Abstract
The transport of glucose across cell membranes is mediated by facilitative glucose transporters. The recently identified Class III glucose transporter GLUT12 is predominantly expressed in insulin-sensitive tissues such as heart, fat, and skeletal muscle. We examined the subcellular localization of GLUT12 in CHO and HEK293 cells stably expressing murine GLUT12. We have previously shown that another Class III glucose transporter, GLUT8, contains a [DE]XXXL[LI] motif that directs it to late endosomal/lysosomal compartments. Despite also having this highly conserved motif in its amino terminus, GLUT12 does not colocalize with GLUT8. Rather, GLUT12 resides in the Golgi network and at the plasma membrane. Furthermore, GLUT8 and GLUT12 exhibit dramatic differences in trafficking from the plasma membrane. Whereas GLUT8 is internalized following its expression at the cell surface, GLUT12 remains largely associated with the plasma membrane. To further explore the trafficking mechanisms, we created mutant constructs to explore the potential role of GLUT12's NH2-terminal dileucine motif in regulating its intracellular sorting. We show that both the GPN and the LL residues within the [DE]XXXL[LI] motif influence the cell surface expression of GLUT12 and conclude that the mechanisms governing the intracellular sorting of GLUT12 are distinct from those regulating the sorting of GLUT8.
Keywords: GLUT12, glucose transport, [DE]XXXL[LI], endocytosis, targeting, lysosome
Introduction
The transport of glucose across the membranes of mammalian cells is mediated by facilitative glucose transporters (GLUTs). The family contains 14 isoforms that differ in substrate specificity, kinetics of transport, and tissue distribution (1, 2). On the basis of sequence similarity, three classes of GLUTs have recently been established, Class I (GLUT-1, -2, -3, -4, and -14), Class II (GLUT-5, -7, -9, and -11) and Class III (GLUT-6, -8, -10, -12, and HMIT). The class III glucose transporter GLUT12 was originally detected in the breast cancer cell line MCF-7 (3). It has subsequently been shown that in adult humans and rodents, the GLUT12 protein is predominantly expressed in insulin-sensitive tissues such as skeletal muscle, fat, and heart (4-7). GLUT12 has also been detected in other cells with high metabolic demands including the uterus, the small intestine, and in both breast and colon carcinomas (5) (8, 9). Recent studies in muscle have shown that concentrations of mRNA for GLUT4, GLUT5, and GLUT12 were much higher than for the other GLUTs, comprising 98% of the total GLUT isoform mRNA in human skeletal muscle (10). This study further demonstrated that GLUT12 protein localizes to the same slow-twitch fiber type as GLUT4 in human skeletal muscle. GLUT12 possesses several putative sorting motifs that are similar to known GLUT4 targeting motifs. Dileucine motifs in the NH2- and the COOH-termini of GLUT12 are found in positions similar to the FQQI and dileucine targeting motifs, respectively, in the NH2- and the COOH-termini of GLUT4 (11, 12). The presence of potential targeting motifs as well as the pattern of GLUT12 expression in adult tissues suggests the possibility that GLUT12 may represent a second insulin-sensitive glucose transport system. However, the physiological role of GLUT12 in insulin-sensitive tissue remains undefined. To gain insight into its potential physiological role, a major aim of this study is to investigate the subcellular localization and targeting of murine GLUT12 (mGLUT12).
The NH2-terminus of GLUT12 contains a putative late endosomal/lysosomal [DE]XXXL[LI] targeting motif. This type of dileucine signal interacts with adaptor proteins to facilitate the targeting of proteins to endosomes, lysosomes, and lysosome-related organelles(13). The Class III glucose transporter GLUT8 is the only other identified member of the glucose transporter family that contains this sorting motif. Recent studies in our lab have demonstrated that this motif in GLUT8 is responsible for sorting the transporter to the late endosome and the lysosome in both CHO and HEK293 cells expressing exogenous GLUT8 (14). The intracellular sorting of GLUT8 is dependent on interactions between its [DE]XXXL[LI] sorting signal and the heterotetrameric adaptor protein AP-2 (15). While it remains unclear whether GLUT8 is directly targeted from the Golgi network to the lysosome or is indirectly targeted to the lysosome following endocytosis from the plasma membrane, a recent study in HeLa cells has demonstrated that GLUT8 endocytosis is regulated via its interactions between its NH2-terminal dileucine motif and the β2-adaptin subunit of AP-2 (14, 15). These findings suggest that the GLUT8 NH2-terminal [DE]XXXL[LI] motif functions as an internalization signal. In agreement with this hypothesis, it has been shown that mutating the LL residues to AA or the acidic E residue to R results in the accumulation of GLUT8 at the cell surface (14-17). AP-2 is the most abundantly found adaptor protein at the plasma membrane and functions as the major endocytic adaptor in mammalian cells (18, 19). Because GLUT12 contains a [DE]XXXL[LI] sorting signal in its amino terminus, it is possible that the trafficking of this transporter is also mediated by interactions between its NH2-terminal dileucine motif and adaptor protein complexes.
In this study we demonstrate that despite containing a similar NH2-terminal binding motif, GLUT12 fails to colocalize with GLUT8 in stably expressing CHO cells. Mutation of the dileucine motif to dialanine results in an intracellular retention of GLUT12. This finding is in contrast to GLUT8, in which the analogous mutation results in mistargeting of the transporter to the plasma membrane (16, 17). Furthermore, mutating the XXX residues found in the NH2-terminal [DE]XXXL[LI] in GLUT12 to the analogous residues in GLUT8 results in mislocalization of GLUT12 from the cell surface to an unidentified intracellular compartment. Conversely, mutating the XXX residues in GLUT8 to the XXX residues found in GLUT12 results in a dramatic mistargeting of GLUT8 to the plasma membrane. Our data suggest that similar NH2-terminal [DE]XXXL[LI] motifs in GLUT8 and GLUT12 differentially direct the trafficking of each transporter to different intracellular compartments. We postulate that GLUT8 and GLUT12 traffic to distinct cellular locations due to interactions between their NH2-terminal dileucine motifs and separate adaptor protein subunits.
Results and Discussion
Specificity of GLUT12 antiserum
To confirm the specificity of the GLUT12 antiserum used in the present study, total protein extracts from HEK293 cells stably transfected with empty vector, GLUT12, or GLUT12-FLAG, were analyzed by Western blotting. Detection of GLUT12 was confirmed by immunoprecipitating GLUT12-FLAG using an anti-FLAG antibody and then immunoblotting with antisera to GLUT12 (Figure 1, lane 1). The GLUT12 immunoreactive band runs with a molecular weight of 60-65 kDa (Figure 1, lanes 1-3). Exposing the total membrane extracts to PNGase F to remove N-linked sugar residues shifts the GLUT12 band to a single band with a molecular weight of 50 kDa (Figure 1, lanes 5-6).
Figure 1. Western blot analysis of GLUT12.
