Abstract
Background
The role of pre-transplant salvage chemotherapy has been controversial in relapsed acute leukemia.
Methods
We investigated post-transplant outcomes in 65 patients with acute leukemia treated with allogeneic hematopoietic cell transplantation (HCT) during first relapse or second remission.
Results
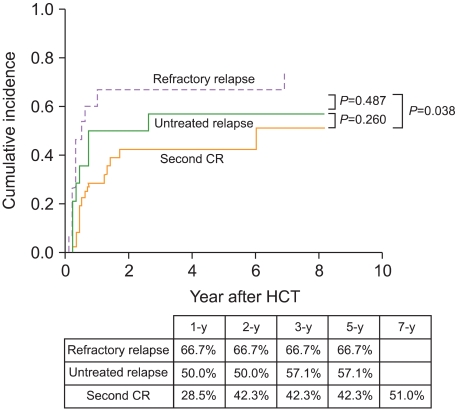
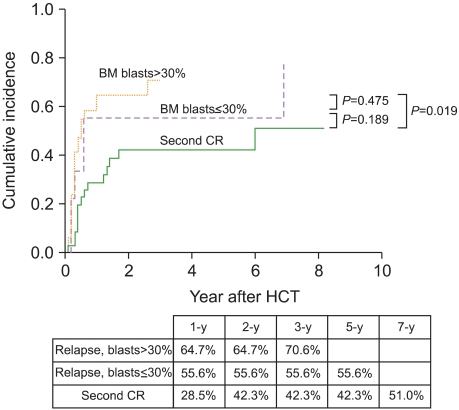
The 5-year cumulative incidence of relapse (CIR) was 52.3%. Multivariate analysis for CIR revealed that patients with unfavorable cytogenetics and those not in remission at the time of HCT had a significantly high CIR (P = 0.031 and P = 0.031, respectively). Allogeneic HCT was performed in 14 patients after first relapse without salvage chemotherapy ("untreated relapse" group), 15 patients failed chemotherapy for reinduction of remission before HCT ("refractory relapse" group), and 36 patients attained second remission with salvage chemotherapy before HCT ("second remission" group). The 5-year CIR for patients in the untreated relapse group (57.1%) was higher than that for those in the second remission group (42.3%), but it was lower than that for patients in the refractory relapse group (66.7%). Among patients who underwent allogeneic HCT in relapse, those with bone marrow (BM) blasts ≤30% had a lower 5-year CIR than those in florid relapse (BM blasts >30%) (57.7% vs. 70.6%).
Conclusion
Our results do not support the role of salvage chemotherapy aimed at re-induction of remission before allogeneic HCT in patients with acute leukemia after first relapse. Patients with early relapse do not appear to benefit from salvage chemotherapy before HCT.
Keywords: Allogeneic HCT, Acute leukemia, First relapse, Second remission
INTRODUCTION
Treatment outcome in young acute leukemia patients has improved over the past 2 decades, with complete remission (CR) rates ranging from 60-80% [1, 2]. However, 50-70% of patients in the first CR eventually relapse, and these patients have poor prognosis. Although a number of chemotherapeutic agents alone and in combination with other drugs have been tested, the CR rate in relapsed patients is very low and any remission with chemotherapy alone is usually short-lived [3-7]. For these patients, allogeneic hematopoietic cell transplantation (HCT) may offer the only chance for cure. The probability of disease-free survival (DFS) 5 years after HCT is about 20% to 30% [8-11]. The timing of allogeneic HCT in patients who relapse after the first CR may be important. Moreover, it is difficult to determine whether reinduction therapy should be attempted or HCT should be directly performed in relapsed patients with histocompatible donors. Several studies have reported contradictory results, with some favoring HCT without reinduction therapy [8, 10, 12, 13], while others emphasized the importance of CR before HCT [11, 14, 15].
In this study, we retrospectively investigated the clinical outcomes of acute leukemia patients who underwent allogeneic HCT after first relapse and analyzed the role of salvage chemotherapy aimed at the reinduction of remission before allogeneic HCT.
