Solid-state NMR is a powerful technique for the investigation of complex biological systems such as membrane proteins and amyloid fibrils. In magic-angle spinning (MAS) NMR, structural information is obtained via the reintroduction of anisotropic interactions.[1] In particular, a large number of homonuclear dipolar recoupling schemes have been developed and applied to record correlation spectra and measure internuclear distances in peptides and proteins.[1] While many recoupling techniques efficiently transfer polarization between directly bonded 13C nuclei, their effectiveness can be reduced significantly when distant 13C spins with weak dipolar couplings are involved. This limitation is generally imposed both by the experimental constraints that must be maintained during long mixing periods and by the inherent complexities of multiple-spin systems such as dipolar truncation,[2, 3] that is the attenuation of weak dipolar couplings by stronger dipolar couplings in the recoupled dipolar Hamiltonian. As a consequence, spin diffusion techniques,[4–8] which circumvent some of these limitations,[9] have been widely utilized to estimate long-range homonuclear structural constraints in protein solid-state NMR studies.[10, 11] In this communication we present an experimental approach that provides highly sensitive long-range correlations between aliphatic 13C nuclei through the combination of isotope dilution and efficient polarization transfer via a band selective radio frequency dipolar recoupling (BASE RFDR) scheme. We demonstrate this method with a sample of PI3-SH3 (the SH3 domain of the p85α subunit of phosphatidylinositol 3 kinase) in amyloid fibril form.
The 13C alternating labeling scheme devised by LeMaster and Kushlan,[12] and introduced to solid-state NMR by Hong,[13] which employs 2-13C1 or 1,3-13C2 glycerol as the carbon source, results in amino acids 13C labeled at approximately every other carbon position (“odd/even” labeling). The approach simplifies 13C-13C correlation spectra by reducing the number of spectral lines and narrows the linewidths due to the elimination of J-couplings. It thus offers significant advantages over uniform 13C labeling.[10] From the perspective of the dipolar recoupling dynamics of the spin system, this type of 13C spin dilution provides two additional benefits. First, relayed polarization transfer, which is dominated by rapid diffusion between directly bonded nuclei in uniformly 13C labeled samples, is partially eliminated in odd/even 13C labeled spin systems; magnetization originating from a given site therefore propagates more directly to fewer spins, resulting in correlation spectra that present improved long-range transfer efficiency between structurally interesting pairs of nuclei. Secondly, the elimination of directly bonded 13C nuclei in most amino acid spin systems results in the partial attenuation of dipolar truncation effects, thus enabling the observation of weak dipolar couplings corresponding to long internuclear distances. However, dipolar truncation is most severe in pulse sequences that generate a first-order recoupled dipolar Hamiltonian and is considerably less pronounced in schemes that utilize second order effects to achieve polarization transfer.[9, 14, 15] Therefore, the enhanced observation of long-range correlations in spin diffusion spectra of odd/even labeled proteins (compared to uniformly labeled samples) is likely due to the reduction of relayed polarization transfer and not to the attenuation of dipolar truncation in these dilute spin systems. In contrast, as examined recently,[3] certain zero-quantum (ZQ) recoupling schemes may benefit directly from the attenuation of dipolar truncation in such spin systems.
In order to explore the advantages of alternating labeling for techniques other than spin diffusion, we investigated the application of efficient recoupling methods to a fibril sample of PI3-SH3 (86 residues) prepared with [2-13C] glycerol as the carbon source (2-PI3-SH3). As may be expected, the elimination of directly bonded nuclei interrupts relayed polarization transfer and attenuates dipolar truncation in broadband radio frequency-driven recoupling[16–20] (RFDR) experiments. The polarization transfer dynamics in this ZQ recoupling approach are heavily dominated by the strongest couplings present, which in the case of 2-13C glycerol labeling are typically the two-bond couplings between sequential 13Cα (i) and 13C’(i-1) resonances. As a consequence, the most prominent cross-peaks in broadband RFDR spectra of 2-PI3-SH3 are medium- and long-range correlations between C’ and aliphatic nuclei, while correlations between distant aliphatic nuclei, rich in structural information, are generally too weak to be observed. A representative broadband RFDR spectrum is shown in the supporting information.