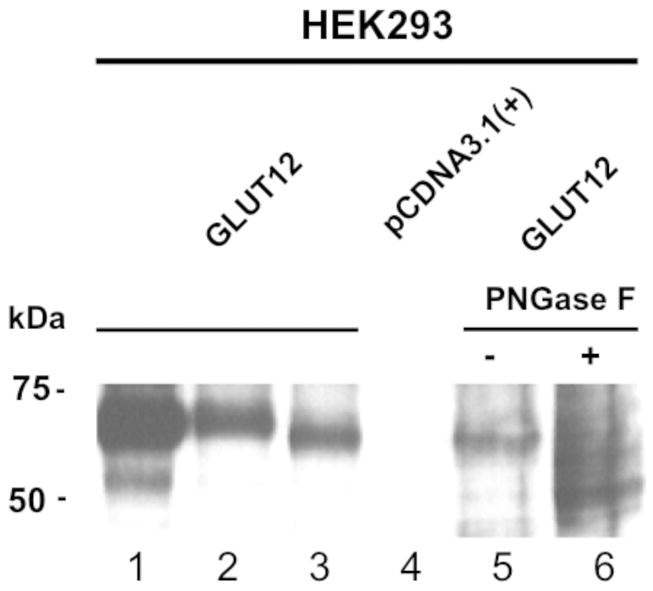
Whole cell lysates were prepared from HEK293 cells transiently transfected with GLUT12FLAG (lane 1 and 2), GLUT12 (lane 3), or empty vector (lane 4). In lane 1, GLUT12FLAG containing cell lysates were subjected to immunoprecipitation with anti-FLAG antibody. Lysates prepared from cells expressing GLUT12 were treated with (lane 6) or without PNGaseF (lane 5) to identify glycosylated and nonglycosylated band size. Proteins were separated by SDS-PAGE, transferred onto nitrocellulose, and probed with the anti-GLUT12 antibody at a dilution of 1:2000.
Co-localization studies of GLUT12
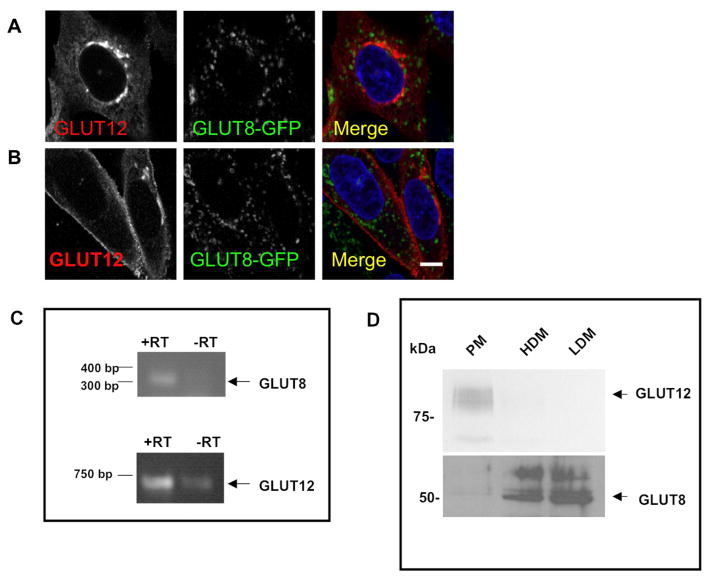
Previous studies from our lab have shown that GLUT8, another GLUT harboring an NH2-terminal [DE]XXXL[LI] motif, localizes to the late endosome and lysosome in CHO and HEK293 cells (14). Therefore, we first sought to determine whether [DE]XXXL[LI] motif in the NH2-terminus of GLUT12 similarly directs the transporter to a late endosomal or lysosomal compartment. To determine whether GLUT8 and GLUT12 localize to the same compartment, the transporters were co-expressed in both CHO and HEK293 cells. Consistent with previous studies, GLUT12 localizes to an intracellular compartment as well as the plasma membrane (3), whereas GLUT8 displays an intracellular localization (Figure 2a and 2b). This pattern of distribution was confirmed by RT-PCR and subcellular fractionation of murine endometrial stromal cells (ESCs) (Figure 2c and d). Recent studies in our lab have shown that both GLUT8 and GLUT12 are endogenously expressed in ESCs (20). GLUT8 has previously been shown to reside in the human endometrium (21). Here we show that GLUT8 and GLUT12 reside in distinct compartments in murine ESCs. GLUT12 predominantly resides in the plasma membrane fraction, whereas GLUT8 is found primarily intracellularly, in the high and low density membrane fractions (Figure 2d). Next we used HEK293 and CHO cells stably overexpressing GLUT12 to identify the intracellular compartment in which GLUT12 resides. This protein colocalizes with the exogenously expressed Rab11, a marker of recycling endosomal compartments (Figure 3a). We further examined whether GLUT12 localizes to a recycling endosomal compartment using transferrin uptake in CHO cells (Supplemental Figure 2). We did not observe significant overlap between GLUT12 and transferrin. It is therefore possible that interactions between GLUT12 and exogenously expressed Rab11-YFP direct GLUT12 into a recycling endosomal compartment. Significant overlap is observed between GLUT12 and the Golgi marker GM130 (Figure 3b) in both CHO and HEK293 cells. In contrast to GLUT8, which resides in the late endosome or the lysosome, GLUT12 does not colocalize with the late endosomal markers Rab7 and Rab9, or Syntaxin 8, a member of the t-SNARE family that is found in late endosomes and lysosomes (Supplemental Figure 1). GLUT12 does not colocalize with EEA1, a marker of the early endosomal compartment (Supplemental Figure 2).
Figure 2. GLUT12 and GLUT8 reside in different compartments.
CHO cells stably expressing GLUT8-GFP were transiently transfected with GLUT12. GLUT12 localizes both to a perinuclear compartment (A) and to the plasma membrane (B), but fails to colocalize with GLUT8. In endometrial stromal cells(C), both GLUT12 and GLUT8 are endogenously expressed. Subcellular fractionation confirms that GLUT12 is predominantly in the plasma membrane (PM) fraction whereas GLUT8 is distributed in the high and low density membrane fractions. Images were taken at 60× magnification.
Figure 3. GLUT12 localizes to the Golgi network.
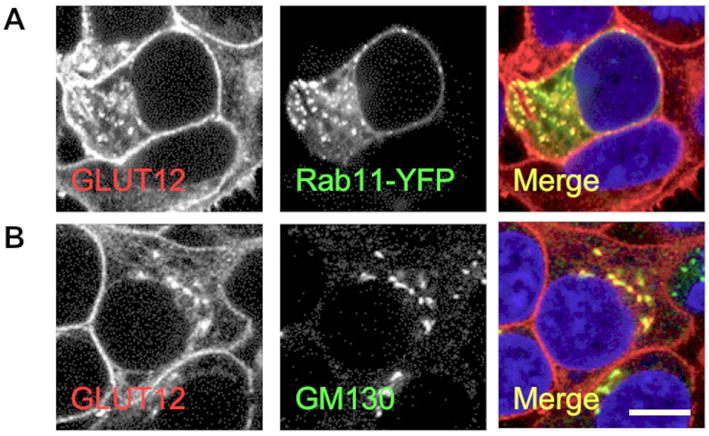
GLUT12 is detected in the Golgi network (A -GM130) and colocalizes with the post-Golgi membrane trafficking Rab11-YFP (B) in stably GLUT12-expressing HEK293 cells. GLUT12 does not colocalized with Syntaxin 8 or Rab7-YFP (C and D). Images were taken at 60× magnification.
