Abstract
Polycystic kidney disease is a common genetic disorder in which fluid-filled cysts displace normal renal tubules. Here we focus on autosomal dominant polycystic kidney disease, which is attributable to mutations in the PKD1 and PKD2 genes and which is characterized by perturbations of renal epithelial cell growth control, fluid transport, and morphogenesis. The mechanisms that connect the underlying genetic defects to disease pathogenesis are poorly understood, but their exploration is shedding new light on interesting cell biological processes and suggesting novel therapeutic targets.
Molecular pathogenesis of autosomal dominant polycystic kidney disease
One’s first glimpse of a specimen from a patient with advanced autosomal dominant polycystic kidney disease (ADPKD) creates a lasting impression. The massive enlargement of the kidney and the substitution of an irregular profusion of glistening cysts for its usual striated architecture are unmistakable hallmarks of a disease afflicting approximately 1 in 1,000 individuals (Torres, 1998; Calvet and Grantham, 2001; Grantham, 2001; Igarashi and Somlo, 2002; Wilson, 2004). The dramatic appearance underscores a single gene’s power to alter grotesquely the morphology of an organ whose structure is normally sublimely intertwined with its function.
ADPKD cysts increase in size and number over the space of decades, displacing and destroying adjacent renal parenchyma, leading ultimately to end-stage renal disease in ∼50% of cases. Cardiovascular, musculoskeletal, and gastrointestinal abnormalities are also associated with ADPKD (Gabow, 1993). The Pkd1 (polycystic kidney disease-1) and Pkd2 (polycystic kidney disease-2) genes encode polycystin-1 (PC1) and polycystin-2 (PC2), respectively. Approximately 85% of ADPKD cases are attributable to mutations in Pkd1, while mutations in Pkd2 account for almost all of the remaining cases. During the past fifteen years an enormous amount of effort has been invested in exploring the functions of the PC1 and PC2 proteins. The return on this investment constitutes something of an embarrassment of riches, in that the polycystin proteins appear to participate in a nearly bewildering array of signaling pathways and regulatory processes, and to reside within a complex collection of subcellular structures. A major goal of current ADPKD research is to elucidate the connections between these cell biological properties of the polycystin proteins and the pathogenesis of the disease that develops when their expression is perturbed.
One of the most intriguing discoveries to emerge from this intense research is the realization that portions of the cellular populations of PC1 and PC2 localize to the primary cilium. ADPKD is the founding member of the “ciliopathies,” a recently defined class of genetic disorders that result from mutations in genes encoding cilia-associated proteins. These disorders are often characterized by the presence of renal cysts as well as by additional pathologies including neural tube defects, retinal malformations, and polydactyly (Badano et al., 2006). Although the cellular and molecular mechanisms responsible for the pathogenesis of ADPKD are still very much the subject of spirited and healthy debate, it has become clear in recent years that understanding ADPKD, and the function or dysfunction of PC1 and PC2, will require an appreciation of these proteins’ roles in the primary cilium.
The polycystin proteins
Polycystin-1 structure and cleavage.
PC1 is a 450-kD protein with a large extracellular N terminus, 11 membrane-spanning domains, and a shorter cytoplasmic C terminus (Hughes et al., 1995; Nims et al., 2003). It is expressed in the epithelial cells of the developing and mature renal tubules, as well as in a variety of other somatic tissues including heart, liver, bone, and endocrine glands (Ward et al., 1996; Ibraghimov-Beskrovnaya et al., 1997; Markowitz et al., 1999; Peters et al., 1999). Expression of PC1 is temporally regulated, with the highest levels found in fetal renal tissue and low but detectable levels present in adult tissue (Chauvet et al., 2002). PC1 is found in the cilium, but also localizes to the lateral domain of the plasma membrane and adhesion complexes in polarized epithelial cells (Ibraghimov-Beskrovnaya et al., 1997; Huan and van Adelsberg, 1999; Yoder et al., 2002; Streets et al., 2009). In addition, PC1 and PC2 may be shed from the apical or ciliary membranes in urinary exosome-like vesicles that can interact with the primary cilium (Hogan et al., 2009).
The large extracellular PC1 N terminus contains 15 PKD repeat motifs, two complete leucine-rich repeat motifs flanked by cysteine-rich sequences, and a C-type lectin domain (Hughes et al., 1995; Bycroft et al., 1999). Many of these domains are crucial for PC1’s functions and play established roles in protein–protein or protein–matrix interactions (van Adelsberg, 1999; Ibraghimov-Beskrovnaya et al., 2000; Babich et al., 2004; Streets et al., 2009). This evidence, combined with PC1’s subcellular localization to the plasma membrane and junctional complexes, supports a role for PC1 in cell–cell and cell–matrix interactions. The extracellular domains of PC1 and PC2 may also participate in sensing fluid flow and pressure in the kidney, as reviewed by Patel and Honoré (2010). The 200 amino acids of the PC1 C-terminal tail (CTT) contain a G protein–binding domain and a coiled-coil domain. The C-terminal tail of PC1 also contains a sequence that is rich in proline, glutamic acid, serine, and threonine (PEST) amino acids, which may facilitate its ubiquitin-mediated degradation (Rechsteiner and Rogers, 1996; Low et al., 2006).
Polycystin-1 undergoes cleavages in both its N- and C-terminal domains (Fig. 1). N-terminal cleavage occurs at the G protein–coupled receptor proteolytic site (GPS), just before the first transmembrane domain (Qian et al., 2002). This is a cis-autoproteolytic cleavage that occurs early in the secretory pathway, and the cleaved PC1 N terminus remains noncovalently attached to the membrane-bound C-terminal fragment (Wei et al., 2007). Not all of the PC1 molecules in a cell are cleaved, generating a heterogeneous population of full-length and GPS-cleaved PC1 proteins (Wei et al., 2007; Yu et al., 2007). To be fully functional PC1 must be able to undergo N-terminal cleavage. Expression of a mutant form of PC1 that cannot undergo GPS cleavage does not rescue PC1-null cultured cells or transgenic mice. In addition, this missense mutation causes ADPKD in humans (Qian et al., 2002; Xu et al., 2007; Yu et al., 2007).
Figure 1.
N- and C-terminal cleavage of the PC1 protein. The N terminus of PC1 is cleaved at the G protein–coupled receptor proteolytic site (GPS), but the extracellular domain remains noncovalently attached to the membrane-bound portion of the protein. Either of two different cleavages can release C-terminal tail fragments that translocate to the nucleus with components of the Wnt pathway, STAT6/p100, and perhaps with other regulators of transcription. At least one of the C-terminal tail cleavages is stimulated by the presence of PC2, and this stimulation requires that PC2 be capable of functioning as an ion channel.
Two other cleavages liberate the cytoplasmic CTT of PC1 (Fig. 1). Chauvet et al. (2004) observed a cleavage that releases an ∼35-kD soluble portion of the tail that accumulates in the nucleus in response to decreased fluid flow in the mouse kidney. Low et al. (2006) observed a second, more distal cleavage that releases a 15-kD fragment of the PC1 cytoplasmic tail that interacts with the transcriptional activator STAT6 and the coactivator p100. Flow cessation increased this PC1 cleavage and nuclear translocation of both the PC1 tail and STAT6 (Low et al., 2006). Interestingly, an increased level of cleaved CTT is observed in cells lining ADPKD cysts (Low et al., 2006). At least one of these C-terminal cleavages is stimulated by the presence of PC2, and this stimulation requires that PC2 be competent to function as an ion channel (Bertuccio et al., 2009). Although the sizes of these fragments have been identified and their production is apparently regulated, the amino acid sequences of both cleavage sites have yet to be determined.
Polycystin-2 structure and channel function.
Polycystin-2 (PC2 or TRP2) is a 968-amino acid protein that spans the membrane six times, with intracellular N and C termini (Mochizuki et al., 1996). PC2 functions as a Ca2+-permeable nonselective cation channel and is homologous to the transient receptor potential family of cation channels (Tsiokas et al., 1999; González-Perrett et al., 2001). Although a portion of PC2 colocalizes with PC1 to the cilium, the majority of the cellular pool of PC2 appears to reside in intracellular compartments, where it may modulate the release of calcium from intracellular stores. The channel activity of the ciliary pool of the PC1–PC2 complex appears to respond to ciliary bending, and may also mediate the cilium’s role in transducing other mechanical or chemical stimuli (Nauli et al., 2003).
