1 program, at × 200 magnification. For each inguinal fat pad examined, 10–20 pictures were taken from 3 different zones of the sample, with at least 100 micrometers distance from each other. Adipocyte size parameters were derived from 3 to 6 representative cells from each picture, depending on the size of the cell, using the public domain National Institutes of Health Scion image program. For each animal, at least 80 cells were analyzed. Representative cells chosen presented a smooth and clear membrane, with no granulation around. A similar methodological approach was described elsewhere (Zagoory-Sharon et al., 2008; Schroeder et al., 2009a; Schroeder et al., 2009b). The estimated number of cells per fat pad was calculated using the average diameter, a density conversion factor (0.915 g/cc), and the mass of the fat pads, as previously described (Ashwell et al., 1976; MacLean et al., 2006).
Estrous cycle
Given that the OLETF females' estrous cycle has been reported to be different than that of LETO controls (Watanobe et al., 2001; Schroeder et al., 2009b), we examined their cycle to assess whether the "abnormalities" were due to their genetic background or a direct consequence of obesity. Vaginal cytology of 35–80 day old OLETF females under chronic food restriction and LETO and OLETF controls was examined daily in the morning (between 07:00–10:00) in a separate set of animals. Females were monitored for the appearance of vaginal opening, and then the daily follow up began. Samples were collected by introduction and immediate extraction of a small amount of phosphate buffer with a micropipette in the rat's vagina. The stage of the estrous cycle (metaestrous, diestrous, pro-estrous or estrous) was determined by examining the appearance and abundance of cells within the vaginal cytology samples. Metaestrous was characterized by leukocytes and clusters of cornified cells, diestrous was characterized by leukocytes and nucleated epithelial cells, pro-estrous was characterized primarily by nucleated epithelial cells, and estrous was characterized by an abundance of cornified cells. In 5-day cycles, either diestrous or estrous appeared twice. Two to six cycles were analyzed per female. The time of the first estrous was recorded, as well as the beginning of regular cycles.
Statistical approach
In Experiment 1, group differences (LETO, OLETF, and OLETF pair-fed) in BW and FE were analyzed by repeated measures ANOVA comparing the 3 groups (independent variable) over the repeated days of measurement. This was followed up by one-way ANOVA’s comparing the 3 groups at each of the ages, with post-hoc Duncan's test (p<0.05) for pairwise comparisons. Group differences in adiposity measures were similarly analyzed by one-way ANOVA’s comparing the 3 groups at each of the ages, with post-hoc Duncan's tests.
In Experiment 2, the figures also present data from the last time-point of Experiment 1 for comparison. All groups were statistically compared. In order to expose further differences that did not reach statistical significance due to the large difference between LETO and OLETF females, follow-up analysis was performed comparing the pair feeding groups with the OLETF controls only, followed by post-hoc Duncan's tests.
Results
Experiment 1: Chronic pair feeding throughout development
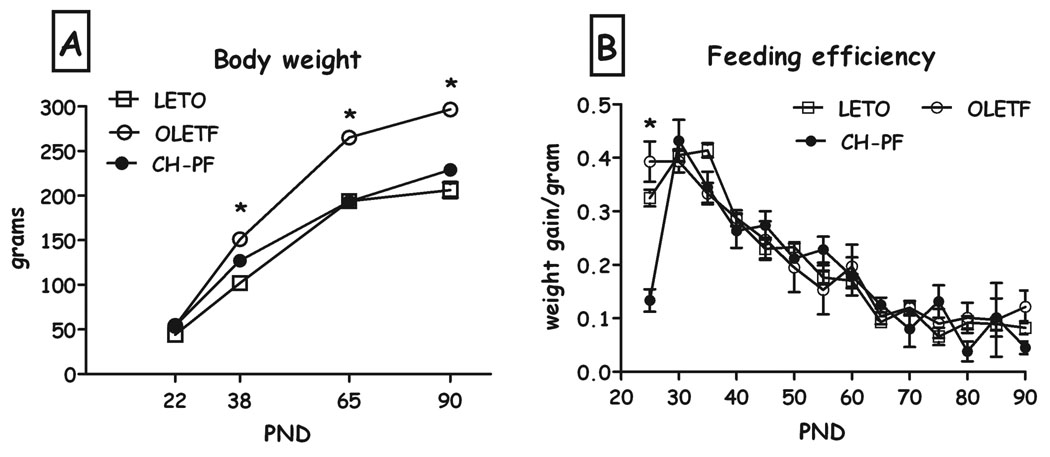
OLETF females under chronic pair feeding weighed significantly less than control OLETF females, starting from PND25 and through PND90 (F(28,22)=5.78, p<0.001 for the time X group interaction; Fig. 1a). FE did not differ between LETO, OLETF and pair fed females (not shown).
Fig. 1.
LETO, OLETF and chronic pair-feeding (CH-PF) OLETF females’ body weight (A) and feeding efficiency (B) on PND 22, 38, 65 & 90. Data are presented as means and SEM. *p<0.05 for significant differences between CH-PF and OLETF controls. N= 6–12 per group.