MATERIALS AND METHODS
1. Patients and transplantation procedure
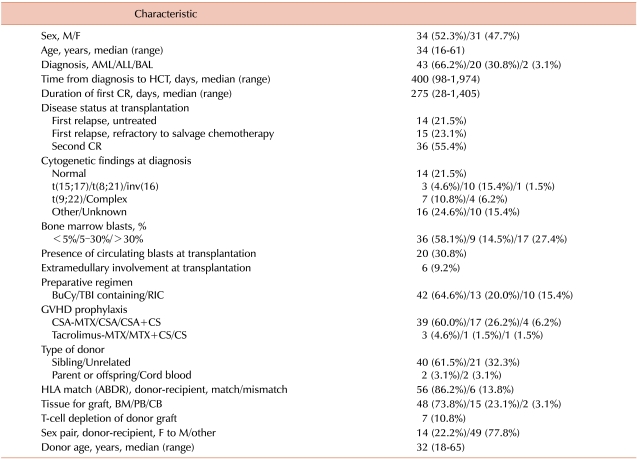
We retrospectively reviewed the records of 65 consecutive adult patients who had received allogeneic HCT for acute leukemia at first relapse or second CR at 3 institutes in Seoul, Korea, between January 1995 and September 2004 (Table 1). Acute myeloid leukemia (AML) was the underlying disease in 43 patients (66.2%), acute lymphoblastic leukemia (ALL) in 20 (30.8%), and biphenotypic acute leukemia in 2 (3.1%). All patients had previously achieved CR and had relapsed after a median CR duration of 275 days (range, 28-1,405 days). The attending physician at each institute decided whether to attempt reinduction therapy or to proceed directly to HCT. Fourteen patients (21.5%) underwent allogeneic HCT after first relapse without salvage chemotherapy aimed at the reinduction of remission ("untreated relapse" group), 15 patients (23.1%) failed to achieve CR with intensive chemotherapy for re-induction of remission before HCT ("refractory relapse" group), and 36 patients (55.4%) attained second CR with salvage chemotherapy before HCT ("second remission" group). Salvage chemotherapy regimens varied: cytarabine plus anthracycline-based regimens were administered in most patients with AML and vincristine, prednisolone plus daunorubicin-based regimens were administered in most patients with ALL. Cytogenetic analyses were successfully performed in 55 patients at diagnosis. Of the 29 patients who were not in remission before HCT, 9 had ≤30% bone marrow (BM) blasts and 17 had >30% blasts; the blast percentage for the other 3 patients was not available.
Table 1.
Patient and transplantation characteristics.
Abbreviations: M, male; F, female; AML, acute myeloid leukemia; ALL, acute lymphoblastic leukemia; BAL, biphenotypic acute leukemia; HCT, hematopoietic cell transplantation; CR, complete remission; BuCy, busulfan-cyclophosphamide; TBI, total body irradiation; RIC, reduced-intensity conditioning; CSA, cyclosporine; MTX, methotrexate; CS, corticosteroid; BM, bone marrow; PB, peripheral blood; CB, cord blood.
Preparative regimens for allogeneic HCT were dependent on the treatment strategy at each institute and the attending physician's discretion (Table 1). Regimens included cyclophosphamide (120 mg/kg) plus oral busulfan (16 mg/kg) or intravenous busulfan (12.8 mg/kg), 12.0 Gy of total body irradiation (TBI) plus cyclophosphamide (120 mg/kg), 10.0-2.0 Gy of TBI plus combinations with other chemotherapeutic agents, and various reduced-intensity conditioning regimens, which fulfilled one of the following criteria: (1) 500 cGy or less of TBI, (2) 9 mg/kg or less of total busulfan dose, (3) 140 mg/m2 or less of total melphalan dose, and (4) regimen included the purine analog fludarabine [16]. Fifty-six donors were HLA-ABDR-matched and 6 donors were mismatched with their respective recipients.
2. Statistical analysis
The probabilities of overall survival (OS) and event-free survival (EFS) were calculated by the Kaplan-Meier method [17] and compared by a log-rank test [18]. For the calculation of EFS, only surviving patients without relapse were censored at the last follow-up date. The effects of variables that changed with time after HCT, such as graft-versus-host disease (GVHD), on survival were analyzed in a time-dependent fashion using Cox regression models [19].