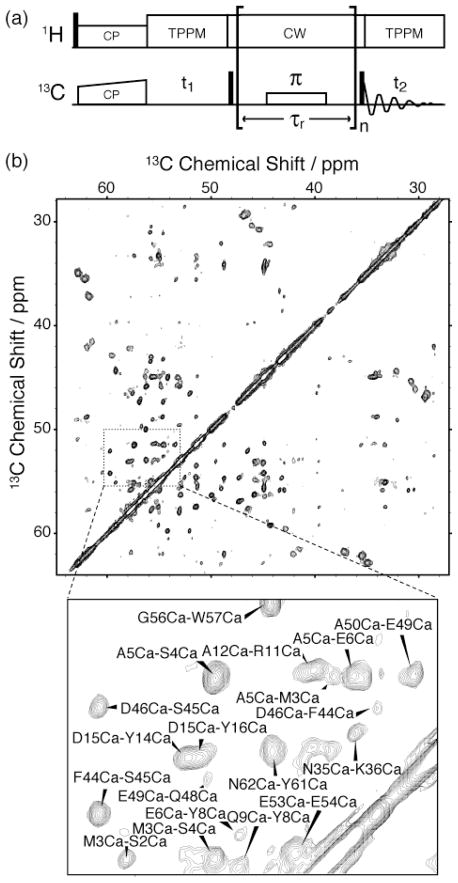
In constrast, the application of RFDR using low-power, band selective, π pulses (Fig. 1(a)) with the minimal bandwidth necessary to cover the aliphatic 13C spectral region yields intense correlation peaks between distant aliphatic nuclei, as illustrated in Fig. 1(b). This spectrum was recorded at a spinning frequency (ωr 2π) of 12.5 kHz and employed 13C recoupling π pulses with a 12.5 kHz rf field (ω1/2π) in a manner similar in appearance to conventional RFDR. During the mixing period (τmix = 17.92 ms), a 80 kHz1H cw decoupling field was applied and the 13C recoupling π pulses followed a 32-step phase sequence (XY-16, YX̄-16) optimal for compensation of chemical shift offsets and rf inhomogeneity for weak π pulses that is based on the XY-16 scheme.[21]
Figure 1.
(a) Band selective RFDR pulse sequence for two-dimensional homonuclear correlation spectroscopy. The recoupling π pulses occupy half or more of the rotor period, τr, and follow a 32-step phase scheme. (b) Long-range aliphatic correlation spectrum of 2-PI3-SH3 amyloid fibrils obtained with 17.92 ms of longitudinal mixing via BASE RFDR. The mixing time was optimized for 3.8 Å, typical internuclear distance of sequential Cα-Cα contacts. The spectrum was recorded in 27 hrs at 16.4 T magnetic field and 12.5 kHz MAS frequency, with 12.5 kHz 13C recoupling π pulses centered at 48 ppm.
The long-range polarization transfer efficiency of this BASE RFDR approach is a product of the interplay between several factors in addition to the attenuation of dipolar truncation afforded by alternating labeling. First, the selective bandwidth of the recoupling π pulses centered on the aliphatic region of the 13C spectrum exclude downfield resonances from the aliphatic-aliphatic recoupling dynamics, resulting in the elimination of relayed polarization transfer via neighboring C’ spins, which enhances the direct polarization transfer between distant aliphatic 13C spins. Concurrently, the application of long 13C π pulses occupying a large fraction of the rotor period entails a significant finite-pulse effect, even for moderate spinning frequencies, which facilitates dipolar recoupling between nuclei with small chemical shift offsets such as aliphatic spins.[18, 19] Furthermore, heteronuclear interference conditions[20] are readily avoided by the application of moderate to strong 1H decoupling fields due to the weak effective 13C rf field employed during mixing, leading to minimal polarization losses during the recoupling period. Finally, numerical simulations indicate that while polarization transfer in BASE RFDR is primarily mediated by direct 13C-13C dipolar interactions, third-spin assisted recoupling[14, 15] (TSAR), which relies on higher-order heteronuclear interactions, is active during these (13C/1H) rf field conditions and is compatible with the longitudinal mixing mechanism of BASE RFDR, potentially enhancing the attainable polarization transfer efficiency. Numerical simulations illustrating finite-pulse and TSAR effects are provided in the supplementary information.