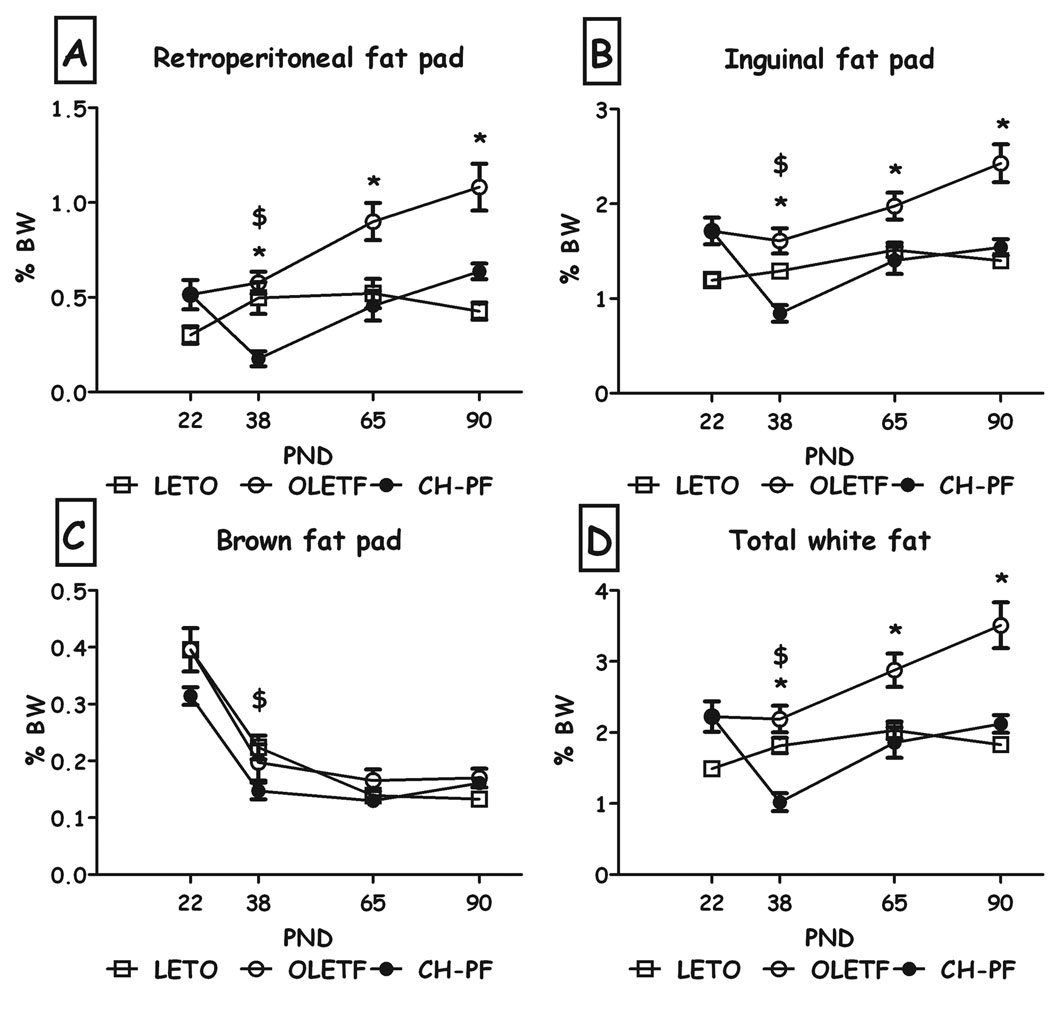
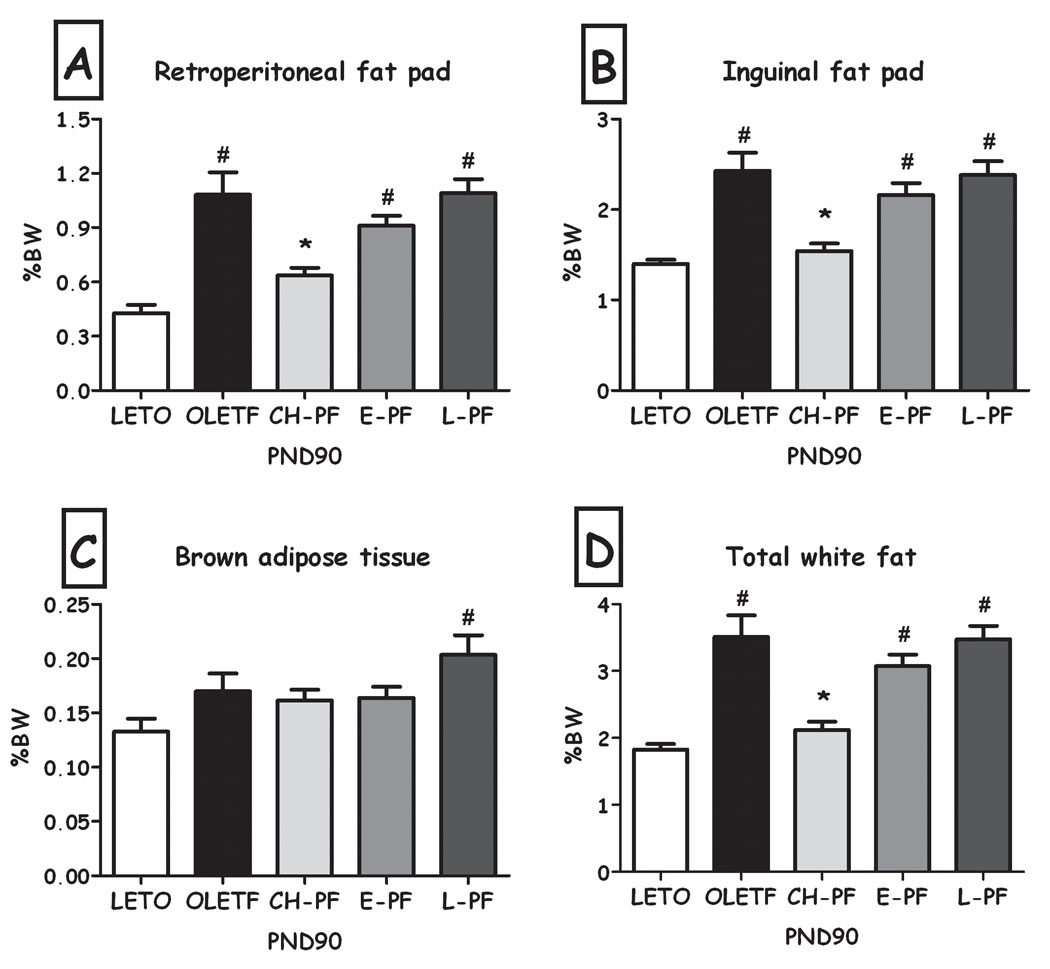
Chronic pair-feeding normalized the weight of the white fat pads (inguinal & retroperitoneal, expressed as percent of the rat's BW) to that of the LETO controls on PND60 and 90; as shown in Fig. 2. On PND38, the manipulation even further reduced fat levels, including BAT, significantly below LETO levels (all Fs>6.41, all p<0.01, Duncan's tests, p<0.05).
Fig. 2.
Percentage weight of the different fat pads of LETO, OLETF and chronic pair-feeding (CH-PF) females on PND 22, 38, 65 & 90 (expressed as percent of BW). Retroperitoneal white fat (A), inguinal fat pad (B), brown fat (C), and total white fat (D). Data are presented as means and SEM. *p<0.05 for significant differences between CH-PF and OLETF controls; $p<0.05 for significant differences between CH-PF and LETO controls. N= 6–12 per group.