For the analysis of the cumulative incidence of relapse (CIR), patients who were alive without relapse were censored, whereas those who died without relapse were counted as competing cause of failure [20]. For the analysis of the cumulative incidence of non-relapse mortality (NRM), patients who relapsed were counted as competing cause of failure. The cumulative incidence was calculated and compared by Gray's method [21].
Statistically or marginally significant prognostic factors identified by univariate analysis were entered into an appropriate multivariate model (Cox proportional hazards regression model for OS and EFS [22] and Gray's method for CIR and NRM).
RESULTS
1. Post-transplant outcomes
Acute graft-versus-host disease (GVHD) occurred in 17 (26.6%) of 64 evaluable patients on median day 26 and chronic GVHD occurred in 15 (27.8%) of 54 evaluable patients on median day 150.
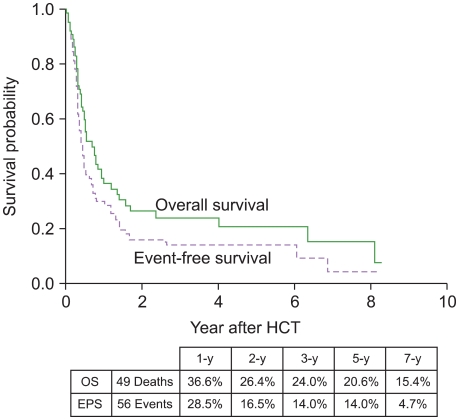
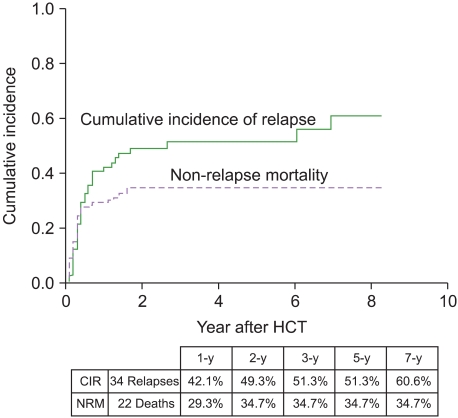
A median follow-up for 673 days (range, 151-3,010 days) among surviving patients indicated that 34 had relapsed and 49 had died. We observed 56 events (deaths or relapses). Of the 49 deaths, 22 were not related to leukemia relapse. These 22 non-relapse deaths were caused by infection (N=10), GVHD (N=4), hepatic venoocclusive disease (N=4), bleeding (N=2), thrombotic thrombocytopenic purpura (N=1), and suicide (N=1). The 5-year probabilities of OS and EFS were 20.6% and 14.0%, respectively (Fig. 1), and the 5-year CIR and NRM were 51.3% and 34.7%, respectively (Fig. 2).
Fig. 1.
Overall survival and event-free survival curves.
Fig. 2.
Cumulative incidences of relapse and non-relapse mortality.