Targeting signal of GLUT12
Using the Clustal W 1.8 alignment tool (http://www.ebi.ac.uk/clustalw/), we performed -an alignment of the NH2-terminal region of GLUT12 using GLUT12 protein sequences available from human, mouse, and cow (Figure 4) (Pubmed protein references NP_660159, NP_849265, and NP_001011683). Similar to GLUT8, the NH2-terminal [DE]XXXL[LI] motif in GLUT12 is highly conserved in each of the species examined. To test whether this motif is involved in sorting GLUT12 to its intracellular compartment or to the plasma membrane, we first mutated the residues LL to AA. Analogous mutations in the Class III glucose transporters GLUT6 and GLUT8 cause retention of the transporters at the plasma membrane (17), suggesting that the NH2-terminal LL residues act as an internalization signal. Using immunofluorescence microscopy, we surprisingly observed less cell surface staining in both HEK293 and CHO cells transiently expressing GLUT12-AA-HA compared to GLUT12-HA (Figure 5A). This decrease in cell surface expression corresponds to an increase in intracellular GLUT12-AA-HA expression. Mutating the LL residues in GLUT12 to AA resulted in a significant decrease in cell surface expression of the transporter as determined by flow cytometry using HEK293 cells transiently expressing GLUT12-AA-HA or GLUT12-HA (Figure 5B). Despite this decrease, some degree of cell surface expression of GLUT12-AA-HA was still seen.
Figure 4. The NH2-terminal region of GLUT12 contains a highly conserved [DE]XXXL[LI] motif.

(A) GLUT12 sequence comparison was performed using Clustal W to align the NH2-terminus of GLUT12 from several species. Highly conserved residues in the motif are highlighted with red letters. (B) Similar [DE]XXXL[LI] motifs are also found in the lysosomal protein LIMPII and in the NH2-terminal region of the Class III glucose transporter GLUT8. The alignment of EXXXLL with the rat dileucine motif is also shown. (C) In this study, the GLUT12 NH2-terminal dileucine motif has been mutated to dialanine as shown in blue letters. In addition, the three amino acids between the acidic residue and the dileucine in the EXXXLL motif in GLUT12 were switched with the equivalent residues from the GLUT8 sequence. The reciprocal mutation in GLUT8 was also generated.
Figure 5. Mutating the EGPNLL motif of mouse GLUT12 to EGPNAA or to ETQPLL decreases the cell surface expression of GLUT12.
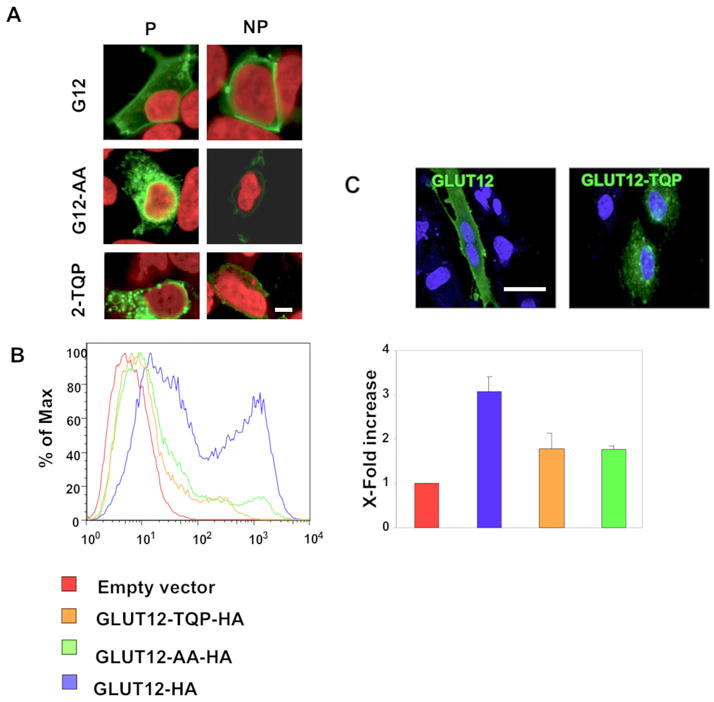
(A). HEK293 cells were transiently transfected with GLUT12-HA, GLUT12-AA-HA, or GLUT12-TQP-HA. Cells were stained with or without prior permeabilization using an HA-antibody. Decreased levels of plasma membrane staining were observed in GLUT12-AA or GLUT12-TQP-expressing cells compared to the cell surface expression levels of wild-type GLUT12. (B) Relative amounts of cell surface-associated GLUT12 were quantified using flow cytometry with HEK293 cells transiently expressing GLUT12-HA, GLUT12-AA-HA, or GLUT12-TQP-HA. In agreement with the immunofluorescence results, GLUT12-AA-HA and GLUT12-TQP-HA exhibit less cell surface expression than wild-type GLUT12. (C) GLUT12-HA and GLUT12-TQP-HA localized to similar compartments in endometrial stromal cells which endogenously express GLUT12. Images were taken at 60× magnification.
Because mutating analogous dileucine residues in the NH2-terminus of GLUT8 and GLUT12 to dialanine differentially affects the cell surface expression levels of each transporter, we next investigated the role that XXX residues play in the intracellular and cell surface targeting of GLUT8 and GLUT12. These XXX residues are the only amino acids that differ between the [DE]XXXL[LI] motifs in GLUT8 and GLUT12. We therefore swapped the XXX residues in GLUT12 for the XXX residues in GLUT8, and vice versa, creating GLUT8-GPN-HA and GLUT12-TQP-HA mutant constructs (Figure 4C). Similar to the LL to AA mutation, mutating GPN to TQP in the GLUT12 construct resulted in increased intracellularly localization of the transporter (Figure 5A). Using flow cytometry on transiently transfected HEK293 cells, we showed a significant decrease in plasma membrane expression levels of GLUT12-TQP-HA relative to GLUT12-HA (Figure 5B). Conversely, mutating TQP to GPN in GLUT8 causes a dramatic mistargeting of the transporter to the plasma membrane (Figure 6A). Flow cytometry using HEK293 cells transiently transfected with either GLUT8-HA or GLUT8-GPN-HA revealed over a three-fold increase in cell surface expression of GLUT8-GPN-HA relative to GLUT8-HA (Figure 6B). These results suggest that the XXX residues in the NH2-terminal [DE]XXXL[LI] motifs of both GLUT8 and GLUT12 play a large role in regulating the normal trafficking of each transporter.
Figure 6. Mutating the ETQPLL motif of mouse GLUT8 to EGPNLL retains the transporter at the plasma membrane.
