Abstract
Background
Acute colonic pseudo-obstruction (ACPO) refers to dilatation of the colon and decreased bowel motility without evidence of mechanical obstruction. Neostigmine, an acetylcholinesterase inhibitor, has been used in patients in whom supportive therapy failed to resolve ACPO. Here, we report the results of administering neostigmine to treat ACPO in children with hematologic malignancies.
Methods
Between September 2005 and December 2009, 10 patients (8 male and 2 female) were diagnosed with ACPO at the Department of Pediatrics, Catholic University of Korea. Diagnosis of ACPO was based on typical clinical features as well as colonic dilatation found on abdominal CT imaging. Neostigmine was administered subcutaneously at a dosage of 0.01 mg/kg/dose (maximum 0.5 mg) twice daily for a maximum of 5 total doses. ACPO was determined to be responsive to neostigmine if the patient showed both stool passage and improvement of clinical symptoms.
Results
The study group included 8 acute lymphoblastic leukemia patients, 1 patient with malignant lymphoma, and 1 patient with juvenile myelomonocytic leukemia. The median age at ACPO diagnosis was 8.5 years (range, 3-14). Overall, 8 patients (80%) showed therapeutic response to neostigmine at a median of 29 hours after the initial administration (range, 1-70). Two patients (20%) showed side effects of grade 2 or above, but none complained of cardiovascular symptoms that required treatment.
Conclusion
In this study, ACPO was diagnosed most often in late-childhood ALL patients. Subcutaneous neostigmine can be used to effectively treat ACPO diagnosed in children with hematologic malignancies without major cardiovascular complications.
Keywords: Acute colonic pseudo-obstruction, Neostigmine, Children, Hematologic malignancies
INTRODUCTION
Acute colonic pseudo-obstruction (ACPO), also known as Ogilvie's syndrome, refers to massive colonic dilatation with combined symptoms and signs of colonic obstruction without overt mechanical blockage. Important predisposing factors for the development of ACPO include trauma, infections, and cardiac disease as well as underlying medical illnesses such as cancer. If left untreated, ACPO may lead to intestinal perforation in 3-15% of cases, with an estimated mortality rate of 50% once spontaneous perforation occurs [1].
Once ACPO is diagnosed, treatment options include conservative management or colonoscopic decompression if symptoms fail to show improvement with initial care. Neostigmine, an acetylcholinesterase inhibitor, has shown efficacy in the treatment of ACPO without exposing the patient to the rare but significant complication of colonic perforation that accompanies colonoscopy, and since the initial study of successful decompression of ACPO using neostigmine [2], several reports have reaffirmed the utility of neostigmine in treating this rare but potentially life-threatening phenomenon [3, 4]. However, the effectiveness of neostigmine in treating ACPO diagnosed solely in a group of hematology patients has yet to be proven.
Intestinal complications are frequently observed in cancer patients. Typhilitis, or inflammation of the cecum, is a necrotizing enterocolitis that occurs in neutropenic patients after chemotherapy. Chemotherapeutic agents as far ranging as vincristine and docetaxel have been implicated in the pathogenesis of megacolon [5, 6].
Here, we report our single-institution experience of administrating neostigmine for the treatment of ACPO that was diagnosed in a series of pediatric patients with hematologic malignancies.
MATERIALS AND METHODS
1. Study population
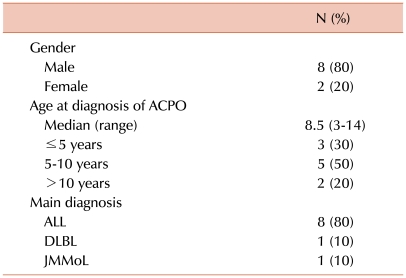
The study group consisted of consecutive hematology patients diagnosed with ACPO between September 2005 (the time period when neostigmine was first administered for the treatment of ACPO) and December 2009 at the Department of Pediatrics, Catholic University of Korea. Overall, 10 patients were diagnosed with ACPO during this period and treated with neostigmine. The major characteristics of this group are outlined in Table 1. The median age at ACPO diagnosis was 8.5 years, and 7 patients (70%) were older than 5 years. Eight patients (80%) had been diagnosed with and treated for acute lymphoblastic leukemia (ALL), 6 of whom had been designated as high-risk or above.
Table 1.
Demographic characteristics of the study group (N=10).