Several domains present in PC2’s N and C termini are responsible for PC2’s protein–protein interactions and Ca2+ sensitivity. At least two domains, one in each cytoplasmic tail, contribute to PC2 oligomerization. Immediately distal to PC2’s last transmembrane domain is a functionally complex region of the C terminus that includes coiled-coil, EF-hand, and ER retention domains. A calcium-binding EF hand domain begins upstream of and extends into the PC1-interacting coiled-coil region (Mochizuki et al., 1996; Qian et al., 1997; Celić et al., 2008). The helix-loop-helix structure of the EF-hand binds Ca2+, permitting the protein to sense or to buffer changes in Ca2+ (Gifford et al., 2007). The PC2 EF-hand has a single Ca2+-binding site with micromolar affinity (Celić et al., 2008). Slightly overlapping with both the coiled-coil and the EF-hand is a sequence that is required for maintaining PC2’s ER and Golgi localization (Cai et al., 1999). A naturally occurring truncation mutation that removes this C-terminal domain, and thus presumably abrogates all of its interactions and regulatory potential, is sufficient to cause ADPKD (Mochizuki et al., 1996).
PC2 is a calcium-activated channel that releases calcium from intracellular stores in response to local increases in calcium concentrations (Vassilev et al., 2001; Koulen et al., 2002). The calcium-conducting pore of PC2 is likely formed by the loop between the fifth and sixth transmembrane domains, with some involvement of the third transmembrane domain (Clapham et al., 2001; Koulen et al., 2002). A missense mutation that perturbs this putative conducting pore (D511V) is causative of ADPKD (Koulen et al., 2002). Fine-tuning PC2’s Ca2+ response and channel properties involves post-translational modifications, such as phosphorylation at S812 by casein kinase II, and binding of protein partners (Cai et al., 2004; Rundle et al., 2004). For a more thorough discussion of PC2 channel activity see the review by Cantiello (2004).
PC2 also indirectly regulates cytoplasmic calcium levels through interactions with two major intracellular Ca2+ channels: the ryanodine receptor and the inositol 1,4,5-trisphosphate receptor (IP3R). The ryanodine receptor mediates calcium-induced calcium release, and PC2 inhibits its function by binding the channel in its open state and decreasing its conductance (Anyatonwu et al., 2007). PC2 also modifies IP3-induced Ca2+ flux through direct binding between the PC2 C terminus and the IP3R (Li et al., 2009).
Polycystin-2 localization and trafficking.
PC2 localizes to several subcellular compartments (Köttgen and Walz, 2005; Tsiokas et al., 2007). The largest pool of PC2 is found in the ER and early Golgi (Cai et al., 1999; Koulen et al., 2002). Functional PC2 is also found at the plasma membrane, where it may exist in complexes with PC1 (Hanaoka et al., 2000; Pelucchi et al., 2006; Yu et al., 2009). Pools of PC2 also reside in more restricted subcellular domains, such as the primary cilium and mitotic spindles (Yoder et al., 2002; Nauli et al., 2003; Rundle et al., 2004; Xu et al., 2007).
A set of very specific signal sequences and trafficking proteins helps establish and maintain PC2 at these subcellular locations. Retention of PC2 in the early secretory pathway involves proteins that bind to the PC2 C terminus. A stretch of acidic amino acids in the protein’s C terminus functions as an ER retention signal by binding phosphofurin acidic cluster–sorting protein (PACS)-1 and PACS-2 (Cai et al., 1999; Köttgen et al., 2005). PACS-2 seems to be capable of ensuring that PC2 remains localized to the ER, whereas PACS-1 brings PC2 from endosomal compartments back to the TGN. The binding between PC2 and the PACS proteins requires PC2 phosphorylation by casein kinase II (CK2) (Köttgen et al., 2005) Facilitating PC2–PACS binding is one of the several roles for CK2 in altering PC2 localization. Experiments in Caenorhabditis elegans show that a mutation that prevents phosphorylation at a CK2 site in the PC2 orthologue protein promotes its localization to cilia, and this localization is prevented by a phospho-mimetic mutation at the same site. Consistent with this model, the calcineurin phosphatase TAX-6 is required for the PC2 orthologue’s ciliary localization in C. elegans (Hu et al., 2006). One of the other CK2 recognition sites in PC2 appears to exert no effect on the protein’s localization, but its phosphorylation is required for PC2 channel function (Cai et al., 2004). Thus, the multiple effects of CK2-mediated phosphorylation demonstrate a potential connection between mechanisms that regulate PC2’s localization and function.
Regulation of PC2 movement from the ER to the Golgi is also controlled by another protein that binds to the PC2 C-terminal tail. PIGEA-14 (polycystin-2 interactor, Golgi- and endoplasmic reticulum–associated protein), also called Chibby, is a 14-kD protein that binds to the Golgi matrix protein GM130. Co-expressing PIGEA-14 with PC2 in culture cells causes a redistribution of PC2 from the ER to the TGN (Hidaka et al., 2004).
Mechanisms for targeting PC2 to the primary cilium and mitotic spindles appear to rely on novel motifs and protein trafficking machinery. A 15-amino acid R6VxP motif at the very beginning of PC2’s N terminus is sufficient to ensure PC2’s localization to the primary cilium (Geng et al., 2006). Targeting PC2 to the mitotic spindle of dividing cells requires mammalian diaphanous 1 (mDia1), which belongs to a protein subfamily involved in cytoskeletal rearrangements and cytokinesis. Interestingly, mDia1 binding to PC2 also modulates PC2’s channel activity and is subject to regulation by growth factors, suggesting an interesting but as-of-yet unexplored connection between PC2 channel function and mitosis (Rundle et al., 2004).
Interaction between PC1 and PC2.
The subcellular localizations of PC1 and PC2 overlap and may, in some locations, be functionally codependent. There is strong colocalization of both proteins to the primary cilium, and they are also found together in the ER (Yoder et al., 2002). Several investigations suggest that PC1 and PC2 may reciprocally affect each other’s surface membrane or ciliary localizations, although the precise nature of this interdependence has varied somewhat among experimental systems (Hanaoka et al., 2000; Grimm et al., 2003; Babich et al., 2004). Studies performed on cells derived from ADPKD cysts indicate that impairing the function of one protein negatively affects the localization of the other: cells expressing an ADPKD-associated PC1 mutation that prevents GPS cleavage have decreased amounts of both PC1 and PC2 in their primary cilia (Xu et al., 2007). An interaction between PC1 and PC2 has also been suggested to be important in creating a functional ion channel, whether through activation of the PC2 protein’s intrinsic channel properties or through emergent channel properties attributable to formation of the complex (Hanaoka et al., 2000; Delmas et al., 2004). Physically, the interaction between the two proteins is thought to occur primarily through their C-terminal cytoplasmic tails (Qian et al., 1997; Tsiokas et al., 1997; Casuscelli et al., 2009). This interaction also appears to influence the proteins’ functional properties, as interaction of PC2 with PC1 decreases the ability of PC1 to activate G proteins (Delmas et al., 2002).
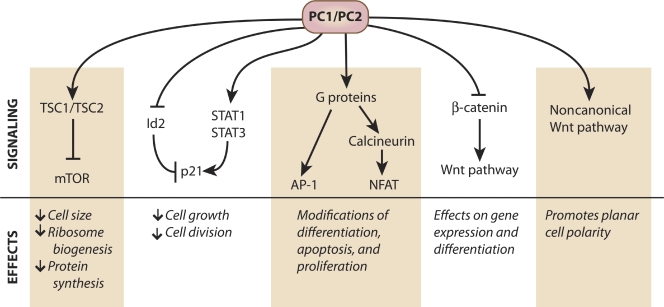
Signaling pathways modified by PC1 and PC2
The polycystin proteins modulate diverse signaling pathways, and there is a long list of proteins known to interact with PC1 or PC2 (Somlo et al., 2008). To summarize broadly, however, the evidence highlights three general themes in the relationship between the PC1 and PC2 proteins and cellular signaling pathways: negative growth regulation, G protein activation, and Wnt pathway modulation (Fig. 2). Although the mechanism for the PC1- or PC2-dependent effect in each case varies, a frequent theme is that the PC1 CTT binds to and negatively regulates the activity of crucial signaling molecules. In some cases this negative regulation happens at the cell membrane, and may be attributable at least in part to the sequestration at the cell surface of signaling protein partners that would otherwise enter the nucleus to modulate signaling. In other cases the cleaved PC1 CTT itself travels to the nucleus, where it appears to influence transcriptional activities. In each case, misregulating PC1 expression or cleavage appears to result in aberrant signaling, which may in turn lead to the abnormal cellular growth behaviors that are likely to contribute to ADPKD pathogenesis.
Figure 2.
PC1 and PC2 affect multiple signaling pathways. Summary of the effects that PC1 and PC2 exert on signaling pathways. Multiple direct and indirect interactions allow the polycystin proteins to inhibit or stimulate pathways involved in cellular growth and differentiation.