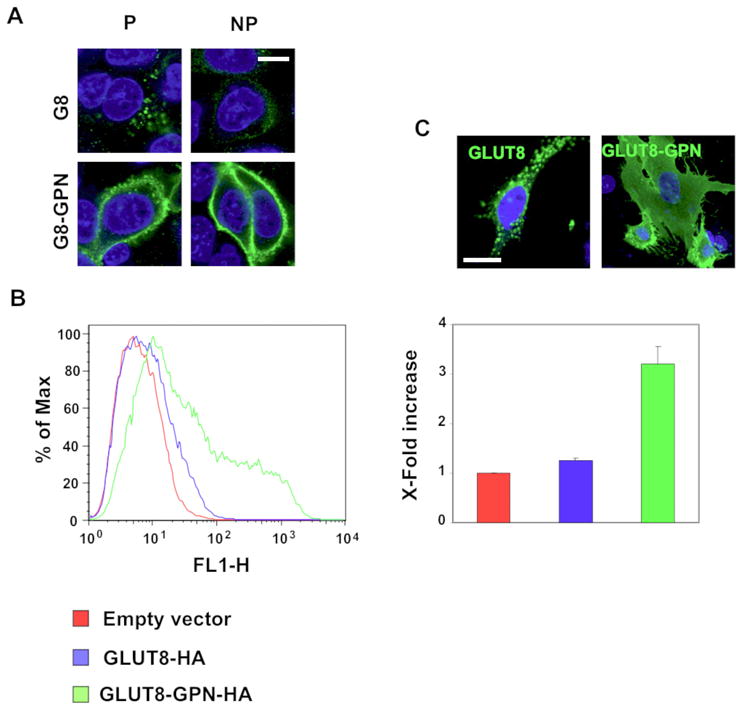
(A) HEK293 cells were transiently transfected with GLUT8-HA or with GLUT8-GPN-HA. Cells were stained with HA-antibody with or without permeabilization. In contrast to wild-type GLUT8, GLUT8-GPN primarily localizes to the plasma membrane. (B) The relative amounts of cell surface-associated GLUT8 were quantified using flow cytometry in HEK293 cells that were transiently transfected with GLUT8-HA or GLUT8-GPN-HA. The GLUT8-GPN-HA mutation increases the amount of protein at the cell surface, suggesting that the mutation may hinder GLUT8 endocytosis. (C) GLUT8-HA and GLUT8-GPN-HA localize to similar compartments in endometrial stromal cells which endogenously express GLUT8. Images were taken at 60× magnification.
To confirm these findings in a cell type endogenously expressing GLUT8 and GLUT12, both GLUT8-HA and GLUT8-GPN-HA plus GLUT12-HA and GLUT12-TQP-HA were overexpressed in murine ESCs. GLUT12-HA localized predominantly at the plasma membrane by confocal immunofluorescent microscopy, whereas, GLUT12-TQP-HA was seen intracellularly (Figure 5C). In contrast, overexpression of GLUT8-HA appeared intracellularly in a lysosomal-like compartment whereas GLUT8-GPN-HA was seen at the plasma membrane (Figure 6C).
Sorting mechanism of GLUT12
Previous studies have shown that the Class III glucose transporter GLUT8 is endocytosed from the cell surface in a dynamin-dependent fashion (14) (15). This dynamin-dependent endocytosis relies on the NH2-terminal dileucine motif. Because GLUT12 harbors a similar NH2-terminal dileucine motif, we sought to examine whether GLUT12 is expressed at the plasma membrane and is subsequently endocytosed. To examine this hypothesis, we used an antibody internalization assay with CHO and HEK293 cells expressing GLUT12-HA. GLUT12-HA localizes to an intracellular compartment and to the plasma membrane in permeabilized and nonpermeabilized CHO cells, respectively (Figure 7A-left panel top row). To compare the endocytosis of GLUT12 and GLUT8, GLUT12-HA- and GLUT8-HA- expressing cells were cultured for one hour in the presence of HA-antibody (Figure 7A and B-left panels middle rows). Only small amounts of endocytosed GLUT12-HA are observed in permeabilized cells whereas the majority of GLUT12-HA remains associated with the plasma membrane. In contrast, the majority of GLUT8-HA is endocytosed and only trace amounts of the transporter were detected at the cell surface of nonpermeabilized cells (Figure 7B). To determine whether an increase in endocytosed GLUT12 is observed following a longer antibody incubation period, cells were cultured for two hours with HA-antibody. In these cells, the majority of GLUT12 continues to remain associated with the cell surface (Figure 7A-left panel bottom row). A small amount of intracellular staining was observed, indicating that some endocytosis of GLUT12 is occurring in these cell lines. GLUT8-HA on the other hand is all found in intracellular compartments with very little cell surface expression in nonpermeabilized cells only (Figure 7B-left panel bottom row).
Figure 7. Internalization of GLUT12 and retention of GLUT8 occur when substitutions are made to the XXXL[LI] residues.
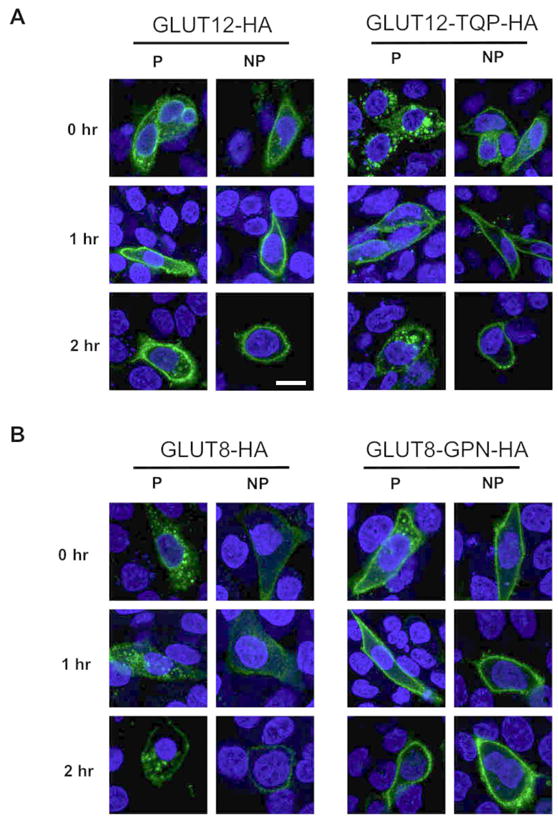
CHO cells were transiently transfected with GLUT12-HA, GLUT12-TQP-HA, GLUT8-HA, or GLUT8-GPN-HA. The steady state localization (0 hr) of the transfected GLUTs in CHO cells was determined by staining permeabilized (P) and nonpermeabilized (NP) cells with an HA antibody. Cells were cultured for one hour with the HA antibody (1 hr) or after rinsing out the antibody, cultured for an additional hour (2 hr). Cells with or without prior permeabilization were then incubated with Alexa-488 to label intracellular or plasma membrane HA antibody. (A) At steady state levels (0 hr), most of GLUT12 localizes to the plasma membrane, whereas a majority of GLUT12-TQP localizes to an intracellular compartment. Following one and two hours of cell surface exposure to an HA-antibody, the majority of GLUT12 remained associated with the cell surface. In contrast to GLUT12, much of the GLUT12-TQP was internalized within the two hours. (B) At steady state levels, the majority of GLUT8 localizes to an intracellular compartment. The GLUT8 that is associated with the cell surface becomes internalized after one and two hour time periods. GLUT8-GPN, on the other hand, resides primarily at the cell surface at its steady state distribution. Similar to GLUT12, most GLUT8-GPN remains associated with the cell surface one and two hours after exposure to an HA-antibody. Images were taken at 60× magnification.