Abbreviations: ACPO, acute colonic pseudo-obstruction; ALL, acute lymphoblastic leukemia; DLBL, diffuse large B-cell lymphoma; JMMoL, juvenile myelomonocytic leukemia.
2. Diagnosis of ACPO
Since literature on the diagnosis of ACPO in the pediatric population is scarce, we implemented certain unique criteria for ACPO diagnosis as follows:
1) Symptoms of bowel obstruction such as constipation, abdominal pain, nausea, and vomiting
2) Findings on abdominal CT imaging consistent with colonic dilatation without overt mechanical obstruction, that characterize ACPO, as confirmed by a pediatric radiologist.
3. Neostigmine administration
The administration protocol was as follows:
1) Neostigmine was given subcutaneously at a dosage of 0.01 mg/kg/dose (maximum, 0.5 mg/dose) twice daily.
2) If no major side effects were noted with initial neostigmine infusion, administration was continued until therapeutic response, defined as stool passage and relief of mechanical obstruction symptoms, was observed. One additional neostigmine infusion was allowed after the patient showed treatment response in order to expedite full relief from ACPO. A maximum of 5 total doses of neostigmine were given. If a patient's symptoms failed to show improvement after the fifth neostigmine infusion, then the patient's ACPO was deemed unresponsive to neostigmine and no further infusion was attempted.
3) With each administration of neostigmine, vital signs and ECG monitoring were undertaken in order to identify possible bradycardia, and atropine was prepared at the bedside. All major side effects were noted and graded according to NCI CTC version 2.0. With regard to abdominal pain, newly developed pain or definite aggravation of previous pain after neostigmine infusion was considered a major side effect.
RESULTS
1. Diagnosis of ACPO
The median time from diagnosis of underlying malignancy to ACPO was 38.5 days (range, 21-121 days). The most common sign or symptom at diagnosis was abdominal pain (7/10, 70%), which was followed by fever (3/10, 30%), vomiting (2/10, 20%), and loose stools (1/10, 10%). The median maximum diameter of the ascending colon on CT imaging at diagnosis was 4.9 cm (range, 4.2-6.9 cm).
2. Patient history at ACPO diagnosis
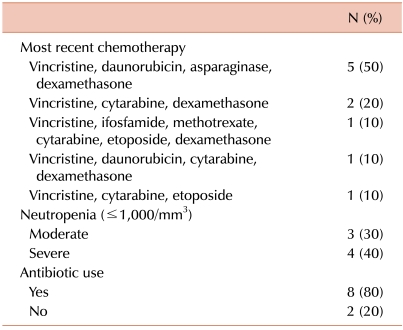
The chemotherapeutic regimens infused immediately prior to the diagnosis of ACPO are listed in Table 2. All 10 patients had previously received chemotherapy including vincristine, with a median of 8.5 days since the last infusion of vincristine (range, 4-10 days). Remission induction chemotherapy for ALL was the most common regimen given prior to ACPO diagnosis (5 patients, 50%), and it was followed by consolidation chemotherapy for ALL (3 patients, 30%). The majority of patients were neutropenic (7 patients, 70%: 3 patients with moderate neutropenia and 4 patients with severe neutropenia) and receiving broad-spectrum antibiotics at the time of ACPO diagnosis. Only 1 patient each had been receiving narcotic analgesics for relief of abdominal pain and had a potassium level <3.0 mEq/L at the time of ACPO diagnosis (data not shown).
Table 2.
Patient history at ACPO diagnosis (N=10).
3. Neostigmine administration and response
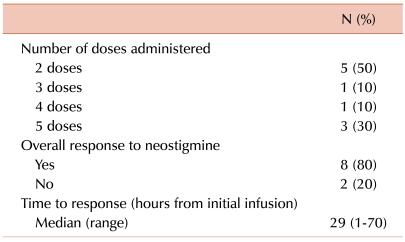
Details of neostigmine administration and responses to treatment are outlined in Table 3. A median of 18.5 hours (range, 4-31 hours) passed between diagnosis of ACPO to initial neostigmine infusion, during which time the patients received conservative management, such as nasogastric suction, laxatives, and enemas. Half of the patients showed improvement in clinical symptoms after 1 day of neostigmine infusion (2 doses). The overall response rate to neostigmine was 80% (8/10); a typical case of ACPO improvement resulting from neostigmine is shown in Fig. 1. The clinical course of the 2 patients whose conditions failed to respond to neostigmine was as follows: one patient, who was diagnosed with Philadelphia chromosome-positive ALL, experienced ACPO during induction chemotherapy. The patient continued to complain of constipation and abdominal pain despite neostigmine infusion, and further infusion was stopped after the fourth dose. This patient passed stools and experienced symptom improvement 105 hours after the last infusion. The second patient, who was also diagnosed with ACPO during remission induction chemotherapy for ALL, received the maximum number of doses (5 doses) without symptom relief but passed stools 77 hours after the last infusion. None of the patients, including the 2 who did not respond to neostigmine, showed recurrence of ACPO during the follow-up period.