2. Prognostic factor analysis
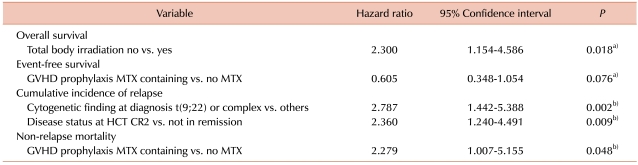
After univariate analyses, TBI (no vs. yes; OS at 5-year, 25.0% vs. 7.7%; P = 0.015), GVHD prophylaxis (methotrexate-containing vs. no methotrexate; 29.9 vs. 6.2%; P = 0.054), HLA mismatch (no vs. yes; 22.2% vs. 11.1%; P = 0.067), and donor-recipient sex pair (female to male vs. other; 0% vs. 27.8%; P = 0.079) were entered into a Cox proportional hazards regression model for OS; duration of first CR (<300 days vs. ≥300 days; EFS at 5-year, 7.8% vs. 19.9%; P = 0.085), TBI (no vs. yes; 17.8% vs. 0%; P = 0.086), and GVHD prophylaxis (methotrexate-containing vs. no methotrexate; 18.3% vs. 5.7%; P = 0.073) were entered into a Cox proportional hazards regression model for EFS; duration of first CR (<300 days vs. ≥300 days; CIR at 5-year, 67.9% vs. 35.5%; P = 0.011), disease status at HCT (second remission vs. not in remission; 42.3% vs. 62.1%; P = 0.040), and cytogenetic findings at diagnosis (t(9;22) or complex vs. others; 81.8% vs. 44.5%; P = 0.018) were entered into a multivariate model by Gray's method for CIR; TBI (no vs. yes; NRM at 5-year, 29.8% vs. 53.9%; P = 0.055) and GVHD prophylaxis (methotrexate-containing vs. no methotrexate; 26.0% vs. 52.3%; P = 0.049) were entered into a multivariate model by Gray's method for NRM. Acute or chronic GVHD did not have a significant influence on post-transplant outcomes in the univariate analyses. Table 2 shows the results of multivariate analyses of prognostic factors. TBI was an independent prognostic factor for OS (hazard ratio [HR], 2.300; 95% confidence interval [CI], 1.154-4.586; P = 0.018). GVHD prophylaxis was a marginally significant prognostic factor for EFS (HR, 0.605; 95% CI, 0.348-1.054; P = 0.076) and an independent prognostic factor for NRM (HR, 2.279; 95% CI, 1.007-5.155; P = 0.048). Cytogenetic findings at diagnosis (HR, 2.787; 95% CI, 1.442-5.388; P = 0.002) and disease status at transplantation (HR, 2.360; 95% CI, 1.240-4.491; P = 0.009) were independent prognostic factors for CIR.
Table 2.
Multivariate analysis of prognostic factors for survival probabilities and cumulative incidences.
Abbreviations: a)Cox proportional hazards regression model; b)Gray's method.
MTX, methotrexate; HCT, hematopoietic cell transplantation; CR2, second complete remission; GVHD, graft-versus-host disease.
3. Role of salvage chemotherapy aimed at reinduction of CR before HCT
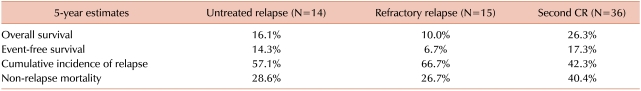
Patients in the second CR before HCT showed a significantly lower relapse rate than those who did not show remission before HCT. However, the latter group was divided into patients in the untreated relapse and refractory relapse groups, depending on whether they received salvage chemotherapy aimed at reinduction of CR before HCT. The 5-year CIR rate in the untreated relapse group (57.1%) was higher than that of the second remission group (42.3%), but it was lower than that for the refractory relapse group (66.7%) (Fig. 3). The median age of patients in second remission group was lower (31.0 vs. 40.5 vs. 43.0 years) and the median duration of the first CR was longer (367 vs. 168 vs. 131 days) than that of patients in the untreated relapse or refractory relapse groups. Among patients who underwent allogeneic HCT in relapse, those with ≤30% BM blasts had a lower 3-year CIR than those in florid relapse (BM blasts >30%) (57.7% vs. 70.6%; Fig. 4). Table 3 shows 5-year estimates of OS, EFS, CIR, and NRM in patients in untreated first relapse, refractory relapse, and second CR groups.
Fig. 3.
Cumulative incidence of relapse relative to salvage chemotherapy aimed at reinduction of complete remission (CR) before hematopoietic cell transplantation: second CR vs. untreated relapse vs. refractory relapse.
Fig. 4.
Cumulative incidence of relapse relative to bone marrow blast percentage before hematopoietic cell transplantation.
Table 3.
Clinical outcomes after allogeneic hematopoietic cell transplantation according to disease status.
Abbreviation: CR: complete remission.
