Abstract
In this issue, Thon et al. (2010. J. Cell Biol. doi: 10.1083/jcb.201006102) demonstrate that newly released platelets exhibit bipolar behavior, shifting back and forth between round cells and multibodied proplatelets (Thon et al., 2010). The authors define this intermediate as a preplatelet and, in doing so, shed new insight into the terminal steps of platelet maturation.
It is commonly accepted that during the final stages of differentiation, megakaryocytes extend cytoplasmic protrusions referred to as proplatelets (Italiano et al., 1999). These extensions are comprised of multiple cell bodies that are connected by thin cytoplasmic shafts. Based on in vitro model systems (Italiano et al., 1999) and more recently by in vivo tracking (Junt et al., 2007), investigators concluded that proplatelets fragment into shorter extensions or single platelets as they enter the circulation. Notably, these anucleate fragments typically exceed platelet dimensions, suggesting that platelets undergo morphological maturation in the circulation. Platelet morphogenesis in the circulation was initially proposed by Behnke and Forer (1998), and more recently, our group (Schwertz et al., 2010) showed that individual human platelets have the innate capacity to morph into multibodied cells that resemble megakaryocyte-derived proplatelets.
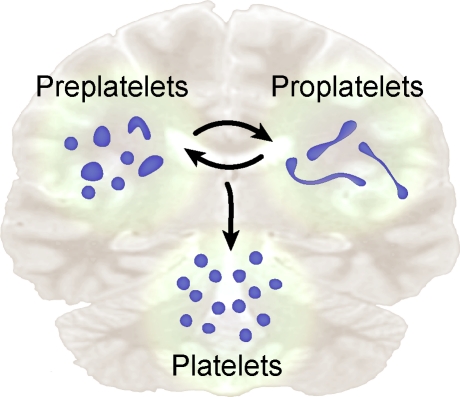
In this issue, using an advanced in vitro mouse model of platelet release from cultured megakaryocytes, Thon et al. glean new information on the final stages of platelet production. Through a series of gradient sedimentation and centrifugation steps, the investigators separated fetal liver–derived megakaryocytes from proplatelet-producing megakaryocytes, released proplatelets, and individual platelets. By enriching for each cell type, they were able to characterize intermediate steps in platelet formation and release. The authors also identified a previously unrecognized intermediate cell, which they termed a preplatelet. Preplatelets are defined as discoid cells that retain the capacity to convert into barbell-shaped proplatelets (Fig. 1). Conversely, the investigators also demonstrated that proplatelets revert to discoid preplatelets.
Figure 1.
Platelet precursors display bipolar behavior. In this issue, Thon et al. (2010) demonstrate that platelet precursors shift back and forth between two morphological poles, one that is discoid and the other that has barbell-like features (i.e., proplatelets). The authors name the discoid precursor a preplatelet. Both preplatelets and proplatelets contribute to the mature platelet pool.
The authors show that these intermediates of thrombopoiesis are present at similar abundance and that their morphology is regulated by microtubules. Manipulation of microtubule function controls the interconversions between barbell-shaped proplatelets and preplatelets. Ultimately, proplatelets or preplatelets that convert into proplatelets divide to generate individual platelets (Fig. 1). Whether a subset of the newly released platelets exhibit a preplatelet phenotype, which would allow them to form barbell-shaped platelets and divide again, is not clear. However, the authors did find that preplatelets only convert to proplatelets if preplatelet diameters are between 2 and 10 µm. This implies that young platelets come in variable sizes and that this morphogenic phenomenon may have size restrictions. The authors found that the lion’s share of preplatelets is between 2 and 4 µm in diameter, which surpasses the mean width of most circulating mouse platelets (Schmitt et al., 2001). If size is the cardinal feature that distinguishes a preplatelet from a mature platelet, then the bulk of mouse platelets may be incapable of dividing in the bloodstream. However, this limitation may not apply to human platelets, whose mean diameter (∼2–3 µm) falls within the diametric range of 69% of mouse preplatelets. Like mouse preplatelets, human platelets of this size are capable of forming new extensions and cell bodies (Schwertz et al., 2010). This raises the possibility that subpopulations of human platelets may be analogous to mouse preplatelets. In either case, the morphological similarities between mouse preplatelets and human platelets that form new cell bodies are striking.