Table 3.
Neostigmine administration and response (N=10).
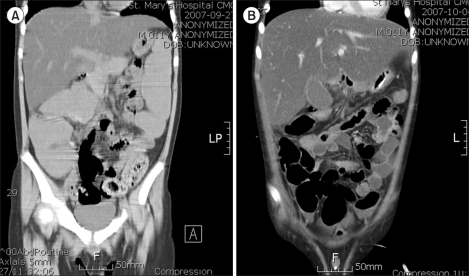
Fig. 1.
An 11-year-old male patient diagnosed with Philadelphia chromosome-positive ALL failed to pass stools for 157 hours before an abdominal CT was undertaken. Initial imaging showed massive colonic dilatation and fecal impaction consistent with ACPO, with the maximum diameter of the ascending colon measuring approximately 6.7 cm (A). Four hours after imaging, the patient was started on subcutaneous neostigmine, which resulted in stool passage 1 hour later. The patient received 1 more infusion for rapid amelioration of symptoms. Follow-up imaging taken 7 days later showed improvement in both colonic dilatation and fecal loading with a decrease in the maximum diameter of the ascending colon to 4.8 cm (B).
4. Side effects of neostigmine infusion
One patient experienced diplopia (grade 2), and another experienced aggravated abdominal pain (grade 2) after neostigmine infusion. Neither of these patients required specific medication for these symptoms. None of the patients had cardiovascular complications such as symptomatic bradycardia or syncope after neostigmine treatment.
DISCUSSION
ACPO can be associated with a wide range of medical conditions and, as such, studies on the use of neostigmine for the treatment of this condition have largely been based on a heterogeneous patient population. Furthermore, literature on ACPO and its treatment in patients with hematologic diseases or children with cancer is scarce, with limited case reports on successful use of neostigmine for decompression of ACPO [7-9].
Our study is unique in that ACPO was diagnosed and treated in a homogeneous group of children with underlying hematologic malignancies who were accrued over a period of more than 4 years. In this patient group, ACPO was most commonly diagnosed in school-aged children with ALL, with the median age at diagnosis being 8.5 years. ACPO tended to be observed soon after initiation of diagnosis and treatment for hematologic malignancy, lessening the possibility that this significant complication resulted from gradual and prolonged accumulation of chemotherapy-related toxicity. Importantly, most of the patients were neutropenic and were receiving antibiotic therapy at the time of ACPO diagnosis, indicating the potential role of post-chemotherapy neutropenia as a triggering factor for this gastrointestinal disorder, as is the case for typhilitis. A case report on the diagnosis and treatment of ACPO in an adult with acute myeloid leukemia also emphasizes neutropenia as an important factor in the development of ACPO and underscores the utility of both cholinergic agents and granulocyte-colony stimulating factor for its resolution [7]. All patients had been previously treated with a chemotherapeutic regimen that included vincristine, and an average of 1 week had passed from the most recent vincristine injection to ACPO diagnosis. However, the ACPO diagnosed in our patients should be differentiated from the constipation that may develop as an adverse effect of vincristine therapy. First, neurotoxicity from vincristine is dose-related and cumulative [10] while, as noted above, these study patients tended to be diagnosed with ACPO early on in their primary disease course without experiencing symptom recurrence despite full chemotherapy treatment. Second, other neurologic symptoms that characterize vincristine neurotoxicity, such as motor and sensory symptoms and autonomic neuropathy in the form of urinary retention [11], were not evident in our patients. Instead of being the sole causative agent of these gastrointestinal symptoms, recent treatment with vincristine may, like neutropenia, act as a significant predisposing factor for the development of ACPO.