Growth regulation.
Elevated cellular growth rates are a hallmark of ADPKD, so it is no surprise that the polycystin proteins negatively regulate cellular growth and division through several pathways, which are diagrammed in Fig. 2 (see also the recent review by Zhou, 2009). One significant effect of PC1 involves inhibition of the mTOR (mammalian target of rapamycin) cascade (Shillingford et al., 2006a; Distefano et al., 2009; Dere et al., 2010). This effect is mediated by the TSC1 and TSC2 (tuberous sclerosis 1 and 2) complex, which acts as a negative regulator of the mTOR complex (Huang and Manning, 2008). The TSC2–TSC1 complex acts as a GTPase-activating protein for the small GTP-binding protein Rheb, which must be in its GTP-bound state in order for the mTOR kinase to function. PC1 decreases mTOR activity by stabilizing the functional TSC1–TSC2 complex via two distinct mechanisms. PC1 decreases ERK-dependent phosphorylation of TSC2 at S664 (Distefano et al., 2009), which allows TSC2 to remain bound to TSC1 (Ma et al., 2005). The TSC1/2 complex is also stabilized by the binding of PC1 to TSC2 at the plasma membrane, protecting TSC2 from phosphorylation by Akt at S939 (Dere et al., 2010), thus allowing the protein complex to continue repressing mTOR signaling (Inoki et al., 2002). The influence of the PC1–TSC2 interaction may not be unidirectional; expression of TSC2 may help PC1 to reach the plasma membrane (Kleymenova et al., 2001).
Cell cycle progression is governed by cyclin-dependent kinases (Cdks), and p21 slows or halts cell cycle progression by inhibiting Cdk2. The polycystin proteins act in concert to positively regulate p21 expression and activity. PC1 can increase p21 levels by binding members of the Janus kinase (JAK) and signal transducers and activators of transcription (STAT) pathway. PC1 activates STAT1 and STAT3, thus elevating p21 levels and decreasing cell growth. This activation requires a PC2-dependent interaction with JAK2, and also requires that PC1 have an intact C terminus (Bhunia et al., 2002). PC2-dependent mechanisms also prevent the nuclear localization of Id2 and E47, two p21-repressing helix-loop-helix proteins (Li et al., 2005). PC2 also reduces cell growth through direct physical interaction with eukaryotic translation elongation initiation factor 2a (eIF2a). This translation factor is activated through phosphorylation by pancreatic ER-resident eIF2a kinase (PERK). PC2 binds both PERK and eIF2a, enhancing eIF2a’s phosphorylation and decreasing cell proliferation (Liang et al., 2008).
G protein activation.
The PC1 CTT contains a highly conserved trimeric G protein activation domain (Parnell et al., 1998). G protein α-subunits activated by PC1 go on to positively regulate the activity of the c-Jun N-terminal kinase (JNK) and the AP-1 transcription factor (Parnell et al., 2002). AP-1 controls differentiation, apoptosis, and proliferation through a complex network of signaling and binding proteins (Shaulian and Karin, 2002). In addition, PC1 activates JNK through PKC-α (Arnould et al., 1998). Abnormal levels of AP-1 activity in tissue from ADPKD cysts support the conclusion that polycystin proteins play an important role in regulating AP-1 (Le et al., 2005).
The interaction between PC1 and G proteins also activates the nuclear factor of activated T cells (NFAT). The NFAT pathway regulates genes involved in apoptosis, growth, cellular differentiation, and cell adaptation (Horsley and Pavlath, 2002). Exogenous expression of PC1 causes NFAT nuclear accumulation, and this effect is enhanced by coexpressing Gαq, a known PC1-binding G protein subunit (Puri et al., 2004). NFAT can act in concert with AP-1 to turn on genes with composite transcription factor binding sites (Macián et al., 2001). Both NFAT and AP-1 are activated by PC1-activated G proteins and it is possible that they may have combinatorial effects; however, there are currently no data supporting cooperativity between activated NFAT and AP-1 in PC1 signaling.
NFAT is connected in interesting ways to calcium signaling and PC2 localization. NFAT is activated by calcineurin which, in turn, is activated by sustained elevation of cytosolic Ca2+ levels. Activated calcineurin dephosphorylates NFAT, leading to its nuclear accumulation. NFAT rephosphorylation by glycogen synthase kinase 3β (GSK-3β) causes NFAT to move back into the cytoplasm (Horsley and Pavlath, 2002). Expressing PC1 presumably activates calcineurin through G proteins, leading to NFAT dephosphorylation and nuclear accumulation. In C. elegans, calcineurin-mediated dephosphorylation of PC2 permits this protein’s ciliary localization (Hu et al., 2006). Puri et al. (2004) found that inhibiting the PC2-modulated inositol triphosphate or ryanodine receptor channels impaired PC1’s ability to regulate NFAT. It is thus tempting to suggest a connection between PC2’s effect on cytoplasmic calcium and the NFAT signaling pathway, the activation of calcineurin, and the localization of PC2. Further research will be needed to unravel this network of interaction.
Canonical and noncanonical Wnt signaling.
The Wnt pathways affect growth, differentiation, and establishment of planar cell polarity. PC1 seems to have a profound influence on both the canonical (β-catenin dependent) and noncanonical (β-catenin independent) components that make up the Wnt signaling network. ADPKD cysts and PC1-null cells manifest up-regulation of Wnt signaling activity markers, suggesting that PC1 exerts a negative effect on this system (Lal et al., 2008; Happé et al., 2009; Song et al., 2009). In the canonical pathway, the presence of the Wnt ligand induces β-catenin stabilization and nuclear translocation, leading to T cell factor (TCF)–dependent transcriptional activity. Cleaved PC1 CTT inhibits this pathway by directly or indirectly binding to β-catenin, moving with it to the nucleus, and reducing its ability to promote TCF-dependent transcription (Lal et al., 2008). PC2 may also regulate the expression of some components of the Wnt pathway. Knocking out PC2 in cultured mouse cells resulted in increased levels of β-catenin protein (Kim et al., 2009). Both PC1 and PC2 can therefore influence canonical Wnt signaling; however, it is currently unclear whether the effects of PC2 knockout on β-catenin levels are a direct result of the lack of PC2, or an indirect effect of PC1 misregulation caused by PC2 absence.
PC1 may also regulate noncanonical Wnt signaling, which is in turn related to the maintenance of planar cell polarity. The cells lining renal tubules generally divide parallel to the tubule’s axis, lengthening the tubule rather than expanding its diameter. Tubule-lining cells in models of polycystic kidney disease, however, show a tendency to divide at an angle to the tubule’s axis, which could lead to expansion of the tubule diameter. This deviation can occur before cysts appear, suggesting that a loss of this planar cell polarity may be a precursor to cyst formation (Fischer et al., 2006; Patel et al., 2008).
Mechanisms of cyst formation
Although ADPKD is genetically dominant at the organismal level, it is recessive at the cellular level. The kidneys of an ADPKD patient who inherits one mutated copy of PC1 or PC2 from a parent will develop and function normally into adulthood. Over time, however, cysts will form in this patient’s kidneys and several studies suggest that the cells that line these cysts will have lost both functional copies of a polycystin gene (Qian et al., 1996; Brasier and Henske, 1997). This indicates that an additional “second hit” somatic mutation may cause cysts to form. According to this model, each cyst arises as a consequence of a distinct somatic mutation event, explaining the disease’s slow progression over the course of decades. Subtler factors may also impact upon disease progression, including the level of PKD1 protein expression, the penetrance of pathogenic alleles, and the stage of kidney development affected by PKD1 mutation (Lu et al., 1997; Reynolds et al., 1999; Pritchard et al., 2000; Lantinga-van Leeuwen et al., 2004; Rossetti et al., 2009). Temporally controlled inactivation of PC1 or PC2 expression in the kidneys of mice has revealed that loss of these proteins in the developing kidney causes far more severe cystic disease than does loss of PC1 or PC2 in the mature kidney (Lantinga-van Leeuwen et al., 2007; Piontek et al., 2007; Takakura et al., 2008). These data suggest that loss of polycystin function during the period of rapid cell growth and division that characterizes post-natal renal development creates a predisposition toward cystogenesis, whereas polycystin function is far less critical after this period of cell proliferation ends.