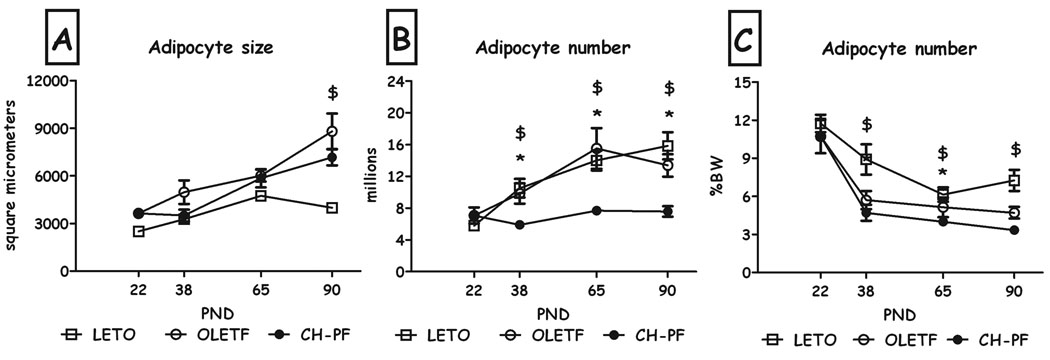
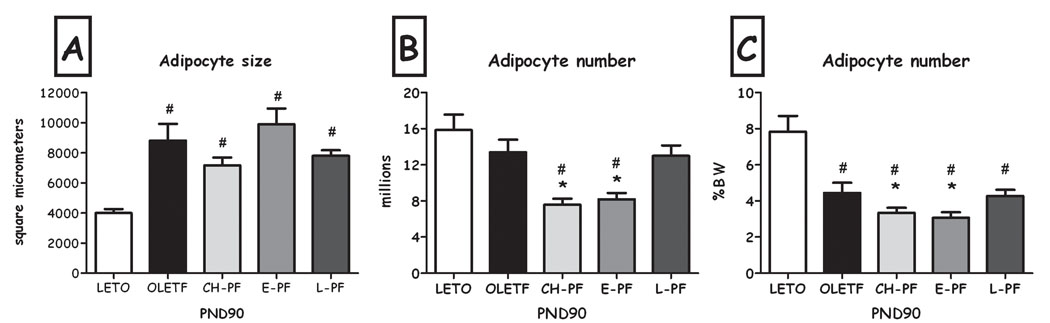
As can be seen in Fig. 3a, chronic pair-feeding did not affect adipocyte size on PND 38, 65 & 90, compared to OLETF controls. In contrast, this manipulation resulted in a drastic decrease in adipocyte number compared to both OLETF and LETO controls, at all 3 ages (Fig. 3b), an effect that remained compared to LETO females even when normalized to body weight (Fig. 3c) (all Fs>6.71, all p<0.05, Duncan's tests).
Figure 3.
Adipocyte size (A), estimated number of adipocytes (B) and adipocyte number (expressed as percent of BW) (C) of LETO, OLETF and chronic pair-feeding (CH-PF) females on PND22, 38, 65 & 90. Data are presented as means and SEM. *p<0.05 for significant differences between CH-PF and OLETF controls; $p<0.05 for significant differences between CH-PF and LETO controls. N= 4–6 per group.
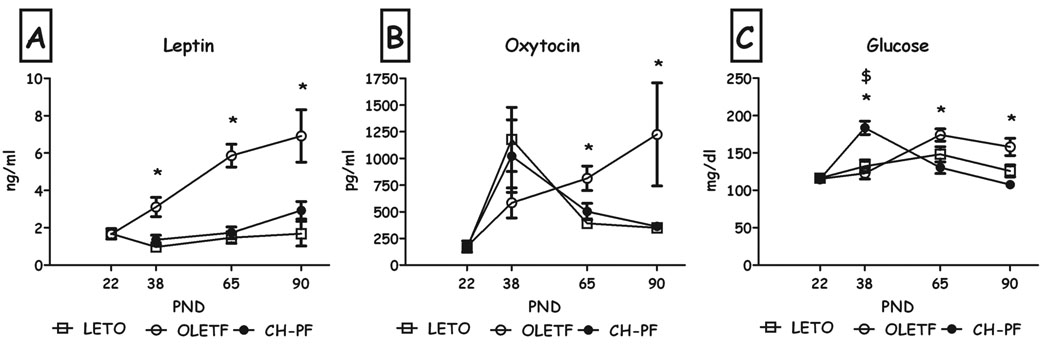
Chronic pair-feeding decreased plasma leptin levels compared to OLETF controls, at all 3 ages (all Fs>8.53, all p<0.01, Duncan's tests; Fig. 4a). While group differences were not found on PND38 in plasma oxytocin levels, pair-feeding significantly normalized oxytocin to LETO levels on PND65 & 90 (both F>4.66, p<0.05; Fig. 4b). Blood glucose levels were significantly higher in chronic pair-feeding than both control groups on PND38, and were normalized to LETO levels on PND65 & 90 (all Fs>9.5, all p<0.01, Duncan's test; Fig. 4c).
Fig. 4.
Plasma leptin (A), oxytocin (B) and glucose (C) levels of LETO, OLETF and chronic pair-feeding (CH-PF) females on PND22, 38, 65 & 90. Data are presented as means and SEM. *p<0.05 for significant differences between CH-PF and OLETF controls; $p<0.05 for significant differences between CH-PF and LETO controls. N= 4–6 per group.
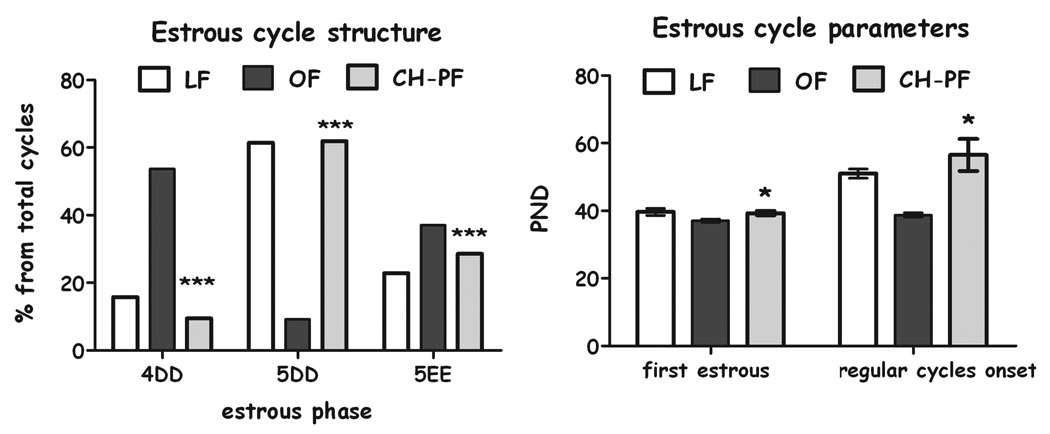
Chronic pair-feeding normalized the structure of the estrous cycle (chi-square=35.06, df=2, p<0.001), Fig 5A) which was abnormal in the OLETF females. Chronic pair feeding also delayed the appearance of the first estrous (F(2,22)=4.66, p<0.05) and the regular cycles (F(2,21)=17.26, p<0.001), normalizing them to LETO controls (Fig. 5B).