Although the number of patients in the study group is small, the overall response of ACPO to neostigmine was impressive: 80% (8/10) of patients showed clinical improvement after neostigmine administration, with most of the responses coming after just 1 day (2 dosages) of treatment. Furthermore, in contrast to previous studies that utilized intravenous injection of neostigmine, we treated the study patients with neostigmine administered subcutaneously in an attempt to minimize the potential for significant cardiovascular complications while maintaining a therapeutic cholinergic effect. Possibly due to this different route of drug administration, none of the patients experienced major side effects that required intervention. However, the median time to response after initial drug administration was also significantly longer (29 hours) than has been reported by that employed intravenous injection of neostigmine. Since the clinical course of this study group was not compared with that of a control group of ACPO patients who did not receive neostigmine, the potential benefit of our medical intervention cannot be fully measured. However, considering that the 2 patients who did not respond to neostigmine started to show symptom improvement 3-4 days after the last infusion, one may speculate that the course of ACPO in children with hematologic malignancies is protracted, possibly lending itself to other major complications such as intestinal perforation and neutropenic shock.
In conclusion, although the number of patients in the study group is limited, our results indicate that subcutaneous neostigmine is both effective and safe for the treatment of ACPO diagnosed in children with hematologic malignancies.
References
- 1.Saunders MD. Acute colonic pseudo-obstruction. Best Pract Res Clin Gastroenterol. 2007;21:671–687. doi: 10.1016/j.bpg.2007.03.001. [DOI] [PubMed] [Google Scholar]
- 2.Ponec RJ, Saunders MD, Kimmey MB. Neostigmine for the treatment of acute colonic pseudo-obstruction. N Engl J Med. 1999;341:137–141. doi: 10.1056/NEJM199907153410301. [DOI] [PubMed] [Google Scholar]
- 3.van der Spoel JI, Oudemans-van Straaten HM, Stoutenbeek CP, Bosman RJ, Zandstra DF. Neostigmine resolves critical illness-related colonic ileus in intensive care patients with multiple organ failure--a prospective, double-blind, placebo-controlled trial. Intensive Care Med. 2001;27:822–827. doi: 10.1007/s001340100926. [DOI] [PubMed] [Google Scholar]
- 4.McNamara R, Mihalakis MJ. Acute colonic pseudo-obstruction: rapid correction with neostigmine in the emergency department. J Emerg Med. 2008;35:167–170. doi: 10.1016/j.jemermed.2007.06.043. [DOI] [PubMed] [Google Scholar]
- 5.Rosenberg RF, Caridi JG. Vincristine-induced megacolon. Gastrointest Radiol. 1983;8:71–73. doi: 10.1007/BF01948092. [DOI] [PubMed] [Google Scholar]
- 6.Williams EV, Drew PJ, Gaffney C, Shrestha BM, Mansel RE. Pancolitis associated with docetaxel: a rare cause of megacolon. Breast. 2001;10:346–347. doi: 10.1054/brst.2000.0237. [DOI] [PubMed] [Google Scholar]
- 7.Breccia M, Girmenia C, Mecarocci S, et al. Ogilvie's syndrome in acute myeloid leukemia: pharmacological approach with neostigmine. Ann Hematol. 2001;80:614–616. doi: 10.1007/s002770100337. [DOI] [PubMed] [Google Scholar]
- 8.Khosla A, Ponsky TA. Acute colonic pseudoobstruction in a child with sickle cell disease treated with neostigmine. J Pediatr Surg. 2008;43:2281–2284. doi: 10.1016/j.jpedsurg.2008.07.030. [DOI] [PubMed] [Google Scholar]
- 9.Kim TS, Lee JW, Kim MJ, et al. Acute colonic pseudo-obstruction in postchemotherapy complication of brain tumor treated with neostigmine. J Pediatr Hematol Oncol. 2007;29:420–422. doi: 10.1097/MPH.0b013e318063ef4b. [DOI] [PubMed] [Google Scholar]
- 10.Legha SS. Vincristine neurotoxicity. Pathophysiology and management. Med Toxicol. 1986;1:421–427. doi: 10.1007/BF03259853. [DOI] [PubMed] [Google Scholar]
- 11.Gomber S, Dewan P, Chhonker D. Vincristine induced neurotoxicity in cancer patients. Indian J Pediatr. 2010;77:97–100. doi: 10.1007/s12098-009-0254-3. [DOI] [PubMed] [Google Scholar]