A critical question is whether the conversion process of preplatelet to proplatelet to platelet occurs in the vasculature. The authors answer the latter half of this question by transfusing labeled proplatelets into mice and demonstrating shortly thereafter that these precursors generate individual, discoid platelets. Presumably, the labeled proplatelets fragmented into single platelets as they encountered shear forces in the bloodstream. Consistent with this notion, Thon et al. (2010) demonstrate that increased shear accelerates proplatelet fission and platelet release in vitro. Establishing that the interconnecting shafts between platelet bodies are cleaved by shear forces adds insight into the final steps of platelet release and may explain why proplatelets are rarely detected in ex vivo platelet preparations that are purified by successive centrifugation steps. However, it also presents a quandary for visualizing the intermediate steps in vivo, especially the conversion of preplatelets to proplatelets. The fragility of interconnections between platelet bodies suggests that shear forces in the bloodstream rapidly sever them after they form. In addition, preplatelets convert to proplatelets at different rates and unpredictable times, so capturing the event in circulating blood will be challenging.
There is no doubt that the work of Thon et al. (2010) pushes the field forward, more clearly defining intermediate stages in platelet maturation. Their work also supplies investigators with state of the art methods for isolating platelet progenitors that come in different sizes and shapes. More importantly, their results support and facilitate new investigative avenues. Among these is determining whether interconversion between preplatelets and proplatelets is a spontaneous event or whether it responds and adjusts to external cues. In addition, is fission triggered by shear forces alone, or are molecular signals involved? As shown by Thon et al. (2010), the shafts of mouse proplatelets form constricted regions that resemble cleavage furrows. Similar restrictions have been observed in multibodied human platelets (Schwertz et al., 2010), and cleavage furrows in dividing nucleated cells form and sunder via specific molecular pathways. Lastly, does this morphogenic process become dysregulated in thrombocytopenic situations and, as a result, lead to differential platelet phenotypes or contribute to decreased platelet counts? Regardless of the answers, the discovery that preplatelets display bipolar behavior reminds us that platelet precursors and their progeny function in unique and unexpected ways.
References
- Behnke O., Forer A. 1998. From megakaryocytes to platelets: platelet morphogenesis takes place in the bloodstream. Eur. J. Haematol. Suppl. 61:3–23 [DOI] [PubMed] [Google Scholar]
- Italiano J.E., Jr, Lecine P., Shivdasani R.A., Hartwig J.H. 1999. Blood platelets are assembled principally at the ends of proplatelet processes produced by differentiated megakaryocytes. J. Cell Biol. 147:1299–1312 10.1083/jcb.147.6.1299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Junt T., Schulze H., Chen Z., Massberg S., Goerge T., Krueger A., Wagner D.D., Graf T., Italiano J.E., Jr, Shivdasani R.A., von Andrian U.H. 2007. Dynamic visualization of thrombopoiesis within bone marrow. Science. 317:1767–1770 10.1126/science.1146304 [DOI] [PubMed] [Google Scholar]
- Schmitt A., Guichard J., Massé J.M., Debili N., Cramer E.M. 2001. Of mice and men: comparison of the ultrastructure of megakaryocytes and platelets. Exp. Hematol. 29:1295–1302 10.1016/S0301-472X(01)00733-0 [DOI] [PubMed] [Google Scholar]
- Schwertz H., Köster S., Kahr W.H., Michetti N., Kraemer B.F., Weitz D.A., Blaylock R.C., Kraiss L.W., Greinacher A., Zimmerman G.A., Weyrich A.S. 2010. Anucleate platelets generate progeny. Blood. 115:3801–3809 10.1182/blood-2009-08-239558 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thon J.N., Montalvo A., Patel-Hett S., Devine M.T., Richardson J.L., Ehrlicher A., Larson M.K., Hoffmeister K., Hartwig J.H., Italiano J.E., Jr 2010. Cytoskeletal mechanics of proplatelet maturation and platelet release. J. Cell Biol. 191:861–874 [DOI] [PMC free article] [PubMed] [Google Scholar]