Fig. 5.
Estrous cycle parameters of LETO, OLETF and CH-PF females. Estrous cycle structure (4d: 4 day cycle, 5DD: 5 day cycle with double diestrous and 5EE: 5 day cycle with double estrous) (A) is presented in percentages from total cycles (chi square ***p<0.001); appearance of first estrous and onset of regular cycles (B) are presented as means and SEM. *p<0.05 for significant differences between CH-PF and OLETF controls. N= 6–12 per group.
Experiment 2: Short term manipulations
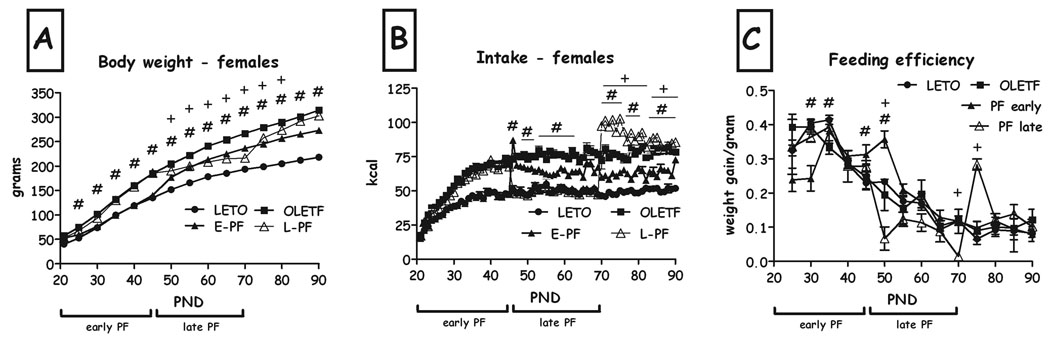
Compared to freely-feeding OLETF controls, short-term diet-restriction manipulations affected body weight over time (F(42,51)=12.98, p<0.001 for the time X group interaction; ANOVAs at all ages F>8.83, all p<0.01; Fig. 6a). Duncan's tests revealed that the early short-term diet reduced BW beyond the food-restriction period, throughout the study. In contrast, the late manipulation reduced BW only during the restriction period (Fig. 6a).
Fig. 6.
Body weight (A), intake (B) and feeding efficiency (C) of LETO, OLETF, chronic (CH-PF), early (E-PF) and late (L-PF) pair-feeding OLETF females from PND22 until PND90. Data are presented as means and SEM. OLETF and LETO FE did no differ and the PF groups were only compared to the OLETF females due to lack of space. #p<0.05 for significant differences between E-PF and OLETF controls and +p<0.05 for significant differences between L-PF and OLETF controls. N= 6–12 per group.
Compared to OLETF controls, short term manipulations affected food intake over time (F(28,16)=55.55, p<0.001 for the time X group interaction; ANOVAs at all ages F>4.61, all p<0.05; Fig. 6b). Duncan's tests revealed that release from the early short-term diet was followed by one day of hyperphagia, after which the females maintained spontaneous eating levels that were consistently below control OLETF intake levels. The late pair-feeding manipulation was followed by sustained hyperphagia that lasted until the termination of the follow-up.
Feeding efficiency (FE) was decreased during the initial week of diet restriction in early PF, and increased immediately after release from food restriction in this group, after which no difference was found in FE from OLETF controls (F(3,26)= 13.90, p<0.001; Fig. 6c). Late restriction produced the following pattern: FE was decreased on PND50 (F(3,26)= 13.90, p<0.001), and exhibited a dramatic increase immediately after release from food restriction, after which no difference was found in FE from OLETF controls (F(3,26)= 16.51, p<0.001, Duncan's test, p<0.05; Fig. 6c).
The two short-term manipulations did not have significant effects on total percent of adipose tissue, compared to OLETF controls (Figure 7). While adipocyte size was not affected by either manipulation (Fig. 8a), adipocyte number was significantly lower after the early, but not the late diet restriction (F(2,11) =7.45, p<0.01; Duncan's test, p<0.05; Fig. 8b). This effect remained significant even when the number of cells was normalized not only to the weight of the inguinal pad, but also to the rat's body weight (Fig. 8c).
Fig. 7.
Percentage weight of the different fat pads of LETO, OLETF, chronic (CH-PF), early (E-PF) and late (L-PF) pair feeding OLETF females on PND90 (expressed as percent of BW). Retroperitoneal white fat (A), inguinal fat pad (B), brown fat (C), and total white fat (D). Data are presented as means and SEM. *p<0.05 for significant differences compared to OLETF controls; # p<0.05 for significant differences compared to LETO controls. N= 6–12 per group.
Fig. 8.
Adipocyte size (A), adipocyte number (B), and adipocyte number (expressed as percent of BW) (C) of LETO, OLETF, chronic (CH-PF), early (E-PF) and late (L-PF) pair-feeding OLETF females on PND90. Data are presented as means and SEM. *p<0.05 for significant differences compared to OLETF controls; # p<0.05 for significant differences compared to LETO controls. N= 6–12 per group.