To determine whether endocytosed GLUT12 is recycling from the cell surface back to the Golgi, we co-stained cells for endocytosed GLUT12 and for markers of the Golgi network (Figure 7C). While GLUT12 appears to recycle to a compartment adjacent to the Golgi, no overlap is observed with GM130 or Syntaxin6 positive compartments, suggesting that GLUT12 may localize to a different Golgi compartment or to a distinct compartment separate from the Golgi network.
In order to confirm that the NH2-terminal [DE]XXXL[LI] motifs are critical for normal trafficking of these Class III transporters, the same HA antibody internalization assays were repeated using the GLUT12-TQP-HA and GLUT8-GPN-HA mutants. At the start of the assay, the overexpressed GLUT12 mutant localized predominantly to an intracellular compartment and less to the plasma membrane in permeabilized and nonpermeabilized CHO cells, respectively (Figure 7A-right panel top row). One hour after incubation with HA antibody, the HA-tagged GLUT12 mutant was seen both at the cell surface as seen in the wildtype GLUT12 however, some degree of GLUT12-TQP-HA was internalized (Figure 7A-right panel middle row). After 2 hours, a significant fraction of GLUT12-TQP-expressing cells displayed HA antibody internalization that was in excess of the internalization observed in GLUT12-expressing cells (Figure 7A-left panel bottom row). Variability of HA antibody internalization, however, was observed in GLUT12-TQP-expressing cells with some cells displaying little or no HA-antibody internalization as is seen in GLUT12-expressing cells. (Figure 7A-left panel bottom row). Swapping GPN for TQP of the XXX motif significantly altered trafficking of GLUT12. In comparison, exchanging TQP for GPN in GLUT8 resulted in retention at the cell surface with very little internalization after 2 hours (Figure 7B-right panel bottom row). This finding differs greatly from that seen with the GLUT8-HA wildtype results, in which the majority of GLUT8-HA tagged is internalized by 2 hours and an extremely low amount is detected at the cell surface (Figure 7B-bottom row left vs right panel).
Conclusions
The Class III glucose transporters GLUT8 and GLUT12 are the only two members of the glucose transporter family that contain a [DE]XXXL[LI] sorting motif. GLUT12 has been shown to localize primarily to an unidentified intracellular compartment in human breast and colon cancer cell lines (3, 8). For these reasons, we initially anticipated that the localization and targeting of GLUT12 would closely resemble that of GLUT8. Surprisingly, we found that despite sharing a similar [DE]XXXL(8) targeting motif, GLUT8 and GLUT12 reside in different locations. GLUT12 is seen predominantly at the plasma membrane, however is also seen in intracellular compartments. GLUT12 localizes to the Golgi network as shown by the significant overlap with endogenous GM130 as well as to recycling endosomes as shown by the overlap with the endosomal marker Rab11. Unlike GLUT8, GLUT12 does not localize to the late endosome or to the lysosome, as demonstrated by a lack of colocalization between GLUT12 and the late endosomal markers Rab7 and Rab9 and the lysosomal marker Syntaxin 8.
Based on our studies we conclude that the [DE]XXXL[LI] consensus sequence in the NH2-terminus of GLUT8 and GLUT12 differentially affects the trafficking of each transporter from the cell membrane. When we performed immunoabsorption assays using CHO cells expressing GLUT8-HA- or GLUT12-HA, we found that while GLUT8 is internalized from the plasma membrane, the majority of GLUT12 remains associated with the cell surface following two hours of cell culture in the presence of HA antibody. Further examination of the [DE]XXXL[LI] motif in GLUT12 showed that the consensus sequence might play a role in regulating the post-Golgi trafficking of GLUT12. Using site-directed mutagenesis to mutate the EGPNLL motif to EGPNAA, we found that this mutation decreases the cell surface expression levels of GLUT12. The decrease in cell surface-associated GLUT12 correlated with a noticeable increase in the intracellular levels of GLUT12. The analogous mutation in GLUT8 results in the retention of GLUT8 at the plasma membrane (16, 17). Because this dileucine motif differentially affects the intracellular and cell surface trafficking of GLUT8 and GLUT12, we examined the role that the XXX residues immediately upstream of the dileucine motif play in regulating the cell surface expression levels of these Class III glucose transporters. Compared to the wild type transporters, we found that GLUT8-GPN aberrantly traffics to the cell surface while GLUT12-TQP localizes to an intracellular compartment and shows a decrease in cell surface expression. This shows that the residues immediately upstream of the dileucine motif are important in regulating the trafficking of GLUT8 and GLUT12 to their respective destinations. Previous studies have revealed that the residues upstream of the dileucine pair may bias the interactions of different dileucine motifs towards specific AP complexes (22, 23). In particular, dileucine motifs containing a proline residue at -1 position may preferentially bind to AP3 (22). Proteins that interact strongly with AP3, such as LIMPII, are sorted directly to the late endosome and the lysosome (24-26). The lysosome-associated glucose transporter GLUT8 contains a proline residue immediately upstream of the dileucine motif, suggesting a possible mechanism for GLUT8 sorting via interactions with AP-3. It is therefore probable that the NH2-terminal dileucine motifs differently affect the trafficking of GLUT8 and GLUT12 through the preferential binding of different adaptor proteins to each motif. Investigating the interactions between the adaptor proteins AP1, AP2, and AP3 and the glucose transporters GLUT8, GLUT12, GLUT12-AA, GLUT8-GPN, and GLUT12-TQP will identify a role for the amino terminal dileucine motif in sorting GLUT8 and GLUT12 to distinct subcellular locations. Better understanding the mechanisms that regulate the trafficking of GLUT12 to the cell surface will allow us to clarify whether GLUT12 functions as a second insulin-responsive glucose transporter in adipose tissue and skeletal muscle.
Materials and Methods
DNA constructs and antibodies
The murine GLUT12 coding sequence was cloned into pcDNA3.1(+) (Invitrogen, Carlsbad, CA, USA) using EcoRI and XhoI restrictions sites. An HA-tag (YPYDVPDYA) was inserted into the large exofacial loop between transmembrane helices 9 and 10 using PCR. Two primary PCRs were performed to generate amplicons containing the HA-tag sequence (TACCCATACGACGTCCCAGACTACGCT) in 5′ and 3′ overhangs. A secondary PCR was performed to fuse the two primary PCR products, and the resulting amplicon was cloned into pcDNA3.1(+) vector. Site-directed mutagenesis was performed to change the amino acids LL (12, 13) to AA and GPN (9, 10, 11) to TQP in mouse GLUT12 using the Quikchange Site-Directed Mutagenesis Kit (Stratagene, La Jolla, CA, USA). The amino acids TQP (10, 11, 12) were also changed to GPN in mouse GLUT8 by using site-directed mutagenesis. All constructs were verified by sequencing. The YFP-rab11 construct was provided by Dr. Phillip Stahl (Washington University in St. Louis). The YFP-rab9 and CFP-rab7 were provided by Dr. Susan Pfeffer. The rab7 cds was subsequently cloned into the YFP vector to visualize by confocal microscopy using a 488-nm laser as described previously (14).