The slow accumulation of cysts throughout adult life may be due to slow accumulation of inactivating “second hit” mutations as a result of a constant somatic mutation rate. It is also possible that, as individuals age, their kidneys are more likely to suffer transient obstructive or ischemic injuries to the tubule epithelial cells. These injuries would then stimulate repair, which involves cellular growth and division. Given the importance of PC1 and PC2 for cellular growth and differentiation, the decreased levels of functional polycystin proteins present in the cells of individuals heterozygous for ADPKD mutation could perturb the repair process and thus lead to cyst formation. Support for this pathway to cystic disease comes from studies in mice subjected to a renal injury, which initiates an up-regulation of cell growth and division. Kidneys heterozygous for PKD1 or PKD2 mutations cannot repair themselves as effectively as kidneys from wild-type mice, and accumulate more tubule dilation and microcysts than wild-type kidneys (Bastos et al., 2009; Prasad et al., 2009). Knocking out PKD1 expression in adult mouse kidneys causes a similar sensitivity to injury (Takakura et al., 2009). These results suggest that injury may be able to initiate cyst formation in heterozygotes without a requirement for a somatic “second hit” mutagenesis event. In addition, injury accelerates cyst formation in mouse models with slowly progressive cystic disease secondary to conditional inactivation of PKD1 or PKD2 in adulthood. It is possible, therefore, that the initiation of cyst formation could hinge upon the occurrence of either a somatic mutagenesis or an injury event, either of which could be seen to constitute a “second hit” that conspires with heterozygosity at one of the PKD loci to cause disease.
Cyst expansion.
The macroscopic consequence of ADPKD progression is the formation of fluid-filled cysts, which constitute a stark contrast to the normally compact arrangement of tubules in a healthy kidney (Fig. 3). At the cellular level, this transformation is predicated upon two alterations: cells must organize themselves to create spherical rather than tubular structures, and the lumens of these structures must fill with fluid in order to expand the consequent cysts. Cysts increase their surface areas primarily by increasing the number of cells that surround the cyst lumens rather than by simply stretching this epithelial layer (Grantham, 1996; Grantham et al., 1987). Thus, one model for the change from tubular to spherical morphology posits that perturbations in planar cell polarity cause tubular epithelial cells to no longer divide along an axis parallel to the tubule lumen, causing tubule expansion rather than elongation. Such a shift was seen in the axes of division in tubules of a rat cystic kidney model (Fischer et al., 2006). A close analysis of precystic tubules in mouse models manifesting kidney-specific inactivation of PKD1 or PKD2, however, showed that cells lining cystic tubules lose orientated division after tubules began to dilate (Nishio et al., 2010). More dramatically, Nishio et al. (2010) also found that misoriented cellular division is not sufficient for cyst development. Mice with a mutation in the ciliary protein fibrocystin have altered mitotic orientation but do not form kidney cysts because the cells that divide out of the epithelial plane migrate back into the tubule lining. Thus, it is likely that defects in planar cell polarity play a role in cyst formation, but losing this polarity may not be the event that initially causes cyst formation.
Figure 3.
Cyst formation at the level of the cell, nephron, and kidney. Defects in the genes encoding PC1 or PC2 lead to aberrant gene transcription, cell proliferation, and ion secretion, which in turn result in the formation of fluid-filled cysts. As cysts balloon out from individual nephrons, their collective effect leads to the displacement of the normal renal parenchyma and the formation of a cyst-filled kidney with reduced functional capacity.
The other critical aspect of cyst formation, which involves the expansion of cyst fluid volume, can be understood as the conversion of the cyst-lining cells from an ion-absorptive to an ion-secretory epithelium. Ion secretion into the lumen then drives paracellular or transcellular osmotic water movement into the cyst, as illustrated in Fig. 3. A prime component of this secretion is Cl− transport stimulated by cAMP (Grantham, 1996). The fluid movement driving cyst formation is stimulated by cAMP and involves the apical cystic fibrosis transmembrane regulator (CFTR) and the basolateral Na+-K+-2Cl− cotransporter NKCC1 (Davidow et al., 1996; Magenheimer et al., 2006; Montesano et al., 2009). PC1 may affect the expression, localization, or activity of Cl− channels. Expressing full-length PC1 with the CFTR channel in cultured MDCK cells decreases CFTR surface localization and cAMP-stimulated channel activity, suggesting that PC1 misregulation in ADPKD may lead to an increase in CFTR activity (Ikeda et al., 2006). Expressing just the C-terminal tail of PC1 seems to enhance Cl− transport, prolonging ATP-stimulated Cl− conductance in transfected collecting duct cells and up-regulating Cl− transport in Xenopus oocytes (Wildman et al., 2003; Chernova et al., 2005). The polycystin proteins may also regulate cAMP levels because cystic disease is associated with misregulation of phosphodiesterases that break down cAMP (Wang et al., 2010).
Emerging treatment strategies
Clearly, no single unifying mechanism relates the normal functions of the polycystin proteins to the pathology that develops in their absence. It is, however, probably safe to assert that the progression of cystic disease is predicated upon perturbations in two fundamental processes. The epithelial cells that line cysts appear to proliferate excessively and these cells secrete rather than absorb fluid and electrolytes. Thus, many current efforts aimed at developing small molecule therapies for ADPKD target one or the other of these derangements (Chang and Ong, 2008; Harris and Torres, 2009; Patel et al., 2009).
Because fluid secretion into cyst lumens is mediated, at least in part, by apical CFTR chloride channels and is stimulated by cAMP, both of these factors may constitute promising molecular targets. The CFTR inhibitor compound CFTRinh172 appears to substantially slow cyst expansion (Yang et al., 2008). Inhibition of a basolateral potassium channel whose activity is required to maintain the electrochemical potential that drives chloride and fluid secretion is also being explored as an approach to blocking cyst fluid accumulation (Albaqumi et al., 2008).
Antidiuretic hormone (ADH), acting through the V2 vasopressin receptor, is a major stimulant of cAMP production in the collecting tubule of the kidney. Tolvaptan, a V2 receptor antagonist, dramatically reduces cyst progression in mouse models of ADPKD (Gattone et al., 2003; Torres et al., 2004; Wang et al., 2005; Torres, 2008). Ocreotide, a somatostatin analogue, also inhibits cAMP accumulation in several cell types and has produced intriguing results in animal models (Masyuk et al., 2007; Hogan et al., 2010).
The observation that inappropriately high mTOR activity may contribute to the excessive growth and proliferation that characterize cystic tissue has prompted investigations into the utility of mTOR inhibitors in the setting of ADPKD (Shillingford et al., 2006b; Wahl et al., 2006; Distefano et al., 2009; Zafar et al., 2009; Dere et al., 2010; Torres et al., 2010). Animal studies have suggested dramatic beneficial effects, although recent clinical trial data suggest that these results are not borne out in ADPKD patients, and the side effects of chronic mTOR inhibition may be substantial enough to further limit its potential utility (Serra et al., 2010; Torres et al., 2010; Walz et al., 2010). Other efforts have directly targeted the regulation of mitosis. Roscovitine, an anti-proliferative drug that blocks Cdks, dramatically slows cyst formation in at least some animal models of PKD (Bukanov et al., 2006).
Additional emerging potential therapies are directed at other interesting targets. These include triptolide, a compound derived from a traditional Chinese herbal therapy (Leuenroth et al., 2008); pioglitazone, a PPAR-γ agonist (Muto et al., 2002; Raphael et al., 2009); and Genz-123346, which blocks glycosyl ceramide synthesis (Natoli et al., 2010). The connection between these compounds’ molecular targets and the pathological processes involved in ADPKD remain to be determined. Further exploring these molecules, however, may reveal promising new pharmacological approaches to treating this disease and may also shed light on as-yet-undiscovered connections between the polycystin proteins and a variety of cellular signaling and metabolic pathways.
Conclusion
ADPKD is a disease that merits the attention of cell biologists. The responsible genes have been identified, but much remains to be learned about the functions of the proteins they encode. Although it is clear that both polycystin-1 and -2 influence and are influenced by a wide array of signaling pathways, the connection between these pathways and the pathogenesis of the disease has yet to be definitively established. Furthermore, critical to any understanding of polycystic kidney disease will be a deeper insight into the nature of a mysterious and fascinating organelle, the primary cilium. Insights into how the polycystins traffic into the cilium, and what they do once they arrive there, will shed light not only on ADPKD, but also on novel and fundamental processes in cell biology.
Acknowledgments
The authors wish to thank all of the members of the Caplan laboratory for helpful discussions and suggestions.
The authors’ work is supported by a fellowship from the National Science Foundation (H.C. Chapin) and a National Institutes of Health grant (DK57328).