DISCUSSION
Although many patients with acute leukemia do not undergo allogeneic HCT during their first CR, it may be the only option for cure after relapse, and allogeneic HCT is performed depending on the disease characteristics and the discretion of the patients of their physicians. Because a small but significant proportion of patients with refractory acute leukemia can be cured by allogeneic HCT [23-27], a clinically important issue in relapsed patients is not whether to perform allogeneic HCT but when this treatment should be performed. Although allogeneic HCT in untreated first relapse has proven to be feasible, with a 23-29% 5-year DFS rate, a 44-57% 5-year relapse rate, and a 44-47% 5-year NRM rate [8, 10], only a few studies have addressed the issue regarding the optimal time for allogeneic HCT in acute leukemia patients who relapse after non-transplantation treatment and who had a suitable hematopoietic cell donor, and different results have been reported in these studies [12, 13, 15]. According to the Société Fançaise de Greffe de Möelle (SFGM) registry data on allogeneic HCT for advanced AML, the survival of patients receiving HCT in untreated relapse or refractory relapse was significantly lower than that of patients undergoing HCT in second or third CR (untreated relapse vs. refractory relapse vs. CR; OS, 14% vs. 11% vs. 35%; DSF, 13% vs. 11% vs. 32%) [15]. However, others have reported that clinical outcomes in AML patients receiving allogeneic HCT during untreated first relapse were similar to or better than those for patients receiving allogeneic HCT in second or subsequent CR (untreated relapse vs. CR; OS, 29% vs. 22%; relapse incidence 23% vs. 47%) [12]. In a study of pediatric patients with advanced AML, allogeneic HCT during second CR resulted in significantly better outcomes than in patients with refractory disease, but the outcomes did not differ significantly between patients in second CR and those in untreated first relapse (untreated relapse vs. refractory relapse vs. CR; DFS, 36% vs. 9% vs. 58%; relapse incidence, 36% vs. 74% vs. 42%) [13]. While the SFGM registry data suggested the importance of CR reinduction before HCT in patients with relapsed acute leukemia, two other studies found that attainment of second CR before HCT did not significantly improve clinical outcomes. Similarly, our results, showing that clinical outcomes of patients in untreated first relapse were between those of patients in refractory relapse and patients in second CR, suggest that salvage chemotherapy aimed at reinduction of CR is not beneficial in patients with relapsed acute leukemia who have suitable HCT donors. Reinduction chemotherapy may serve to select chemosensitive patients with better prognosis rather than improving clinical outcomes. Furthermore, reinduction chemotherapy can result in mortality or serious morbidity before HCT. All of the above cited studies, including ours, however, were retrospective in design, emphasizing the necessity for randomized prospective studies to determine the optimal time for allogeneic HCT in relapsed acute leukemia patients. Moreover, our findings, that patients in early relapse (BM blasts ≤30%) had lower CIR rates than those in florid relapse (BM blasts >30%), were similar one study [12] but different from another [8].
The duration of the first CR is an important prognostic factor in relapsed acute leukemia patients [28]. We analyzed the effects of the duration of the first CR on post-transplant outcomes, and univariate analysis indicated that it was a significant risk factor for CIR, although the significance was not confirmed by multivariate analysis. Cytogenetic results at diagnosis have been shown to predict the outcomes of pre- and post-remission therapy for acute leukemia patients [29-31], as well as being associated with outcomes after allogeneic HCT in adults with AML in the first CR [32-34] or primary refractory AML [24] and in pediatric patients with advanced AML [13]. Multivariate analysis showed that the presence of unfavorable cytogenetic abnormalities (Philadelphia chromosome or complex karyotype) was associated with high CIR after HCT, indicating that unfavorable cytogenetics is a risk factor favoring allogeneic HCT for adult patients with advanced acute leukemia and suggesting that allogeneic HCT should be performed prior to relapse. Leukemia relapse is a common cause of treatment failure after allogeneic HCT for advanced acute leukemia. One approach to reduce the risk of relapse is intensification of the preparative regimen. This approach showed promising results in some studies [8, 35], probably due to an increase in drug toxicities [14, 36]. In our study, the use of TBI-containing preparative regimens resulted in lower OS, mainly due to an increase in NRM. Although the results cannot be generalized to other populations because of the limitations of a retrospective study, this finding suggests that a more refined approach, rather than merely intensifying the preparative regimen, can reduce the risk of relapse without increasing NRM. Our study showed that patients receiving methotrexate-containing regimens (usually cyclosporine or tacrolimus plus methotrexate) showed a significantly lower NRM and a tendency toward higher EFS than patients not receiving methotrexate (i.e., cyclosporine or tacrolimus alone) (Table 3). Our results were similar to those of other studies [11, 27], suggesting that optimal GVHD prophylaxis is essential even in patients with advanced acute leukemia. Furthermore, we failed to show antileukemic effects of GVHD (acute or chronic)(data not shown). However, some studies showed the importance of graft-versus-leukemia effects in advanced acute leukemia [10, 15, 36] and efforts are being focused on post-HCT immune modulation to increase graft-versus-leukemia effects without worsening GVHD.