The polyclonal GLUT12 antibody used in this study targets the COOH-terminal peptide sequence CGRGQSQRPSPDT of mouse GLUT12. The GLUT12 antibody was peptide purified and used at a concentration of 10 ug/mL for immunofluorescence studies and at a dilution of 1:2000 in 1% milk in TBST for western blot analysis. For co-localization and immuno-absorption experiments, the following antibodies were used: Syntaxin 8, GM130, and EEA1 antibodies (BD Biosciences, San Diego, CA, USA), and monoclonal mouse (Covance Research Products Inc, Berkeley, CA, USA) and rat-HA antibodies (Roche Applied Science, Indianapolis, IN, USA). Fluorescent-labeled and HRP-conjugated secondary antibodies were purchased from Molecular Probes (Eugene, OR, USA) and Pierce (Rockford, IL, USA). Alexa 546-conjugated transferrin and FITC-Dextran were purchased from Molecular Probes.
Western blotting and PNGase F treatment
The expression of GLUT12 in transiently and stably transfected HEK293 and CHO cells was verified using western blot analysis of whole cell lysates. Cells were washed in PBS and lysed in cell lysis buffer (50 mM Tris-HCl, 150 mM NaCl, 1mM EDTA, 0.5% NP-40, and 0.2% Glycerol) containing proteinase inhibitors. Whole cell lysates were separated using SDS-PAGE electrophoresis and transferred to nitrocellulose paper. Non-specific antibody binding was blocked in 5% milk in TBST (10 mM Tris-HCl, 100 mM NaCl, and 0.1% Tween). The GLUT12 antibody was applied overnight at 4°C in 1% milk in TBST (1:2000). Membranes were then probed with HRP-conjugated secondary antibodies and detected using SuperSignal Dura chemiluminescence substrate (Pierce). To verify the specificity of the GLUT12 antibody and to identify the glycosylated and unglycosylated band sizes for GLUT12, 20 μg of unboiled whole cell lysates were treated with PNGase F (NEB) at 37°C for one hour as described in the manufacturers instructions. Following PNGase F treatment cell lysates were subjected to western blot analysis.
Animal care
The mouse studies were approved by the Animal Studies Committee at Washington University School of Medicine and conform to the Guide for the Care and Use of Laboratory Animals published by the National Institute of Health. Female C57BL/6NCr (National Cancer Institute) mice of 7-8 weeks of age were used for isolating endometrial stromal cells (ESCs). Mice were given food and water ad libitum and were maintained on a 12 hour light/dark cycle.
Isolation of murine endometrial stromal cells
Female mice were sacrificed and uterine horns were dissected out and placed in Hanks Balanced Salt Solution (HBSS). Uteri were cut open longitudinally and washed in HBSS for five minutes. Following the wash, uteri placed in Dulbecco's modified Eagle's Medium:F12 medium (DMEM:F12) without phenol red and supplemented with 2 g/liter Collagenase Type I (Gibco). Uteri were then cut into small pieces (1 mm3) and incubated at 37°C for 60-90 minutes. The uteri were agitated by vortexing the solution every 15 minutes throughout the incubation. Following the incubation, the solution was passed through a 40 um sieve (BD Falcon) and centrifuged for five minutes at 250 × g. The ESCs were resuspended in DMEM:F12 without phenol red supplemented with 2% charcoal dextran-stripped calf serum and penicillin-streptomycin and were plated in 6-well cell culture dishes.
RNA extraction, Reverse Transcription-PCR, and PCR amplification
Total RNA was extracted from murine endometrial stromal cells using TRIzol® reagent (Invitrogen) according to the manufacturer's instructions. Total RNA was then DNase-treated using DNA-free™ (Ambion). cDNA was synthesized from the total RNA using SuperScript™ III Reverse Transcriptase (Invitrogen). The absence of reverse transcriptase was used as an internal control for each cDNA synthesis reaction. GLUT8 and GLUT12 were detected in endometrial stromal cells by reverse-transcription-PCR using Klentaq LA polymerase (Wayne Barnes, Washington University). To identify GLUT8 the following primers were used: 5′-TCTCCATGACGCTGCTGAACTCTT-3′ and 5′-TCGGGTGTGATCATGGTGTTCAGT-3′. GLUT12 was amplified using the following primers: 5′-GCCTCCTGGTGGGTTATGAAC-3′ and 5′-CCTCATGTTGTCCTTAGAGCG-3′. The fresh PCR products were ligated into a pCR®2.1-TOPO® cloning vector and sequenced to confirm the identity of the amplified GLUT8 and GLUT12 bands.
Subcellular fractionation
Subcellular fractionation was performed as described previously (14) To isolate plasma membrane (PM), high-density microsome (HDM), and low-density microsome (LDM) fractions, endometrial stromal cells were washed twice in PBS and then rinsed in cold (4°C) homogenization buffer (20 mM Tris, 1 mM EDTA, and 255 mM sucrose, pH 7.4). Cells were scraped into homogenization buffer containing proteinase inhibitors, homogenized in a dounce homogenizer, and centrifuged at 600 × g for 10 minutes. The supernatent was then centrifuged at 14,000 × g for 15 minutes and the resulting pellet was resuspended in homogenization buffer and overlaid onto an equal volume of 38.5% sucrose gradient and then centrifuged at 100,000 × g for 1 hour. The PM fraction was collected at the interphase of the sample and the sucrose cushion and centrifuged for 1 hour at 50,000 × g. The supernatent from the 14,000 × g spin was centrifuged at 50,000 × g for 30 minutes to obtain the HDM fraction. The resulting supernatent from the 50,000 × g spin was centrifuged an additional time at 180,000 × g for 45 minutes to isolate the LDM fraction. The membrane pellets were resuspended in 10 mM Tris and stored at -80°C prior to analysis. Protein was quantified using BCA reagent (Biorad) and the fractions were examined using western blotting analysis.
Cell culture and transfections
HEK293 and CHO cells were maintained in DMEM supplemented with 10% fetal bovine serum (FBS), nonessential amino acids, penicillin-streptomycin, and sodium-pyruvate. Endometrial stromal cells were maintained in DMEM:F12 without phenol red and supplemented with 2% charcoal dextran-stripped calf serum and penicillin-streptomycin. Cells were transfected with Fugene 6 (Roche) according to manufacturer's instructions, and stably expressing cells were selected for using 1 mg/mL G418. Immunofluorescence and western blot analysis were used to verify stable expression for GLUT12, GLUT12-FLAG, and GLUT12-HA in CHO and HEK293 cells.