Footnotes
Abbreviations used in this paper:
- ADPKD
- autosomal dominant polycystic kidney disease
- CFTR
- cystic fibrosis transmembrane regulator
- CTT
- C-terminal tail
- mTOR
- mammalian target of rapamycin
- NFAT
- nuclear factor of activated T cells
- PC
- polycystin
- PKD
- polycystic kidney disease
- STAT
- signal transducers and activators of transcription
References
- Albaqumi M., Srivastava S., Li Z., Zhdnova O., Wulff H., Itani O., Wallace D.P., Skolnik E.Y. 2008. KCa3.1 potassium channels are critical for cAMP-dependent chloride secretion and cyst growth in autosomal-dominant polycystic kidney disease. Kidney Int. 74:740–749 10.1038/ki.2008.246 [DOI] [PubMed] [Google Scholar]
- Anyatonwu G.I., Estrada M., Tian X., Somlo S., Ehrlich B.E. 2007. Regulation of ryanodine receptor-dependent calcium signaling by polycystin-2. Proc. Natl. Acad. Sci. USA. 104:6454–6459 10.1073/pnas.0610324104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnould T., Kim E., Tsiokas L., Jochimsen F., Grüning W., Chang J.D., Walz G. 1998. The polycystic kidney disease 1 gene product mediates protein kinase C alpha-dependent and c-Jun N-terminal kinase-dependent activation of the transcription factor AP-1. J. Biol. Chem. 273:6013–6018 10.1074/jbc.273.11.6013 [DOI] [PubMed] [Google Scholar]
- Babich V., Zeng W.Z., Yeh B.I., Ibraghimov-Beskrovnaya O., Cai Y., Somlo S., Huang C.L. 2004. The N-terminal extracellular domain is required for polycystin-1-dependent channel activity. J. Biol. Chem. 279:25582–25589 10.1074/jbc.M402829200 [DOI] [PubMed] [Google Scholar]
- Badano J.L., Mitsuma N., Beales P.L., Katsanis N. 2006. The ciliopathies: an emerging class of human genetic disorders. Annu. Rev. Genomics Hum. Genet. 7:125–148 10.1146/annurev.genom.7.080505.115610 [DOI] [PubMed] [Google Scholar]
- Bastos A.P., Piontek K., Silva A.M., Martini D., Menezes L.F., Fonseca J.M., Fonseca I.I., Germino G.G., Onuchic L.F. 2009. Pkd1 haploinsufficiency increases renal damage and induces microcyst formation following ischemia/reperfusion. J. Am. Soc. Nephrol. 20:2389–2402 10.1681/ASN.2008040435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertuccio C.A., Chapin H.C., Cai Y., Mistry K., Chauvet V., Somlo S., Caplan M.J. 2009. Polycystin-1 C-terminal cleavage is modulated by polycystin-2 expression. J. Biol. Chem. 284:21011–21026 10.1074/jbc.M109.017756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhunia A.K., Piontek K., Boletta A., Liu L., Qian F., Xu P.N., Germino F.J., Germino G.G. 2002. PKD1 induces p21(waf1) and regulation of the cell cycle via direct activation of the JAK-STAT signaling pathway in a process requiring PKD2. Cell. 109:157–168 10.1016/S0092-8674(02)00716-X [DOI] [PubMed] [Google Scholar]
- Brasier J.L., Henske E.P. 1997. Loss of the polycystic kidney disease (PKD1) region of chromosome 16p13 in renal cyst cells supports a loss-of-function model for cyst pathogenesis. J. Clin. Invest. 99:194–199 10.1172/JCI119147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bukanov N.O., Smith L.A., Klinger K.W., Ledbetter S.R., Ibraghimov-Beskrovnaya O. 2006. Long-lasting arrest of murine polycystic kidney disease with CDK inhibitor roscovitine. Nature. 444:949–952 10.1038/nature05348 [DOI] [PubMed] [Google Scholar]
- Bycroft M., Bateman A., Clarke J., Hamill S.J., Sandford R., Thomas R.L., Chothia C. 1999. The structure of a PKD domain from polycystin-1: implications for polycystic kidney disease. EMBO J. 18:297–305 10.1093/emboj/18.2.297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai Y., Maeda Y., Cedzich A., Torres V.E., Wu G., Hayashi T., Mochizuki T., Park J.H., Witzgall R., Somlo S. 1999. Identification and characterization of polycystin-2, the PKD2 gene product. J. Biol. Chem. 274:28557–28565 10.1074/jbc.274.40.28557 [DOI] [PubMed] [Google Scholar]
- Cai Y., Anyatonwu G., Okuhara D., Lee K.B., Yu Z., Onoe T., Mei C.L., Qian Q., Geng L., Wiztgall R., et al. 2004. Calcium dependence of polycystin-2 channel activity is modulated by phosphorylation at Ser812. J. Biol. Chem. 279:19987–19995 10.1074/jbc.M312031200 [DOI] [PubMed] [Google Scholar]
- Calvet J.P., Grantham J.J. 2001. The genetics and physiology of polycystic kidney disease. Semin. Nephrol. 21:107–123 10.1053/snep.2001.20929 [DOI] [PubMed] [Google Scholar]
- Cantiello H.F. 2004. Regulation of calcium signaling by polycystin-2. Am. J. Physiol. Renal Physiol. 286:F1012–F1029 10.1152/ajprenal.00181.2003 [DOI] [PubMed] [Google Scholar]
- Casuscelli J., Schmidt S., DeGray B., Petri E.T., Celić A., Folta-Stogniew E., Ehrlich B.E., Boggon T.J. 2009. Analysis of the cytoplasmic interaction between polycystin-1 and polycystin-2. Am. J. Physiol. Renal Physiol. 297:F1310–F1315 10.1152/ajprenal.00412.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Celić A., Petri E.T., Demeler B., Ehrlich B.E., Boggon T.J. 2008. Domain mapping of the polycystin-2 C-terminal tail using de novo molecular modeling and biophysical analysis. J. Biol. Chem. 283:28305–28312 10.1074/jbc.M802743200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang M.Y., Ong A.C. 2008. Autosomal dominant polycystic kidney disease: recent advances in pathogenesis and treatment. Nephron. Physiol. 108:1–7 10.1159/000112495 [DOI] [PubMed] [Google Scholar]
- Chauvet V., Qian F., Boute N., Cai Y., Phakdeekitacharoen B., Onuchic L.F., Attié-Bitach T., Guicharnaud L., Devuyst O., Germino G.G., Gubler M.C. 2002. Expression of PKD1 and PKD2 transcripts and proteins in human embryo and during normal kidney development. Am. J. Pathol. 160:973–983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chauvet V., Tian X., Husson H., Grimm D.H., Wang T., Hiesberger T., Hieseberger T., Igarashi P., Bennett A.M., Ibraghimov-Beskrovnaya O., et al. 2004. Mechanical stimuli induce cleavage and nuclear translocation of the polycystin-1 C terminus. J. Clin. Invest. 114:1433–1443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chernova M.N., Vandorpe D.H., Clark J.S., Alper S.L. 2005. Expression of the polycystin-1 C-terminal cytoplasmic tail increases Cl channel activity in Xenopus oocytes. Kidney Int. 68:632–641 10.1111/j.1523-1755.2005.00441.x [DOI] [PubMed] [Google Scholar]
- Clapham D.E., Runnels L.W., Strübing C. 2001. The TRP ion channel family. Nat. Rev. Neurosci. 2:387–396 10.1038/35077544 [DOI] [PubMed] [Google Scholar]
- Davidow C.J., Maser R.L., Rome L.A., Calvet J.P., Grantham J.J. 1996. The cystic fibrosis transmembrane conductance regulator mediates transepithelial fluid secretion by human autosomal dominant polycystic kidney disease epithelium in vitro. Kidney Int. 50:208–218 10.1038/ki.1996.304 [DOI] [PubMed] [Google Scholar]
- Delmas P., Nomura H., Li X.G., Lakkis M., Luo Y., Segal Y., Fernández-Fernández J.M., Harris P., Frischauf A.M., Brown D.A., Zhou J. 2002. Constitutive activation of G-proteins by polycystin-1 is antagonized by polycystin-2. J. Biol. Chem. 277:11276–11283 10.1074/jbc.M110483200 [DOI] [PubMed] [Google Scholar]
- Delmas P., Nauli S.M., Li X., Coste B., Osorio N., Crest M., Brown D.A., Zhou J. 2004. Gating of the polycystin ion channel signaling complex in neurons and kidney cells. FASEB J. 18:740–742 [DOI] [PubMed] [Google Scholar]