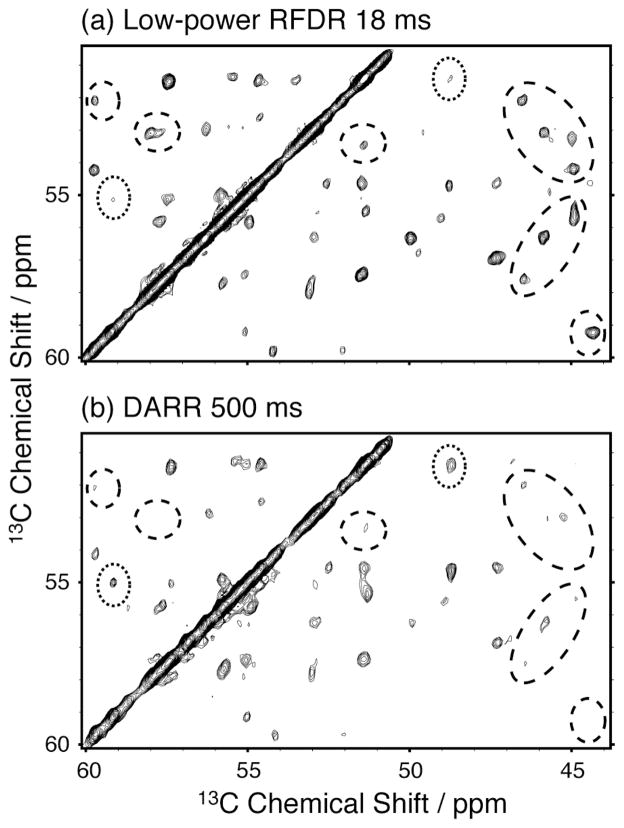
Fig. 2 demonstrates that BASE RFDR leads to improved sensitivity in aliphatic-aliphatic correlation spectra of 2-PI3-SH3 compared to that obtained with proton-driven spin diffusion[5, 6] or DARR[7, 8] for cross-peaks corresponding to internuclear distances of ~3.8 Å and above, arising from sequential inter-residue 13C-13C couplings and longer contacts. However, a few specific exceptions, where cross-peak intensities are attenuated, can be observed most likely as a consequence of strong dipolar truncation effects remaining in certain spin systems in 2-PI3-SH3. We must note that while mixing times in Fig. 2 were optimized for Cα-Cα contacts (~3.8 Å) for direct comparison, in the case of diffusion experiments these cross-peaks build up slowly at such long mixing times (300–500 ms) that longer contacts could potentially be observed.
Figure 2.
Long-range aliphatic correlations in 2-PI3-SH3 obtained with 17.92 ms of BASE RFDR (a) and 500 ms of DARR (b). Both spectra were recorded in 6.8 hrs under similar experimental conditions to those described for Fig. 1(b). BASE RFDR shows improved overall polarization transfer efficiency, highlighted by dashed ellipses. Dotted ellipses mark a few exceptions, likely the result of remaining dipolar truncation for certain spin systems. See the supporting information for full spectral views.
Optimal experimental conditions for efficient low-power recoupling are determined by the spectral bandwidth required. In the case of aliphatic carbons at 16.4 T (700 MHz 1H Larmor frequency), with a spectral dispersion of ~10 kHz, 13C rf fields of similar magnitude are required for efficient recoupling within the aliphatic region. At the same time, the rotor-synchronized 13C recoupling π pulses must occupy a significant fraction of the rotor period (half or more) in order to produce the most favorable finite-pulse effects. Thus, for 12 kHz 13C π pulses, BASE RFDR can be performed at MAS frequencies ranging from ~12 kHz to 24 kHz, the latter being the windowless recoupling irradiation limit in which the recoupling π pulse occupies the entire rotor period. Conversely, at 12 kHz spinning frequency, BASE RFDR can be applied with 13C rf fields ranging from 6 to 12 kHz.
The aliphatic-aliphatic polarization transfer approach described in this communication and demonstrated in studies of PI3-SH3 fibrils has potential applications to a variety of aspects of protein MAS NMR, including sequential resonance assignment, long-range constraints generation, and distance measurements. The BASE RFDR pulse scheme is an extension of RFDR[16–20] into a regime where the majority of the rotor period during the mixing time is occupied by weak 13C irradiation, at moderate spinning frequencies. While the efficiency of BASE RFDR has been delineated here and exploited to procure highly sensitive long-range aliphatic correlations, further methodological investigation of this dipolar recoupling scheme is currently in progress.
Experimental Section
The sample of 2-PI3-SH3 fibrils was prepared as described previously[22] but using [2-13C]glycerol and NaH13CO3 as the sole sources of carbon. The total amount of protein in each packed sample was approximately 8 mg. All experiments were performed using a custom-designed spectrometer operating at 700 MHz 1H Larmor frequency (courtesy of Dr. David J. Ruben at the Francis Bitter Magnet Laboratory, Massachusetts Institute of Technology) equipped with a 3.2 mm Varian/Chemagnetics probe (Palo Alto, CA). Correlation experiments consisted of 1H-13C cross-polarization, chemical shift evolution, longitudinal homonuclear mixing, and detection periods. TPPM[23] decoupling (100 kHz 1H rf field) was applied during the evolution and detection periods. Such high-power decoupling was desirable for improved resolution, but not strictly necessary for experimental performance. Further data acquisition details are provided in the supporting information.
Supplementary Material
Footnotes
This work was supported by National Institutes of Health Grants EB-003151 and EB-002026 and the Wellcome and Leverhulme Trusts. We thank Drs. G. de Paëpe, P. van der Wel and J. Lewandowski for stimulating discussions and Dr. Janet Kumita for advice on the preparation of samples.