Confocal Microscopy
HEK293 and CHO cells were grown on cover slips and rinsed with PBS before being fixed with 3% paraformaldehyde in PBS for 10 minutes. The cells were rinsed with PBS and then permeabilized in 0.1% Tween for 5 minutes. Cells were rinsed in PBS and nonspecific antibody binding was blocked by incubating cells in 20% normal goat serum in 2% BSA/PBS for 30 minutes. Primary antibodies diluted in 2% BSA/PBS were then applied for one hour at room temperature or overnight at 4 °C. Cells were washed three times with PBS. Alexa Fluor 488 and Alexa Fluor 546 were used as secondary antibodies at dilutions of 1:250 and 1:100 in 2% BSA/PBS, respectively, and were applied to cells at room temperature for 20 minutes. Cells were washed three times in PBS and then the nuclei were counterstained with TOPRO-iodide (Molecular Probes) diluted 1:500 in PBS for 10 minutes. Cells were mounted on slides using Vectashield (Vector Labs, Burlingame, CA, USA) and examined by confocal microscopy using a Nikon C1 microscope.
Alexa 546-conjugated Transferrin and FITC-Dextran were used to look for colocalization of GLUT12 and the recycling endosome and lysosome, respectively, as described previously. Cells were incubated with Alexa 546-conjugated Transferrin for 30 minutes to label the recycling endosomes. To label the lysosomes, cells were cultured in 1 mg/mL of FITC-Dextran for 6 hours, followed by an additional 2 hours in culture after washing FITC-Dextran out of the cell culture media. The cells were then processed for immunofluorescence staining and confocal microscopy as described above.
Flow cytometry to examine effects of [DE]XXXL[LI] mutants on PM expression of GLUT12 and GLUT8
The effects of mutating GLUT12 and GLUT8 NH2-terminal [DE]XXXL[LI] motifs to EGPNAA (GLUT12), ETQPLL (GLUT12), and EGPNLL (GLUT8) were quantitatively measured using flow cytometry. HEK293 and CHO cells were transiently transfected with GLUT12-HA, GLUT12-AA-HA, GLUT12-TQP-HA, GLUT8-HA, or GLUT8-GPN-HA constructs. Cells were analyzed twenty-four hours after transfection by confocal microscopy or flow cytometry. For flow cytometry, transfected cells were rinsed in PBS and lifted using Cell Dissociation Solution (Sigma). Cells were then resuspended in 10% FCS in PBS staining buffer (SB) and transferred to a V-bottom 96-well plate. Subsequent steps were performed at 4°C. Cells were blocked for thirty minutes in 3% NGS in SB. Cells were then incubated in mouse monoclonal HA-antibody for one hour at a dilution of 1:1000 in SB. The cells were washed two times in SB before a thirty minute incubation with goat anti-mouse Alexa488 antibody at a dilution of 1:200 in SB. Following two more washes with SB, cells were resuspended in 200 μL of SB and analyzed using a FACS Calibur flow cytometer. An arbitrary gate was drawn and the percentage of cells within the gate was decided using CELL QUEST software (BD Biosciences).
Internalization assay for GLUT12-HA, GLUT12-TQP-HA, GLUT8-HA and GLUT8-GPN-HA
Endocytosis of GLUT8, GLUT8-GPN, GLUT12 and GLUT12-TQP was compared using CHO cells expressing GLUT8-HA, GLUT8-GPN-HA, GLUT12-HA or GLUT12-TQP-HA constructs. These GLUT constructs contain an HA-tag in the large exofacial loop between transmembrane helices 9 and 10. CHO cells were grown on coverslips and transiently transfected with either GLUT12-HA, GLUT12-TQP-HA, GLUT8-HA or GLUT8-GPN-HA as described above. Twenty-four hours after transfection the cells were incubated for one hour with 8 uL/mL monoclonal HA antibody (Covance). The cells were rinsed three times in PBS to remove any unbound HA antibody and were cultured for an additional hour without antibody. Cells were then fixed for ten minutes in 3% paraformaldehyde. Following fixation, cells were either permeabilized to examine the degree of GLUT12 internalization or stained without permeabilization to examine the cell surface expression of the transporter. Nonspecific antibody binding was blocked in 20% normal goat serum in 2% BSA/PBS for 30 minutes and cells were probed with goat anti-mouse Alexa-Fluor 488 in 2% BSA/PBS for 30 minutes. The cells were then mounted in Vectashield and analyzed by confocal microscopy.
Supplementary Material
GLUT12 does not localize to a late endosomal compartment in stably GLUT12-expressing CHO cells overexpressing YFP-rab9 (A). Similarly, GLUT12 is also not found in the lysosomal membranes surrounding FITC-Dextran in CHO cells treated with FITC-Dextran (B). Images were taken at 60× magnification.
GLUT12 does not colocalize with markers of recycling endosomes identified using endocytosed Transferrin (A) or early endosomes (B --- EEA1). Images were taken at 60× magnification.
Acknowledgments
This work was funded by an American Diabetes Association Research Grant (KHM) as well as NIH (DK070351). This work was also supported by NIH Neuroscience Blueprint Core Grant NS057105 to Washington University and the Bakewell Family Foundation. The authors would like to thank Dr. Robert Augustin for his critical reading and suggestions.
References
- 1.Joost HG, Thorens B. The extended GLUT-family of sugar/polyol transport facilitators: nomenclature, sequence characteristics, and potential function of its novel members (review) Molecular membrane biology. 2001;18(4):247–256. doi: 10.1080/09687680110090456. [DOI] [PubMed] [Google Scholar]
- 2.Uldry M, Thorens B. The SLC2 family of facilitated hexose and polyol transporters. Pflugers Arch. 2004;447(5):480–489. doi: 10.1007/s00424-003-1085-0. [DOI] [PubMed] [Google Scholar]
- 3.Rogers S, Macheda ML, Docherty SE, Carty MD, Henderson MA, Soeller WC, Gibbs EM, James DE, Best JD. Identification of a novel glucose transporter-like protein-GLUT-12. Am J Physiol Endocrinol Metab. 2002;282(3):E733–738. doi: 10.1152/ajpendo.2002.282.3.E733. [DOI] [PubMed] [Google Scholar]
- 4.Macheda ML, Kelly DJ, Best JD, Rogers S. Expression during rat fetal development of GLUT12--a member of the class III hexose transporter family. Anat Embryol (Berl) 2002;205(5-6):441–452. doi: 10.1007/s00429-002-0263-8. [DOI] [PubMed] [Google Scholar]
- 5.Zhou Y, Kaye PL, Pantaleon M. Identification of the facilitative glucose transporter 12 gene Glut12 in mouse preimplantation embryos. Gene Expr Patterns. 2004;4(6):621–631. doi: 10.1016/j.modgep.2004.04.010. [DOI] [PubMed] [Google Scholar]