Our study has several limitations, primarily due to its retrospective design. The study population included both AML and ALL, and the heterogeneity may mislead the conclusion because graft-versus-leukemia effects are different between AML and ALL. We included, however, both disease entities because the treatment strategy and issue of salvage chemotherapy in relapsed patients before HCT are similar. We could not perform subgroup analyses due to limited number of patients. The transplantation procedures were variable in their conditioning regimens, GVHD prophylaxis, T-cell depletion of hematopoietic cells before infusion, and source of hematopoietic cells. Moreover, the 3 participating institutes differed in their treatment policy after first relapse. A prospective randomized trial is required to determine the optimal time for allogeneic HCT in relapsed acute leukemia patients. In addition, the role of novel therapeutic agents such as hypomethylating agents, tipifarnib, or clofarabine before HCT in relapsed acute leukemia should be investigated in future trials.
In summary, our results do not support the role of salvage chemotherapy aimed at reinduction of remission before allogeneic HCT in patients with acute leukemia after first relapse and suggest that at least the patients with early relapse do not appear to benefit from salvage chemotherapy before HCT.
References
- 1.Löwenberg B, Downing JR, Burnett A. Acute myeloid leukemia. N Engl J Med. 1999;341:1051–1062. doi: 10.1056/NEJM199909303411407. [DOI] [PubMed] [Google Scholar]
- 2.Rowe JM. What is the best induction regimen for acute myelogenous leukemia? Leukemia. 1998;12(Suppl 1):S16–S19. [PubMed] [Google Scholar]
- 3.Estey EH. Treatment of relapsed and refractory acute myelogenous leukemia. Leukemia. 2000;14:476–479. doi: 10.1038/sj.leu.2401568. [DOI] [PubMed] [Google Scholar]
- 4.Ferrara F, Palmieri S, Mele G. Prognostic factors and therapeutic options for relapsed or refractory acute myeloid leukemia. Haematologica. 2004;89:998–1008. [PubMed] [Google Scholar]
- 5.Lee JH, Choi SJ, Lee JH, et al. Continuous infusion intermediate-dose cytarabine, mitoxantrone, plus etoposide for refractory or early relapsed acute myelogenous leukemia. Leuk Res. 2006;30:204–210. doi: 10.1016/j.leukres.2005.06.013. [DOI] [PubMed] [Google Scholar]
- 6.Leopold LH, Willemze R. The treatment of acute myeloid leukemia in first relapse: a comprehensive review of the literature. Leuk Lymphoma. 2002;43:1715–1727. doi: 10.1080/1042819021000006529. [DOI] [PubMed] [Google Scholar]
- 7.Stanisic S, Kalaycio M. Treatment of refractory and relapsed acute myelogenous leukemia. Expert Rev Anticancer Ther. 2002;2:287–295. doi: 10.1586/14737140.2.3.287. [DOI] [PubMed] [Google Scholar]
- 8.Brown RA, Wolff SN, Fay JW, et al. High-dose etoposide, cyclophosphamide, and total body irradiation with allogeneic bone marrow transplantation for patients with acute myeloid leukemia in untreated first relapse: a study by the North American Marrow Transplant Group. Blood. 1995;85:1391–1395. [PubMed] [Google Scholar]
- 9.Buckner CD, Clift RA, Thomas ED, et al. Allogeneic marrow transplantation for patients with acute non-lymphoblastic leukemia in second remission. Leuk Res. 1982;6:395–399. doi: 10.1016/0145-2126(82)90103-5. [DOI] [PubMed] [Google Scholar]
- 10.Clift RA, Buckner CD, Appelbaum FR, et al. Allogeneic marrow transplantation during untreated first relapse of acute myeloid leukemia. J Clin Oncol. 1992;10:1723–1729. doi: 10.1200/JCO.1992.10.11.1723. [DOI] [PubMed] [Google Scholar]