Supporting information for this article is available on the WWW under http://www.angewandte.org or from the author.
Contributor Information
Marvin J. Bayro, Francis Bitter Magnet Laboratory and Department of Chemistry, Massachusetts Institute of Technology Cambridge, MA 02139 (USA)
Dr. Thorsten Maly, Francis Bitter Magnet Laboratory and Department of Chemistry, Massachusetts Institute of Technology Cambridge, MA 02139 (USA)
Dr. Neil R. Birkett, Department of Chemistry, University of Cambridge, Lensfield Road, Cambridge, CB2 1EW (UK)
Prof. Christopher M. Dobson, Department of Chemistry, University of Cambridge, Lensfield Road, Cambridge, CB2 1EW (UK)
Prof. Robert G. Griffin, Francis Bitter Magnet Laboratory and Department of Chemistry, Massachusetts Institute of Technology Cambridge, MA 02139 (USA), Fax: (+01) 617-253-5478, rgg@mit.edu
References
- 1.Griffin RG. Nature Struc Biol. 1998;5(Suppl):508. doi: 10.1038/749. [DOI] [PubMed] [Google Scholar]
- 2.Costa PR. PhD thesis. Massachusetts Institute of Technology; Cambridge, MA: 1996. [Google Scholar]
- 3.Bayro MJ, Huber M, Ramachandran R, Davenport TC, Meier BH, Ernst M, Griffin RG. J Chem Phys. 2009;130:114506. doi: 10.1063/1.3089370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bloembergen N. Physica. 1949;15:386. [Google Scholar]
- 5.Szeverenyi NM, Sullivan MJ, Maciel GE. J Magn Reson. 1982;47:462. [Google Scholar]
- 6.Meier BH. In: Advances in Magnetic and Optical Resonance. Warren WS, editor. Vol. 18. 1999. p. 1. [Google Scholar]
- 7.Takegoshi K, Nakamura S, Terao T. Chem Phys Lett. 2001;344:631. [Google Scholar]
- 8.Morcombe CR, Gaponenko V, Byrd RA, Zilm KW. J Am Chem Soc. 2004;126:7196. doi: 10.1021/ja047919t. [DOI] [PubMed] [Google Scholar]
- 9.Grommek A, Meier BH, Ernst M. Chem Phys Lett. 2006;427:404. [Google Scholar]
- 10.Castellani F, van Rossum B, Diehl A, Schubert M, Rehbein K, Oschkinat H. Nature. 2002;420:98. doi: 10.1038/nature01070. [DOI] [PubMed] [Google Scholar]
- 11.Manolikas T, Herrmann T, Meier BH. J Am Chem Soc. 2008;130:3959. doi: 10.1021/ja078039s. [DOI] [PubMed] [Google Scholar]
- 12.LeMaster D, Kushlan D. J Am Chem Soc. 1996;118:9255. [Google Scholar]
- 13.Hong M. J Magn Reson. 1999;139:389. doi: 10.1006/jmre.1999.1805. [DOI] [PubMed] [Google Scholar]
- 14.De Paëpe G, Lewandowski JR, Loquet A, Böckmann A, Griffin RG. J Chem Phys. 2008;129:245101. doi: 10.1063/1.3036928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lewandowski JR, De Paëpe G, Griffin RG. J Am Chem Soc. 2007;129:728. doi: 10.1021/ja0650394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bennett AE, Griffin RG, Ok JH, Vega S. J Chem Phys. 1992;96:8624. [Google Scholar]
- 17.Bennett AE, Rienstra CM, Griffiths JM, Zhen W, Lansbury J, Griffin RG. J Chem Phys. 1998;108:9463. [Google Scholar]
- 18.Boender GJ, Vega S, de Groot HJM. J Chem Phys. 2000;112:1096. [Google Scholar]
- 19.Ishii Y. J Chem Phys. 2001;114:8473. [Google Scholar]
- 20.Bayro MJ, Ramachandran R, Caporini MA, Eddy MT, Griffin RG. J Chem Phys. 2008;128:052321. doi: 10.1063/1.2834736. [DOI] [PubMed] [Google Scholar]
- 21.Gullion T, Baker DB, Conradi MS. J Magn Reson. 1990;89:479. [Google Scholar]
- 22.Guijarro JI, Sunde M, Jones JA, Campbell ID, Dobson CM. Proc Natl Acad Sci USA. 1998;95:4224. doi: 10.1073/pnas.95.8.4224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bennett AE, Rienstra CM, Auger M, Lakshmi KV, Griffin RG. J Chem Phys. 1995;103:6951. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.