- Dere R., Wilson P.D., Sandford R.N., Walker C.L. 2010. Carboxy terminal tail of polycystin-1 regulates localization of TSC2 to repress mTOR. PLoS One. 5:e9239 10.1371/journal.pone.0009239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Distefano G., Boca M., Rowe I., Wodarczyk C., Ma L., Piontek K.B., Germino G.G., Pandolfi P.P., Boletta A. 2009. Polycystin-1 regulates extracellular signal-regulated kinase-dependent phosphorylation of tuberin to control cell size through mTOR and its downstream effectors S6K and 4EBP1. Mol. Cell. Biol. 29:2359–2371 10.1128/MCB.01259-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer E., Legue E., Doyen A., Nato F., Nicolas J.F., Torres V., Yaniv M., Pontoglio M. 2006. Defective planar cell polarity in polycystic kidney disease. Nat. Genet. 38:21–23 10.1038/ng1701 [DOI] [PubMed] [Google Scholar]
- Gabow P.A. 1993. Autosomal dominant polycystic kidney disease. N. Engl. J. Med. 329:332–342 10.1056/NEJM199307293290508 [DOI] [PubMed] [Google Scholar]
- Gattone V.H., II, Wang X., Harris P.C., Torres V.E. 2003. Inhibition of renal cystic disease development and progression by a vasopressin V2 receptor antagonist. Nat. Med. 9:1323–1326 10.1038/nm935 [DOI] [PubMed] [Google Scholar]
- Geng L., Okuhara D., Yu Z.H., Tian X., Cai Y.Q., Shibazaki S., Somlo S. 2006. Polycystin-2 traffics to cilia independently of polycystin-1 by using an N-terminal RVxP motif. J. Cell Sci. 119:1383–1395 10.1242/jcs.02818 [DOI] [PubMed] [Google Scholar]
- Gifford J.L., Walsh M.P., Vogel H.J. 2007. Structures and metal-ion-binding properties of the Ca2+-binding helix-loop-helix EF-hand motifs. Biochem. J. 405:199–221 10.1042/BJ20070255 [DOI] [PubMed] [Google Scholar]
- González-Perrett S., Kim K., Ibarra C., Damiano A.E., Zotta E., Batelli M., Harris P.C., Reisin I.L., Arnaout M.A., Cantiello H.F. 2001. Polycystin-2, the protein mutated in autosomal dominant polycystic kidney disease (ADPKD), is a Ca2+-permeable nonselective cation channel. Proc. Natl. Acad. Sci. USA. 98:1182–1187 10.1073/pnas.021456598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grantham J.J. 1996. The etiology, pathogenesis, and treatment of autosomal dominant polycystic kidney disease: recent advances. Am. J. Kidney Dis. 28:788–803 10.1016/S0272-6386(96)90378-9 [DOI] [PubMed] [Google Scholar]
- Grantham J.J. 2001. Polycystic kidney disease: from the bedside to the gene and back. Curr. Opin. Nephrol. Hypertens. 10:533–542 10.1097/00041552-200107000-00008 [DOI] [PubMed] [Google Scholar]
- Grantham J.J., Geiser J.L., Evan A.P. 1987. Cyst formation and growth in autosomal dominant polycystic kidney disease. Kidney Int. 31:1145–1152 10.1038/ki.1987.121 [DOI] [PubMed] [Google Scholar]
- Grimm D.H., Cai Y., Chauvet V., Rajendran V., Zeltner R., Geng L., Avner E.D., Sweeney W., Somlo S., Caplan M.J. 2003. Polycystin-1 distribution is modulated by polycystin-2 expression in mammalian cells. J. Biol. Chem. 278:36786–36793 10.1074/jbc.M306536200 [DOI] [PubMed] [Google Scholar]
- Hanaoka K., Qian F., Boletta A., Bhunia A.K., Piontek K., Tsiokas L., Sukhatme V.P., Guggino W.B., Germino G.G. 2000. Co-assembly of polycystin-1 and -2 produces unique cation-permeable currents. Nature. 408:990–994 10.1038/35050128 [DOI] [PubMed] [Google Scholar]
- Happé H., Leonhard W.N., van der Wal A., van de Water B., Lantinga-van Leeuwen I.S., Breuning M.H., de Heer E., Peters D.J.M. 2009. Toxic tubular injury in kidneys from Pkd1-deletion mice accelerates cystogenesis accompanied by dysregulated planar cell polarity and canonical Wnt signaling pathways. Hum. Mol. Genet. 18:2532–2542 10.1093/hmg/ddp190 [DOI] [PubMed] [Google Scholar]
- Harris P.C., Torres V.E. 2009. Polycystic kidney disease. Annu. Rev. Med. 60:321–337 10.1146/annurev.med.60.101707.125712 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hidaka S., Könecke V., Osten L., Witzgall R. 2004. PIGEA-14, a novel coiled-coil protein affecting the intracellular distribution of polycystin-2. J. Biol. Chem. 279:35009–35016 10.1074/jbc.M314206200 [DOI] [PubMed] [Google Scholar]
- Hogan M.C., Manganelli L., Woollard J.R., Masyuk A.I., Masyuk T.V., Tammachote R., Huang B.Q., Leontovich A.A., Beito T.G., Madden B.J., et al. 2009. Characterization of PKD protein-positive exosome-like vesicles. J. Am. Soc. Nephrol. 20:278–288 10.1681/ASN.2008060564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hogan M.C., Masyuk T.V., Page L.J., Kubly V.J., Bergstralh E.J., Li X., Kim B., King B.F., Glockner J., Holmes D.R., III, et al. 2010. Randomized clinical trial of long-acting somatostatin for autosomal dominant polycystic kidney and liver disease. J. Am. Soc. Nephrol. 21:1052–1061 10.1681/ASN.2009121291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horsley V., Pavlath G.K. 2002. NFAT: ubiquitous regulator of cell differentiation and adaptation. J. Cell Biol. 156:771–774 10.1083/jcb.200111073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu J., Bae Y.K., Knobel K.M., Barr M.M. 2006. Casein kinase II and calcineurin modulate TRPP function and ciliary localization. Mol. Biol. Cell. 17:2200–2211 10.1091/mbc.E05-10-0935 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huan Y., van Adelsberg J. 1999. Polycystin-1, the PKD1 gene product, is in a complex containing E-cadherin and the catenins. J. Clin. Invest. 104:1459–1468 10.1172/JCI5111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang J., Manning B.D. 2008. The TSC1-TSC2 complex: a molecular switchboard controlling cell growth. Biochem. J. 412:179–190 10.1042/BJ20080281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes J., Ward C.J., Peral B., Aspinwall R., Clark K., San Millán J.L., Gamble V., Harris P.C. 1995. The polycystic kidney disease 1 (PKD1) gene encodes a novel protein with multiple cell recognition domains. Nat. Genet. 10:151–160 10.1038/ng0695-151 [DOI] [PubMed] [Google Scholar]
- Ibraghimov-Beskrovnaya O., Dackowski W.R., Foggensteiner L., Coleman N., Thiru S., Petry L.R., Burn T.C., Connors T.D., Van Raay T., Bradley J., et al. 1997. Polycystin: in vitro synthesis, in vivo tissue expression, and subcellular localization identifies a large membrane-associated protein. Proc. Natl. Acad. Sci. USA. 94:6397–6402 10.1073/pnas.94.12.6397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ibraghimov-Beskrovnaya O., Bukanov N.O., Donohue L.C., Dackowski W.R., Klinger K.W., Landes G.M. 2000. Strong homophilic interactions of the Ig-like domains of polycystin-1, the protein product of an autosomal dominant polycystic kidney disease gene, PKD1. Hum. Mol. Genet. 9:1641–1649 10.1093/hmg/9.11.1641 [DOI] [PubMed] [Google Scholar]
- Igarashi P., Somlo S. 2002. Genetics and pathogenesis of polycystic kidney disease. J. Am. Soc. Nephrol. 13:2384–2398 10.1097/01.ASN.0000028643.17901.42 [DOI] [PubMed] [Google Scholar]
- Ikeda M., Fong P.Y., Cheng J., Boletta A., Qian F., Zhang X.M., Cai H., Germino G.G., Guggino W.B. 2006. A regulatory role of polycystin-1 on cystic fibrosis transmembrane conductance regulator plasma membrane expression. Cell. Physiol. Biochem. 18:9–20 10.1159/000095133 [DOI] [PubMed] [Google Scholar]
- Inoki K., Li Y., Zhu T.Q., Wu J., Guan K.L. 2002. TSC2 is phosphorylated and inhibited by Akt and suppresses mTOR signalling. Nat. Cell Biol. 4:648–657 10.1038/ncb839 [DOI] [PubMed] [Google Scholar]