- 6.Wood IS, Hunter L, Trayhurn P. Expression of Class III facilitative glucose transporter genes (GLUT-10 and GLUT-12) in mouse and human adipose tissues. Biochemical and biophysical research communications. 2003;308(1):43–49. doi: 10.1016/s0006-291x(03)01322-6. [DOI] [PubMed] [Google Scholar]
- 7.Miller PJ, Finucane KA, Hughes M, Zhao FQ. Cloning and expression of bovine glucose transporter GLUT12. Mamm Genome. 2005;16(11):873–883. doi: 10.1007/s00335-005-0080-5. [DOI] [PubMed] [Google Scholar]
- 8.Chandler JD, Williams ED, Slavin JL, Best JD, Rogers S. Expression and localization of GLUT1 and GLUT12 in prostate carcinoma. Cancer. 2003;97(8):2035–2042. doi: 10.1002/cncr.11293. [DOI] [PubMed] [Google Scholar]
- 9.Rogers S, Chandler JD, Clarke AL, Petrou S, Best JD. Glucose transporter GLUT12-functional characterization in Xenopus laevis oocytes. Biochemical and biophysical research communications. 2003;308(3):422–426. doi: 10.1016/s0006-291x(03)01417-7. [DOI] [PubMed] [Google Scholar]
- 10.Stuart CA, Yin D, Howell ME, Dykes RJ, Laffan JJ, Ferrando AA. Hexose transporter mRNAs for GLUT4, GLUT5, and GLUT12 predominate in human muscle. Am J Physiol Endocrinol Metab. 2006;291(5):E1067–1073. doi: 10.1152/ajpendo.00250.2006. [DOI] [PubMed] [Google Scholar]
- 11.Piper RC, Tai C, Kulesza P, Pang S, Warnock D, Baenziger J, Slot JW, Geuze HJ, Puri C, James DE. GLUT-4 NH2 terminus contains a phenylalanine-based targeting motif that regulates intracellular sequestration. The Journal of cell biology. 1993;121(6):1221–1232. doi: 10.1083/jcb.121.6.1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Verhey KJ, Birnbaum MJ. A Leu-Leu sequence is essential for COOH-terminal targeting signal of GLUT4 glucose transporter in fibroblasts. The Journal of biological chemistry. 1994;269(4):2353–2356. [PubMed] [Google Scholar]
- 13.Bonifacino JS, Traub LM. Signals for sorting of transmembrane proteins to endosomes and lysosomes. Annu Rev Biochem. 2003;72:395–447. doi: 10.1146/annurev.biochem.72.121801.161800. [DOI] [PubMed] [Google Scholar]
- 14.Augustin R, Carayannopoulos MO, Dowd LO, Phay JE, Moley JF, Moley KH. Identification and characterization of human glucose transporter-like protein-9 (GLUT9): alternative splicing alters trafficking. The Journal of biological chemistry. 2004;279(16):16229–16236. doi: 10.1074/jbc.M312226200. [DOI] [PubMed] [Google Scholar]
- 15.Schmidt U, Briese S, Leicht K, Schurmann A, Joost HG, Al-Hasani H. Endocytosis of the glucose transporter GLUT8 is mediated by interaction of a dileucine motif with the beta2-adaptin subunit of the AP-2 adaptor complex. J Cell Sci. 2006;119(Pt 11):2321–2331. doi: 10.1242/jcs.02943. [DOI] [PubMed] [Google Scholar]
- 16.Ibberson M, Uldry M, Thorens B. GLUTX1, a novel mammalian glucose transporter expressed in the central nervous system and insulin-sensitive tissues. The Journal of biological chemistry. 2000;275(7):4607–4612. doi: 10.1074/jbc.275.7.4607. [DOI] [PubMed] [Google Scholar]
- 17.Lisinski I, Schurmann A, Joost HG, Cushman SW, Al-Hasani H. Targeting of GLUT6 (formerly GLUT9) and GLUT8 in rat adipose cells. Biochem J. 2001;358(Pt 2):517–522. doi: 10.1042/0264-6021:3580517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bonifacino JS, Lippincott-Schwartz J. Coat proteins: shaping membrane transport. Nat Rev Mol Cell Biol. 2003;4(5):409–414. doi: 10.1038/nrm1099. [DOI] [PubMed] [Google Scholar]
- 19.Brodsky FM, Chen CY, Knuehl C, Towler MC, Wakeham DE. Biological basket weaving: formation and function of clathrin-coated vesicles. Annu Rev Cell Dev Biol. 2001;17:517–568. doi: 10.1146/annurev.cellbio.17.1.517. [DOI] [PubMed] [Google Scholar]
- 20.Frolova A, Flessner L, Chi M, Kim ST, Foyouzi-Yousefi N, Moley KH. GLUT1 is differentially regulated by progesterone and estrogen in murine and human endometrial stromal cells. Endocrinology. 2008 doi: 10.1210/en.2008-1081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Goldman NA, Katz EB, Glenn AS, Weldon RH, Jones JG, Lynch U, Fezzari MJ, Runowicz CD, Goldberg GL, Charron MJ. GLUT1 and GLUT8 in endometrium and endometrial adenocarcinoma. Mod Pathol. 2006;19(11):1429–1436. doi: 10.1038/modpathol.3800656. [DOI] [PubMed] [Google Scholar]
- 22.Rodionov DG, Honing S, Silye A, Kongsvik TL, von Figura K, Bakke O. Structural requirements for interactions between leucine-sorting signals and clathrin-associated adaptor protein complex AP3. The Journal of biological chemistry. 2002;277(49):47436–47443. doi: 10.1074/jbc.M207149200. [DOI] [PubMed] [Google Scholar]
- 23.Doray B, Lee I, Knisely J, Bu G, Kornfeld S. The gamma/sigma1 and alpha/sigma2 hemicomplexes of clathrin adaptors AP-1 and AP-2 harbor the dileucine recognition site. Mol Biol Cell. 2007;18(5):1887–1896. doi: 10.1091/mbc.E07-01-0012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Vega MA, Rodriguez F, Segui B, Cales C, Alcalde J, Sandoval IV. Targeting of lysosomal integral membrane protein LIMP II. The tyrosine-lacking carboxyl cytoplasmic tail of LIMP II is sufficient for direct targeting to lysosomes. The Journal of biological chemistry. 1991;266(25):16269–16272. [PubMed] [Google Scholar]
- 25.Vega MA, Segui-Real B, Garcia JA, Cales C, Rodriguez F, Vanderkerckhove J, Sandoval IV. Cloning, sequencing, and expression of a cDNA encoding rat LIMP II, a novel 74-kDa lysosomal membrane protein related to the surface adhesion protein CD36. The Journal of biological chemistry. 1991;266(25):16818–16824. [PubMed] [Google Scholar]
- 26.Janvier K, Kato Y, Boehm M, Rose JR, Martina JA, Kim BY, Venkatesan S, Bonifacino JS. Recognition of dileucine-based sorting signals from HIV-1 Nef and LIMP-II by the AP-1 gamma-sigma1 and AP-3 delta-sigma3 hemicomplexes. The Journal of cell biology. 2003;163(6):1281–1290. doi: 10.1083/jcb.200307157. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
GLUT12 does not localize to a late endosomal compartment in stably GLUT12-expressing CHO cells overexpressing YFP-rab9 (A). Similarly, GLUT12 is also not found in the lysosomal membranes surrounding FITC-Dextran in CHO cells treated with FITC-Dextran (B). Images were taken at 60× magnification.
GLUT12 does not colocalize with markers of recycling endosomes identified using endocytosed Transferrin (A) or early endosomes (B --- EEA1). Images were taken at 60× magnification.