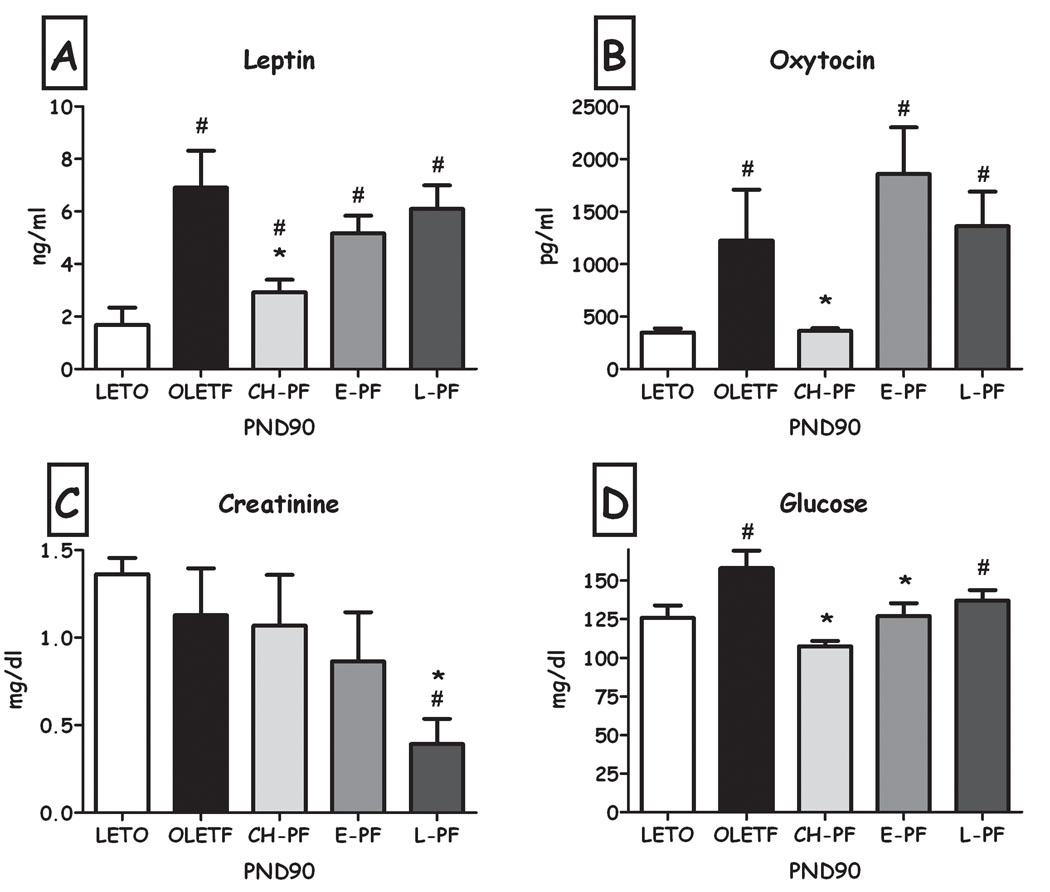
Plasma leptin and oxytocin were not significantly affected by short-term manipulations (Fig. 9a&b). However, the late short term manipulation reduced creatinine levels compared to both LETO and OLETF controls ((F(4,23) =3.98, p<0.05; Duncan's test, p<0.05; Fig. 9c) and the early short-term PF significantly reduced blood glucose levels compared to OLETF controls (Duncan's test, p<0.05; the ANOVA only tended towards significance, p=0.086; Fig. 9d).
Fig. 9.
Plasma leptin (A), Oxytocin (B), Creatinine (C) and Glucose (D) levels of LETO, OLETF, chronic (CH-PF), early (E-PF) and late (L-PF) pair-feeding OLETF females on PND90. Data are presented as means and SEM. *p<0.05 for significant differences compared to OLETF controls; # p<0.05 for significant differences compared to LETO controls. N= 4–6 per group.
Discussion
Chronic food restriction produced fast and effective normalization of body weight and adipose tissue in the females as previously reported if males. Specifically, PND38 represents an exception, where PF females presented even lower adiposity (and normalized adipocyte size) and increased blood glucose than LETO controls. We have previously dedicated some discussion to this developmental period, where OLETF and LETO become closer for the only time during development, with LETO rats presenting a transitory increase in FE and with OLETF rats presenting a transitory lack of increase in body fat, adipocyte area and drastic changes in hypothalamic gene expression (only males have been examined) (Schroeder et al., 2009b). All of this led us to conclude that some re-organization of both central and peripheral parameters take place around this age, and we believed that intervening during this period of time would have long term implications for the obesity in the OLETF females. Chronic food restriction normalized the total degree of adiposity in female OLETF rats, but in a different manner than that observed in the LETO females. In the LETO strain, the low adiposity is integrated by a relatively large amount of small fat cells, while the "normalized Chronic -PF" OLETF females presented a small amount of large fat cells.
During persistent positive energy balance, adipogenesis may become impaired after initial adipocyte hypertrophy, and then further hypertrophy may result in fat cell dysfunction. In obesity, the development of metabolic complications is closely related to how fat is stored and not only to the amount of fat stored (hypertrophy versus hyperplasia) (Ailhaud, 2006; Bays et al., 2008). The enlargement of adipocytes is associated with substantial changes in metabolic functions (such as leptin and insulin resistance) and it has been hypothesized that such alterations may contribute to the health risks of obesity. Adipocytes are known to release a variety of adipokines, including cytokines, chemokines, and many other biologically active molecules (Ailhaud, 2006). These secreted products may be involved in the development of a chronic low-grade inflammatory state, which may underlie the pathogenesis of the metabolic and cardiovascular complications of obesity. Large fat cells have been found to secrete higher amounts of adipokines such as leptin, IL-6, IL-8, TNF-alpha and adiponectin (among others), compared to small cells (Skurk et al., 2007). In contrast to the increased cell size, obese subjects tend to have a reduced number of adipocytes (Gustafson et al., 2009; Isakson et al., 2009). The amount of pre-adipocytes that can undergo differentiation is reduced in hypertrophic obesity (Permana et al., 2004; Isakson et al., 2009) and their capacity to differentiate to adipose cells appears to be negatively correlated with both BMI and adipocyte cell size of the donors (Isakson et al., 2009). This profile is consistent with our recent work showing that OLETF females have hypertrophic obesity, but with significantly reduced relative number of cells compared to LETO controls (Schroeder et al., 2009b). We now observe that despite the body weight and adiposity normalization of OLETF females under chronic food restriction, their adipocyte size remained high, and the main source of inguinal fat reduction was an even greater reduction (compared to the already low fat cell number in the OLETF females) in cell number (both in estimated number and normalized to body weight). Accordingly, leptin levels (which reflect fat cell size), while significantly reduced, remained higher than in LETO controls.
We saw a similar pattern in the early pair-feeding group despite the 45-day post-diet recovery time. Fat cell size remained high, cell number was significantly reduced and leptin levels were high. It appears that the restricted feeding intervention during this critical time for adipocyte hyperplasia in the females was not effective in altering the structure of the fat tissues to make it "healthier". This is especially true because beyond a possible reorganization of central hypothalamic gene expression that may have led to the decrease in voluntary intake and body weight observed in the early pair-feeding group after the release to ad-libitum food access; peripheral adipose tissues were completely restored by the end of the follow up. However, CNS and ANS regulation of energy expenditure are integrated to achieve balanced energy homeostasis depending on physiologic needs (Redinger, 2009). Our results in the early pair-feeding group, where adiposity was not reduced despite the moderation in food intake and body weight, suggest the involvement of mechanisms beyond the central control of feeding in the recovery of adiposity that should not be overlooked when intending to understand and treat female obesity.