- Kim I., Ding T.B., Fu Y.L., Li C.X., Cui L., Li A., Lian P.W., Liang D., Wang D.W., Guo C.Y., et al. 2009. Conditional mutation of Pkd2 causes cystogenesis and upregulates beta-catenin. J. Am. Soc. Nephrol. 20:2556–2569 10.1681/ASN.2009030271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleymenova E., Ibraghimov-Beskrovnaya O., Kugoh H., Everitt J., Xu H., Kiguchi K., Landes G., Harris P., Walker C. 2001. Tuberin-dependent membrane localization of polycystin-1: a functional link between polycystic kidney disease and the TSC2 tumor suppressor gene. Mol. Cell. 7:823–832 10.1016/S1097-2765(01)00226-X [DOI] [PubMed] [Google Scholar]
- Köttgen M., Walz G. 2005. Subcellular localization and trafficking of polycystins. Pflugers Arch. 451:286–293 10.1007/s00424-005-1417-3 [DOI] [PubMed] [Google Scholar]
- Köttgen M., Benzing T., Simmen T., Tauber R., Buchholz B., Feliciangeli S., Huber T.B., Schermer B., Kramer-Zucker A., Höpker K., et al. 2005. Trafficking of TRPP2 by PACS proteins represents a novel mechanism of ion channel regulation. EMBO J. 24:705–716 10.1038/sj.emboj.7600566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koulen P., Cai Y., Geng L., Maeda Y., Nishimura S., Witzgall R., Ehrlich B.E., Somlo S. 2002. Polycystin-2 is an intracellular calcium release channel. Nat. Cell Biol. 4:191–197 10.1038/ncb754 [DOI] [PubMed] [Google Scholar]
- Lal M., Song X., Pluznick J.L., Di Giovanni V., Merrick D.M., Rosenblum N.D., Chauvet V., Gottardi C.J., Pei Y., Caplan M.J. 2008. Polycystin-1 C-terminal tail associates with beta-catenin and inhibits canonical Wnt signaling. Hum. Mol. Genet. 17:3105–3117 10.1093/hmg/ddn208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lantinga-van Leeuwen I.S., Dauwerse J.G., Baelde H.J., Leonhard W.N., van de Wal A., Ward C.J., Verbeek S., Deruiter M.C., Breuning M.H., de Heer E., Peters D.J. 2004. Lowering of Pkd1 expression is sufficient to cause polycystic kidney disease. Hum. Mol. Genet. 13:3069–3077 10.1093/hmg/ddh336 [DOI] [PubMed] [Google Scholar]
- Lantinga-van Leeuwen I.S., Leonhard W.N., van der Wal A., Breuning M.H., de Heer E., Peters D.J. 2007. Kidney-specific inactivation of the Pkd1 gene induces rapid cyst formation in developing kidneys and a slow onset of disease in adult mice. Hum. Mol. Genet. 16:3188–3196 10.1093/hmg/ddm299 [DOI] [PubMed] [Google Scholar]
- Le N.H., van der Wal A., van der Bent P., Lantinga-van Leeuwen I.S., Breuning M.H., van Dam H., de Heer E., Peters D.J. 2005. Increased activity of activator protein-1 transcription factor components ATF2, c-Jun, and c-Fos in human and mouse autosomal dominant polycystic kidney disease. J. Am. Soc. Nephrol. 16:2724–2731 10.1681/ASN.2004110913 [DOI] [PubMed] [Google Scholar]
- Leuenroth S.J., Bencivenga N., Igarashi P., Somlo S., Crews C.M. 2008. Triptolide reduces cystogenesis in a model of ADPKD. J. Am. Soc. Nephrol. 19:1659–1662 10.1681/ASN.2008030259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X., Luo Y., Starremans P.G., McNamara C.A., Pei Y., Zhou J. 2005. Polycystin-1 and polycystin-2 regulate the cell cycle through the helix-loop-helix inhibitor Id2. Nat. Cell Biol. 7:1202–1212 [DOI] [PubMed] [Google Scholar]
- Li Y., Santoso N.G., Yu S., Woodward O.M., Qian F., Guggino W.B. 2009. Polycystin-1 interacts with inositol 1,4,5-trisphosphate receptor to modulate intracellular Ca2+ signaling with implications for polycystic kidney disease. J. Biol. Chem. 284:36431–36441 10.1074/jbc.M109.068916 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang G.Q., Yang J.W., Wang Z.C., Li Q., Tang Y., Chen X.Z. 2008. Polycystin-2 down-regulates cell proliferation via promoting PERK-dependent phosphorylation of eIF2alpha. Hum. Mol. Genet. 17:3254–3262 10.1093/hmg/ddn221 [DOI] [PubMed] [Google Scholar]
- Low S.H., Vasanth S., Larson C.H., Mukherjee S., Sharma N., Kinter M.T., Kane M.E., Obara T., Weimbs T. 2006. Polycystin-1, STAT6, and P100 function in a pathway that transduces ciliary mechanosensation and is activated in polycystic kidney disease. Dev. Cell. 10:57–69 10.1016/j.devcel.2005.12.005 [DOI] [PubMed] [Google Scholar]
- Lu W., Peissel B., Babakhanlou H., Pavlova A., Geng L., Fan X., Larson C., Brent G., Zhou J. 1997. Perinatal lethality with kidney and pancreas defects in mice with a targetted Pkd1 mutation. Nat. Genet. 17:179–181 10.1038/ng1097-179 [DOI] [PubMed] [Google Scholar]
- Ma L., Chen Z., Erdjument-Bromage H., Tempst P., Pandolfi P.P. 2005. Phosphorylation and functional inactivation of TSC2 by Erk implications for tuberous sclerosis and cancer pathogenesis. Cell. 121:179–193 10.1016/j.cell.2005.02.031 [DOI] [PubMed] [Google Scholar]
- Macián F., López-Rodríguez C., Rao A. 2001. Partners in transcription: NFAT and AP-1. Oncogene. 20:2476–2489 10.1038/sj.onc.1204386 [DOI] [PubMed] [Google Scholar]
- Magenheimer B.S., St John P.L., Isom K.S., Abrahamson D.R., De Lisle R.C., Wallace D.P., Maser R.L., Grantham J.J., Calvet J.P. 2006. Early embryonic renal tubules of wild-type and polycystic kidney disease kidneys respond to cAMP stimulation with cystic fibrosis transmembrane conductance regulator/Na(+),K(+),2Cl(-) Co-transporter-dependent cystic dilation. J. Am. Soc. Nephrol. 17:3424–3437 10.1681/ASN.2006030295 [DOI] [PubMed] [Google Scholar]
- Markowitz G.S., Cai Y., Li L., Wu G., Ward L.C., Somlo S., D’Agati V.D. 1999. Polycystin-2 expression is developmentally regulated. Am. J. Physiol. 277:F17–F25 [DOI] [PubMed] [Google Scholar]
- Masyuk T.V., Masyuk A.I., Torres V.E., Harris P.C., Larusso N.F. 2007. Octreotide inhibits hepatic cystogenesis in a rodent model of polycystic liver disease by reducing cholangiocyte adenosine 3′,5′-cyclic monophosphate. Gastroenterology. 132:1104–1116 10.1053/j.gastro.2006.12.039 [DOI] [PubMed] [Google Scholar]
- Mochizuki T., Wu G., Hayashi T., Xenophontos S.L., Veldhuisen B., Saris J.J., Reynolds D.M., Cai Y., Gabow P.A., Pierides A., et al. 1996. PKD2, a gene for polycystic kidney disease that encodes an integral membrane protein. Science. 272:1339–1342 10.1126/science.272.5266.1339 [DOI] [PubMed] [Google Scholar]
- Montesano R., Ghzili H., Carrozzino F., Rossier B.C., Féraille E. 2009. cAMP-dependent chloride secretion mediates tubule enlargement and cyst formation by cultured mammalian collecting duct cells. Am. J. Physiol. Renal Physiol. 296:F446–F457 10.1152/ajprenal.90415.2008 [DOI] [PubMed] [Google Scholar]
- Muto S., Aiba A., Saito Y., Nakao K., Nakamura K., Tomita K., Kitamura T., Kurabayashi M., Nagai R., Higashihara E., et al. 2002. Pioglitazone improves the phenotype and molecular defects of a targeted Pkd1 mutant. Hum. Mol. Genet. 11:1731–1742 10.1093/hmg/11.15.1731 [DOI] [PubMed] [Google Scholar]
- Natoli T.A., Smith L.A., Rogers K.A., Wang B., Komarnitsky S., Budman Y., Belenky A., Bukanov N.O., Dackowski W.R., Husson H., et al. 2010. Inhibition of glucosylceramide accumulation results in effective blockade of polycystic kidney disease in mouse models. Nat. Med. 16:788–792 10.1038/nm.2171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nauli S.M., Alenghat F.J., Luo Y., Williams E., Vassilev P., Li X., Elia A.E., Lu W., Brown E.M., Quinn S.J., et al. 2003. Polycystins 1 and 2 mediate mechanosensation in the primary cilium of kidney cells. Nat. Genet. 33:129–137 10.1038/ng1076 [DOI] [PubMed] [Google Scholar]