- 11.Doney K, Hägglund H, Leisenring W, Chauncey T, Appelbaum FR, Storb R. Predictive factors for outcome of allogeneic hematopoietic cell transplantation for adult acute lymphoblastic leukemia. Biol Blood Marrow Transplant. 2003;9:472–481. doi: 10.1016/s1083-8791(03)00149-6. [DOI] [PubMed] [Google Scholar]
- 12.Appelbaum FR, Clift RA, Buckner CD, et al. Allogeneic marrow transplantation for acute nonlymphoblastic leukemia after first relapse. Blood. 1983;61:949–953. [PubMed] [Google Scholar]
- 13.Nemecek ER, Gooley TA, Woolfrey AE, Carpenter PA, Matthews DC, Sanders JE. Outcome of allogeneic bone marrow transplantation for children with advanced acute myeloid leukemia. Bone Marrow Transplant. 2004;34:799–806. doi: 10.1038/sj.bmt.1704689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bortin MM, Gale RP, Kay HE, Rimm AA. Bone marrow transplantation for acute myelogenous leukemia. Factors associated with early mortality. JAMA. 1983;249:1166–1175. [PubMed] [Google Scholar]
- 15.Michallet M, Thomas X, Vernant JP, et al. Long-term outcome after allogeneic hematopoietic stem cell transplantation for advanced stage acute myeloblastic leukemia: a retrospective study of 379 patients reported to the Société Française de Greffe de Möelle (SFGM) Bone Marrow Transplant. 2000;26:1157–1163. doi: 10.1038/sj.bmt.1702690. [DOI] [PubMed] [Google Scholar]
- 16.Giralt S, Logan B, Rizzo D, et al. Reduced-intensity conditioning for unrelated donor progenitor cell transplantation: long-term follow-up of the first 285 reported to the national marrow donor program. Biol Blood Marrow Transplant. 2007;13:844–852. doi: 10.1016/j.bbmt.2007.03.011. [DOI] [PubMed] [Google Scholar]
- 17.Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc. 1958;53:457–481. [Google Scholar]
- 18.Peto R, Pike MC, Armitage P, et al. Design and analysis of randomized clinical trials requiring prolonged observation of each patient. II. analysis and examples. Br J Cancer. 1977;35:1–39. doi: 10.1038/bjc.1977.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Prentice RL, Kalbfleisch JD, Peterson AV, Jr, Flournoy N, Farewell VT, Breslow NE. The analysis of failure times in the presence of competing risks. Biometrics. 1978;34:541–554. [PubMed] [Google Scholar]
- 20.Seal HL. Studies in the history of probability and statistics: XXXV. Multiple decrements or competing risks. Biometrika. 1977;64:429–439. [Google Scholar]
- 21.Gray RJ. A class of K-sample tests for comparing the cumulative incidence of a completing risk. Ann Stat. 1988;16:1141–1154. [Google Scholar]
- 22.Cox DR. Regression models and life tables. J R Stat Soc B. 1972;34:187–202. [Google Scholar]
- 23.Biggs JC, Horowitz MM, Gale RP, et al. Bone marrow transplants may cure patients with acute leukemia never achieving remission with chemotherapy. Blood. 1992;80:1090–1093. [PubMed] [Google Scholar]
- 24.Fung HC, Stein A, Slovak M, et al. A long-term follow-up report on allogeneic stem cell transplantation for patients with primary refractory acute myelogenous leukemia: impact of cytogenetic characteristics on transplantation outcome. Biol Blood Marrow Transplant. 2003;9:766–771. doi: 10.1016/j.bbmt.2003.08.004. [DOI] [PubMed] [Google Scholar]