The late pair-feeding did not provide any sustained beneficial outcomes. Instead of reducing adiposity in the long term, late dieting produced robust "rebound" hyperphagia after release from the diet that lasted more than 2 weeks. It appears that early PF successfully lowered the innate threshold of body mass and fat and achieved a central re-organization of the orexigenic and anorexigenic pathways, while late PF did not. Accordingly, OLETF females efficiently over-consumed food to achieve their genetically determined phenotype and completely recovered from the diet (while losing lean body mass, as reflected by the low creatinine).
The FE calculation revealed a general pattern of weight-gain in both early and late groups that was determined by overall caloric intake. During the first days after release from the diet, a significant surge in efficiency was detected, presumably resulting from the combination of the increase in intake together with reduced expenditure of energy and a higher caloric efficiency of regain that may be linked with suppressed lipid utilization early in the relapse process. In the late PF group, FE tended to be lower than in controls during the period of food restriction; probably representing a lack of compensation of energy expenditure decrease as a result of the diet. In accordance with previous reports on effective weight regain after dieting performed later in life, females in this group presented a massive increase in intake and FE after release from food restriction compared to the other groups, and their relapse to the obese state was the most accelerated.
Regarding Oxytocin, central and peripheral concentrations are related and elevated in pathological states of energy balance such as obesity (Stock et al., 1989; Smith, 2006). Peripheral oxytocin in obese humans (Stock et al., 1989), in a rodent model of diet induced obesity (Northway et al., 1989) and in the OLETF rat (Zagoory-Sharon et al., 2008; Schroeder et al., 2009b) were reported as being significantly higher than in control subjects. One study further reported that oxytocin levels decreased significantly following gastric banding, a procedure that induced weight loss in the patients (Stock et al., 1989). During adolescence, we found a transitory surge in oxytocin levels, especially in the LETO strain and in the OLETF chronic PF females, which was in accordance with our previous findings in males and females of both strains at this age (Schroeder et al., 2009b), but the high levels expected from adult OLETF females were absent in the chronic PF animals, suggesting that high Oxytocin levels may be related to hyper-adiposity, hyperleptinemia, or even to their reduced voluntary intake. Accordingly, levels on the early and late PF manipulations were high. While the mechanism by which oxytocin exerts its effects on energy balance are still largely unknown, the present findings further support the disruption in other systems (such as leptin) related to CCK that become dysregulated in the OLETF strain with the development of obesity that may further contribute to the worsening of their obese phenotype.
In humans, menstruation appearance is closely related to percent body fat with obese adolescents usually presenting early puberty compared to lean girls (Wang, 2002). OLETF females also showed an early appearance of the first estrous and also an abnormal estrous cycle structure, as compared with LETO controls (Schroeder et al., 2009b). Chronic pair feeding normalized the estrous cycle structure to that of LETO rats and also delayed the appearance of the first estrous and the first regular cycle, suggesting that these disruptions derive from the OLETF's obesity levels and not from the genetic mutation itself. The influence of these changes on the OLETF females' fertility problems (Watanobe et al., 2001) remains to be explored.
Finally, we expected that chronic dieting would produce normalization in body fat as previously observed in OLETF males (Bi et al., 2001; Schroeder et al., 2010) as a consequence of a reduction in fat cell hypertrophy (Schroeder et al., 2010). Male OLETF rats showed a marked normalization in plasma leptin and oxytocin just like the females, but the normalization in adiposity was related to a reduction in cell size without affecting cell number. Moreover, early short term dieting even increased their cell number, normalizing them to the levels in LETO controls (Schroeder et al., 2010). In addition, we observed a pattern of adipocyte number development that differed between the sexes in both strains. Adipocyte hypertrophy was evident in OLETFs of both sexes from PND7, but a significantly higher level of adipocyte hyperplasia was observed only in the OLETF females in the pre-weaning to PND48 period (Schroeder et al., 2009a; Schroeder et al., 2009b). In the males, the period of highest hyperplasia occurred between the weaning day and PND65 (Schroeder et al., 2009b). These last findings may have strong implications for the adiposity related responses to early dietary interventions in both sexes during early development.
Treatments for adult overweight are often unsuccessful in the long term. We still believe in the potential of early interventions to achieve significant, long lasting outcomes, but the improvement obtained in the present study from chronic dieting resulted from a very drastic regimen that, to be realistic, would be too hard to implement in human patients. The reduction in voluntary intake seen in the females of the early group may have promising implications for late life obesity levels and health that were beyond our follow up period. Further research on young obese females is needed in order to discover the most appropriate timing and type of intervention that could lead to sustained improvement in obesity in genetically predisposed individuals.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aggoun Y. Obesity, metabolic syndrome, and cardiovascular disease. Pediatr. Res. 2007;61:653–659. doi: 10.1203/pdr.0b013e31805d8a8c. [DOI] [PubMed] [Google Scholar]
- Ailhaud G. Adipose tissue as a secretory organ: from adipogenesis to the metabolic syndrome. C. R. Biol. 2006;329:570–577. doi: 10.1016/j.crvi.2005.12.012. discussion 653-575. [DOI] [PubMed] [Google Scholar]
- Ashwell M, Priest P, Bondoux M, Sowter C, McPherson CK. Human fat cell sizing--a quick, simple method. J. Lipid Res. 1976;17:190–192. [PubMed] [Google Scholar]
- Barkan AL, Dimaraki EV, Jessup SK, Symons KV, Ermolenko M, Jaffe CA. Ghrelin secretion in humans is sexually dimorphic, suppressed by somatostatin, and not affected by the ambient growth hormone levels. J. Clin. Endocrinol. Metab. 2003;88:2180–2184. doi: 10.1210/jc.2002-021169. [DOI] [PubMed] [Google Scholar]
- Barker DJ. In utero programming of chronic disease. Clin. Sci. (Lond) 1998;95:115–128. [PubMed] [Google Scholar]
- Barker DJ. Fetal programming of coronary heart disease. Trends Endocrinol. Metab. 2002;13:364–368. doi: 10.1016/s1043-2760(02)00689-6. [DOI] [PubMed] [Google Scholar]
- Bays H, Blonde L, Rosenson R. Adiposopathy: how do diet, exercise and weight loss drug therapies improve metabolic disease in overweight patients? Expert. Rev. Cardiovasc. Ther. 2006;4:871–895. doi: 10.1586/14779072.4.6.871. [DOI] [PubMed] [Google Scholar]
- Bays HE, Gonzalez-Campoy JM, Bray GA, Kitabchi AE, Bergman DA, Schorr AB, Rodbard HW, Henry RR. Pathogenic potential of adipose tissue and metabolic consequences of adipocyte hypertrophy and increased visceral adiposity. Expert. Rev. Cardiovasc. Ther. 2008;6:343–368. doi: 10.1586/14779072.6.3.343. [DOI] [PubMed] [Google Scholar]
- Bi S, Ladenheim EE, Schwartz GJ, Moran TH. A role for NPY overexpression in the dorsomedial hypothalamus in hyperphagia and obesity of OLETF rats. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2001;281:R254–R260. doi: 10.1152/ajpregu.2001.281.1.R254. [DOI] [PubMed] [Google Scholar]
- Bjorntorp PA. Sex differences in the regulation of energy balance with exercise. Am. J. Clin. Nutr. 1989;49:958–961. doi: 10.1093/ajcn/49.5.958. [DOI] [PubMed] [Google Scholar]
- Blevins JE, Eakin TJ, Murphy JA, Schwartz MW, Baskin DG. Oxytocin innervation of caudal brainstem nuclei activated by cholecystokinin. Brain. Res. 2003;993:30–41. doi: 10.1016/j.brainres.2003.08.036. [DOI] [PubMed] [Google Scholar]
- Blumberg S, Haba D, Schroeder M, Smith GP, Weller A. Independent ingestion and microstructure of feeding patterns in infant rats lacking CCK-1 receptors. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2006;290:R208–R218. doi: 10.1152/ajpregu.00379.2005. [DOI] [PubMed] [Google Scholar]
- Cortright RN, Chandler MP, Lemon PW, DiCarlo SE. Daily exercise reduces fat, protein and body mass in male but not female rats. Physiol Behav. 1997;62:105–111. doi: 10.1016/s0031-9384(97)00148-0. [DOI] [PubMed] [Google Scholar]
- Espelund U, Hansen TK, Hojlund K, Beck-Nielsen H, Clausen JT, Hansen BS, Orskov H, Jorgensen JO, Frystyk J. Fasting unmasks a strong inverse association between ghrelin and cortisol in serum: studies in obese and normal-weight subjects. J. Clin. Endocrinol. Metab. 2005;90:741–746. doi: 10.1210/jc.2004-0604. [DOI] [PubMed] [Google Scholar]
- Gayle DA, Desai M, Casillas E, Beloosesky R, Ross MG. Gender-specific orexigenic and anorexigenic mechanisms in rats. Life Sci. 2006;79:1531–1536. doi: 10.1016/j.lfs.2006.04.015. [DOI] [PubMed] [Google Scholar]
- Geary NaL J. Sex differences in the brain, from genes to behavior. Oxford: Oxford University Press; 2008. Sex differences in energy metabolism, obesity and eating behavior. [Google Scholar]
- Gustafson B, Gogg S, Hedjazifar S, Jenndahl L, Hammarstedt A, Smith U. Inflammation and impaired adipogenesis in hypertrophic obesity in man. Am J Physiol Endocrinol Metab. 2009 doi: 10.1152/ajpendo.00377.2009. in press. [DOI] [PubMed] [Google Scholar]
- Hakansson ML, Brown H, Ghilardi N, Skoda RC, Meister B. Leptin receptor immunoreactivity in chemically defined target neurons of the hypothalamus. Neurosci. 1998;18:559–572. doi: 10.1523/JNEUROSCI.18-01-00559.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedley AA, Ogden CL, Johnson CL, Carroll MD, Curtin LR, Flegal KM. Prevalence of overweight and obesity among US children, adolescents, and adults, 1999–2002. JAMA. 2004;291:2847–2850. doi: 10.1001/jama.291.23.2847. [DOI] [PubMed] [Google Scholar]
- Isakson P, Hammarstedt A, Gustafson B, Smith U. Impaired preadipocyte differentiation in human abdominal obesity: role of Wnt, tumor necrosis factor-alpha, and inflammation. Diabetes. 2009;58:1550–1557. doi: 10.2337/db08-1770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawano K, Hirashima T, Mori S, Natori T. OLETF (Otsuka Long-Evans Tokushima Fatty) rat: a new NIDDM rat strain. Diabetes Res. Clin. Pract. 1994;24 Suppl:S317–S320. doi: 10.1016/0168-8227(94)90269-0. [DOI] [PubMed] [Google Scholar]
- Kawano K, Hirashima T, Mori S, Saitoh Y, Kurosumi M, Natori T. Spontaneous long-term hyperglycemic rat with diabetic complications. Otsuka Long-Evans Tokushima Fatty (OLETF) strain. Diabetes. 1992;41:1422–1428. doi: 10.2337/diab.41.11.1422. [DOI] [PubMed] [Google Scholar]
- Legato MJ. Gender-specific aspects of obesity. Int. J. Fertil. Womens Med. 1997;42:184–197. [PubMed] [Google Scholar]
- MacLean PS, Higgins JA, Jackman MR, Johnson GC, Fleming-Elder BK, Wyatt HR, Melanson EL, Hill JO. Peripheral metabolic responses to prolonged weight reduction that promote rapid, efficient regain in obesity-prone rats. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2006;290:R1577–R1588. doi: 10.1152/ajpregu.00810.2005. [DOI] [PubMed] [Google Scholar]
- Man ZW, Hirashima T, Mori S, Kawano K. Decrease in triglyceride accumulation in tissues by restricted diet and improvement of diabetes in Otsuka Long-Evans Tokushima fatty rats, a non-insulin-dependent diabetes model. Metabolism. 2000;49:108–114. doi: 10.1016/s0026-0495(00)90913-2. [DOI] [PubMed] [Google Scholar]