- 25.Kataoka I, Kami M, Takahashi S, et al. The Japan Society for Hematopoietic Cell Transplantation Working Party. Clinical impact of graft-versus-host disease against leukemias not in remission at the time of allogeneic hematopoietic stem cell transplantation from related donors. Bone Marrow Transplant. 2004;34:711–719. doi: 10.1038/sj.bmt.1704659. [DOI] [PubMed] [Google Scholar]
- 26.Singhal S, Powles R, Henslee-Downey PJ, et al. Allogeneic transplantation from HLA-matched sibling or partially HLA-mismatched related donors for primary refractory acute leukemia. Bone Marrow Transplant. 2002;29:291–295. doi: 10.1038/sj.bmt.1703373. [DOI] [PubMed] [Google Scholar]
- 27.Wong R, Shahjahan M, Wang X, et al. Prognostic factors for outcomes of patients with refractory or relapsed acute myelogenous leukemia or myelodysplastic syndromes undergoing allogeneic progenitor cell transplantation. Biol Blood Marrow Transplant. 2005;11:108–114. doi: 10.1016/j.bbmt.2004.10.008. [DOI] [PubMed] [Google Scholar]
- 28.Copelan EA, McGuire EA. The biology and treatment of acute lymphoblastic leukemia in adults. Blood. 1995;85:1151–1168. [PubMed] [Google Scholar]
- 29.Grimwade D, Walker H, Oliver F, et al. The Medical Research Council Adult and Children's Leukaemia Working Parties. The importance of diagnostic cytogenetics on outcome in AML: analysis of 1,612 patients entered into the MRC AML 10 trial. Blood. 1998;92:2322–2333. [PubMed] [Google Scholar]
- 30.Slovak ML, Kopecky KJ, Cassileth PA, et al. Karyotypic analysis predicts outcome of preremission and postremission therapy in adult acute myeloid leukemia: a Southwest Oncology Group/Eastern Cooperative Oncology Group Study. Blood. 2000;96:4075–4083. [PubMed] [Google Scholar]
- 31.Chalandon Y, Barnett MJ, Horsman DE, et al. Influence of cytogenetic abnormalities on outcome after allogeneic bone marrow transplantation for acute myeloid leukemia in first complete remission. Biol Blood Marrow Transplant. 2002;8:435–443. doi: 10.1053/bbmt.2002.v8.pm12234169. [DOI] [PubMed] [Google Scholar]
- 32.Ferrant A, Labopin M, Frassoni F, et al. Acute Leukemia Working Party of the European Group for Blood and Marrow Transplantation (EBMT) Karyotype in acute myeloblastic leukemia: prognostic significance for bone marrow transplantation in first remission: a European Group for Blood and Marrow Transplantation study. Blood. 1997;90:2931–2938. [PubMed] [Google Scholar]
- 33.Keating S, Suciu S, de Witte T, et al. The European Organization for Research and Treatment of Cancer (EORTC) and the Gruppo Italiano Malattie Ematologiche Maligne dell' Adulto (GIMEMA) Leukemia Cooperative Groups. Prognostic factors of patients with acute myeloid leukemia (AML) allografted in first complete remission: an analysis of the EORTC-GIMEMA AML 8A trial. Bone Marrow Transplant. 1996;17:993–1001. [PubMed] [Google Scholar]
- 34.Long GD, Amylon MD, Stockerl-Goldstein KE, et al. Fractionated total-body irradiation, etoposide, and cyclophosphamide followed by allogeneic bone marrow transplantation for patients with high-risk or advanced-stage hematological malignancies. Biol Blood Marrow Transplant. 1997;3:324–330. [PubMed] [Google Scholar]
- 35.Clift RA, Buckner CD, Appelbaum FR, et al. Allogeneic marrow transplantation in patients with acute myeloid leukemia in first remission: a randomized trial of two irradiation regimens. Blood. 1990;76:1867–1871. [PubMed] [Google Scholar]
- 36.Appelbaum FR. Graft versus leukemia (GVL) in the therapy of acute lymphoblastic leukemia (ALL) Leukemia. 1997;11(Suppl 4):S15–S17. [PubMed] [Google Scholar]