- Miller JL, Silverstein JH. Management approaches for pediatric obesity. Nat. Clin. Pract. Endocrinol. Metab. 2007;3:810–818. doi: 10.1038/ncpendmet0669. [DOI] [PubMed] [Google Scholar]
- Moran TH, Bi S. Hyperphagia and obesity of OLETF rats lacking CCK1 receptors: developmental aspects. Dev. Psychobiol. 2006;48:360–367. doi: 10.1002/dev.20149. [DOI] [PubMed] [Google Scholar]
- Nakamura H, Kihara Y, Tashiro M, Kanagawa K, Shirohara H, Yamamoto M, Yoshikawa H, Fukumitsu K, Hirohata Y, Otsuki M. Defects of cholecystokinin (CCK)-A receptor gene expression and CCK-A receptor-mediated biological functions in Otsuka Long-Evans Tokushima Fatty (OLETF) rats. J. Gastroenterol. 1998;33:702–709. doi: 10.1007/s005350050158. [DOI] [PubMed] [Google Scholar]
- Nance DM, Bromley B, Barnard RJ, Gorski RA. Sexually dimorphic effects of forced exercise on food intake and body weight in the rat. Physiol. Behav. 1977;19:155–158. doi: 10.1016/0031-9384(77)90173-1. [DOI] [PubMed] [Google Scholar]
- Northway MG, Morris M, Geisinger KR, MacLean DB. Effects of a gastric implant on body weight and gastrointestinal hormones in cafeteria diet obese rats. Physiol. Behav. 1989;45:331–335. doi: 10.1016/0031-9384(89)90135-2. [DOI] [PubMed] [Google Scholar]
- Olson BR, Drutarosky MD, Stricker EM, Verbalis JG. Brain oxytocin receptor antagonism blunts the effects of anorexigenic treatments in rats: evidence for central oxytocin inhibition of food intake. Endocrinology. 1991a;129:785–791. doi: 10.1210/endo-129-2-785. [DOI] [PubMed] [Google Scholar]
- Olson BR, Drutarosky MD, Stricker EM, Verbalis JG. Brain oxytocin receptors mediate corticotropin-releasing hormone-induced anorexia. Am. J. Physiol. 1991b;260:R448–R452. doi: 10.1152/ajpregu.1991.260.2.R448. [DOI] [PubMed] [Google Scholar]
- Olson BR, Hoffman GE, Sved AF, Stricker EM, Verbalis JG. Cholecystokinin induces c-fos expression in hypothalamic oxytocinergic neurons projecting to the dorsal vagal complex. Brain Res. 1992;569:238–248. doi: 10.1016/0006-8993(92)90635-m. [DOI] [PubMed] [Google Scholar]
- Ostergard T, Hansen TK, Nyholm B, Gravholt CH, Djurhuus CB, Hosoda H, Kangawa K, Schmitz O. Circulating ghrelin concentrations are reduced in healthy offspring of Type 2 diabetic subjects, and are increased in women independent of a family history of Type 2 diabetes. Diabetologia. 2003;46:134–136. doi: 10.1007/s00125-002-0985-4. [DOI] [PubMed] [Google Scholar]
- Park SY, Choi GH, Choi HI, Ryu J, Jung CY, Lee W. Calorie restriction improves whole-body glucose disposal and insulin resistance in association with the increased adipocyte-specific GLUT4 expression in Otsuka Long-Evans Tokushima fatty rats. Arch. Biochem. Biophys. 2005;436:276–284. doi: 10.1016/j.abb.2005.01.010. [DOI] [PubMed] [Google Scholar]
- Permana PA, Nair S, Lee YH, Luczy-Bachman G, Vozarova De Courten B, Tataranni PA. Subcutaneous abdominal preadipocyte differentiation in vitro inversely correlates with central obesity. Am. J. Physiol. Endocrinol. Metab. 2004;286:E958–E962. doi: 10.1152/ajpendo.00544.2003. [DOI] [PubMed] [Google Scholar]
- Plagemann A. Perinatal nutrition and hormone-dependent programming of food intake. Horm. Res. 2006;65 Suppl 3:83–89. doi: 10.1159/000091511. [DOI] [PubMed] [Google Scholar]
- Redinger RN. Fat storage and the biology of energy expenditure. Transl. Res. 2009;154:52–60. doi: 10.1016/j.trsl.2009.05.003. [DOI] [PubMed] [Google Scholar]
- Schroeder M, Lavi-Avnon Y, Dagan M, Zagoory-Sharon O, Moran TH, Weller A. Diurnal and nocturnal nursing behavior in the OLETF rat. Dev. Psychobiol. 2007a;49:323–333. doi: 10.1002/dev.20206. [DOI] [PubMed] [Google Scholar]
- Schroeder M, Lavi-Avnon Y, Zagoory-Sharon O, Moran TH, Weller A. Preobesity in the infant OLETF rat: the role of suckling. Dev. Psychobiol. 2007b;49:685–691. doi: 10.1002/dev.20235. [DOI] [PubMed] [Google Scholar]
- Schroeder M, Moran TH, Weller A. Attenuation of obesity by early life food-restriction in genetically hyperphagic male OLETF rats: Peripheral mechanisms. Horm. Behav. 2010;57(4–5):455–462. doi: 10.1016/j.yhbeh.2010.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroeder M, Shbiro L, Zagoory-Sharon O, Moran TH, Weller A. Toward an animal model of childhood-onset obesity: follow-up of OLETF rats during pregnancy and lactation. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2009a;296:R224–R232. doi: 10.1152/ajpregu.90697.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroeder M, Zagoory-Sharon O, Lavi-Avnon Y, Moran TH, Weller A. Weight gain and maternal behavior in CCK1 deficient rats. Physiol. Behav. 2006;89:402–409. doi: 10.1016/j.physbeh.2006.07.008. [DOI] [PubMed] [Google Scholar]
- Schroeder M, Zagoory-Sharon O, Shbiro L, Marco A, Hyun J, Moran TH, Bi S, Weller A. Development of obesity in the Otsuka Long-Evans Tokushima Fatty rat. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2009b;297:R1749–R1760. doi: 10.1152/ajpregu.00461.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skurk T, Alberti-Huber C, Herder C, Hauner H. Relationship between adipocyte size and adipokine expression and secretion. J. Clin. Endocrinol. Metab. 2007;92:1023–1033. doi: 10.1210/jc.2006-1055. [DOI] [PubMed] [Google Scholar]
- Smith GP. Cholecystokinin and treatment of meal size: proof of principle. Obesity (Silver Spring) 2006;14 Suppl 4:168S–170S. doi: 10.1038/oby.2006.300. [DOI] [PubMed] [Google Scholar]
- Stock S, Granstrom L, Backman L, Matthiesen AS, Uvnas-Moberg K. Elevated plasma levels of oxytocin in obese subjects before and after gastric banding. Int. J. Obes. 1989;13:213–222. [PubMed] [Google Scholar]
- Taylor PD, Poston L. Developmental programming of obesity in mammals. Exp. Physiol. 2007;92:287–298. doi: 10.1113/expphysiol.2005.032854. [DOI] [PubMed] [Google Scholar]
- Ur E, Wilkinson DA, Morash BA, Wilkinson M. Leptin immunoreactivity is localized to neurons in rat brain. Neuroendocrinology. 2002;75:264–272. doi: 10.1159/000054718. [DOI] [PubMed] [Google Scholar]