- Nims N., Vassmer D., Maser R.L. 2003. Transmembrane domain analysis of polycystin-1, the product of the polycystic kidney disease-1 (PKD1) gene: evidence for 11 membrane-spanning domains. Biochemistry. 42:13035–13048 10.1021/bi035074c [DOI] [PubMed] [Google Scholar]
- Nishio S., Tian X., Gallagher A.R., Yu Z.H., Patel V., Igarashi P., Somlo S. 2010. Loss of oriented cell division does not initiate cyst formation. J. Am. Soc. Nephrol. 21:295–302 10.1681/ASN.2009060603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parnell S.C., Magenheimer B.S., Maser R.L., Rankin C.A., Smine A., Okamoto T., Calvet J.P. 1998. The polycystic kidney disease-1 protein, polycystin-1, binds and activates heterotrimeric G-proteins in vitro. Biochem. Biophys. Res. Commun. 251:625–631 10.1006/bbrc.1998.9514 [DOI] [PubMed] [Google Scholar]
- Parnell S.C., Magenheimer B.S., Maser R.L., Zien C.A., Frischauf A.M., Calvet J.P. 2002. Polycystin-1 activation of c-Jun N-terminal kinase and AP-1 is mediated by heterotrimeric G proteins. J. Biol. Chem. 277:19566–19572 10.1074/jbc.M201875200 [DOI] [PubMed] [Google Scholar]
- Patel A., Honoré E. 2010. Polycystins and renovascular mechanosensory transduction. Nat Rev Nephrol. 6:530–538 10.1038/nrneph.2010.97 [DOI] [PubMed] [Google Scholar]
- Patel V., Li L., Cobo-Stark P., Shao X., Somlo S., Lin F., Igarashi P. 2008. Acute kidney injury and aberrant planar cell polarity induce cyst formation in mice lacking renal cilia. Hum. Mol. Genet. 17:1578–1590 10.1093/hmg/ddn045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel V., Chowdhury R., Igarashi P. 2009. Advances in the pathogenesis and treatment of polycystic kidney disease. Curr. Opin. Nephrol. Hypertens. 18:99–106 10.1097/MNH.0b013e3283262ab0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelucchi B., Aguiari G., Pignatelli A., Manzati E., Witzgall R., Del Senno L., Belluzzi O. 2006. Nonspecific cation current associated with native polycystin-2 in HEK-293 cells. J. Am. Soc. Nephrol. 17:388–397 10.1681/ASN.2004121146 [DOI] [PubMed] [Google Scholar]
- Peters D.J., van de Wal A., Spruit L., Saris J.J., Breuning M.H., Bruijn J.A., de Heer E. 1999. Cellular localization and tissue distribution of polycystin-1. J. Pathol. 188:439–446 [DOI] [PubMed] [Google Scholar]
- Piontek K., Menezes L.F., Garcia-Gonzalez M.A., Huso D.L., Germino G.G. 2007. A critical developmental switch defines the kinetics of kidney cyst formation after loss of Pkd1. Nat. Med. 13:1490–1495 10.1038/nm1675 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prasad S., McDaid J.P., Tam F.W., Haylor J.L., Ong A.C. 2009. Pkd2 dosage influences cellular repair responses following ischemia-reperfusion injury. Am. J. Pathol. 175:1493–1503 10.2353/ajpath.2009.090227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pritchard L., Sloane-Stanley J.A., Sharpe J.A., Aspinwall R., Lu W., Buckle V., Strmecki L., Walker D., Ward C.J., Alpers C.E., et al. 2000. A human PKD1 transgene generates functional polycystin-1 in mice and is associated with a cystic phenotype. Hum. Mol. Genet. 9:2617–2627 10.1093/hmg/9.18.2617 [DOI] [PubMed] [Google Scholar]
- Puri S., Magenheimer B.S., Maser R.L., Ryan E.M., Zien C.A., Walker D.D., Wallace D.P., Hempson S.J., Calvet J.P. 2004. Polycystin-1 activates the calcineurin/NFAT (nuclear factor of activated T-cells) signaling pathway. J. Biol. Chem. 279:55455–55464 10.1074/jbc.M402905200 [DOI] [PubMed] [Google Scholar]
- Qian F., Watnick T.J., Onuchic L.F., Germino G.G. 1996. The molecular basis of focal cyst formation in human autosomal dominant polycystic kidney disease type I. Cell. 87:979–987 10.1016/S0092-8674(00)81793-6 [DOI] [PubMed] [Google Scholar]
- Qian F., Germino F.J., Cai Y., Zhang X., Somlo S., Germino G.G. 1997. PKD1 interacts with PKD2 through a probable coiled-coil domain. Nat. Genet. 16:179–183 10.1038/ng0697-179 [DOI] [PubMed] [Google Scholar]
- Qian F., Boletta A., Bhunia A.K., Xu H., Liu L., Ahrabi A.K., Watnick T.J., Zhou F., Germino G.G. 2002. Cleavage of polycystin-1 requires the receptor for egg jelly domain and is disrupted by human autosomal-dominant polycystic kidney disease 1-associated mutations. Proc. Natl. Acad. Sci. USA. 99:16981–16986 10.1073/pnas.252484899 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raphael K.L., Strait K.A., Stricklett P.K., Baird B.C., Piontek K., Germino G.G., Kohan D.E. 2009. Effect of pioglitazone on survival and renal function in a mouse model of polycystic kidney disease. Am. J. Nephrol. 30:468–473 10.1159/000242432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rechsteiner M., Rogers S.W. 1996. PEST sequences and regulation by proteolysis. Trends Biochem. Sci. 21:267–271 [PubMed] [Google Scholar]
- Reynolds D.M., Hayashi T., Cai Y., Veldhuisen B., Watnick T.J., Lens X.M., Mochizuki T., Qian F., Maeda Y., Li L., et al. 1999. Aberrant splicing in the PKD2 gene as a cause of polycystic kidney disease. J. Am. Soc. Nephrol. 10:2342–2351 [DOI] [PubMed] [Google Scholar]
- Rossetti S., Kubly V.J., Consugar M.B., Hopp K., Roy S., Horsley S.W., Chauveau D., Rees L., Barratt T.M., van’t Hoff W.G., et al. 2009. Incompletely penetrant PKD1 alleles suggest a role for gene dosage in cyst initiation in polycystic kidney disease. Kidney Int. 75:848–855 10.1038/ki.2008.686 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rundle D.R., Gorbsky G., Tsiokas L. 2004. PKD2 interacts and co-localizes with mDia1 to mitotic spindles of dividing cells: role of mDia1 IN PKD2 localization to mitotic spindles. J. Biol. Chem. 279:29728–29739 10.1074/jbc.M400544200 [DOI] [PubMed] [Google Scholar]
- Serra A.L., Poster D., Kistler A.D., Krauer F., Raina S., Young J., Rentsch K.M., Spanaus K.S., Senn O., Kristanto P., et al. 2010. Sirolimus and kidney growth in autosomal dominant polycystic kidney disease. N. Engl. J. Med. 363:820–829 10.1056/NEJMoa0907419 [DOI] [PubMed] [Google Scholar]
- Shaulian E., Karin M. 2002. AP-1 as a regulator of cell life and death. Nat. Cell Biol. 4:E131–E136 10.1038/ncb0502-e131 [DOI] [PubMed] [Google Scholar]
- Shillingford J.M., Murcia N.S., Larson C.H., Low S.H., Hedgepeth R., Brown N., Flask C.A., Novick A.C., Goldfarb D.A., Kramer-Zucker A., et al. 2006a. The mTOR pathway is regulated by polycystin-1, and its inhibition reverses renal cystogenesis in polycystic kidney disease. Proc. Natl. Acad. Sci. USA. 103:5466–5471 10.1073/pnas.0509694103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shillingford J.M., Murcia N.S., Larson C.H., Low S.H., Hedgepeth R., Brown N., Flask C.A., Novick A.C., Goldfarb D.A., Kramer-Zucker A., et al. 2006b. The mTOR pathway is regulated by polycystin-1, and its inhibition reverses renal cystogenesis in polycystic kidney disease. Proc. Natl. Acad. Sci. USA. 103:5466–5471 10.1073/pnas.0509694103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somlo S., Torres V., Caplan M. 2008. Autosomal dominant polycystic kidney disease and inherited cystic diseases. In The Kidney Vol. 2 Alpern R.J., Hebert S.C., Elsevier, New York, NY: 2283–2314 [Google Scholar]
- Song X.W., Di Giovanni V., He N., Wang K.R., Ingram A., Rosenblum N.D., Pei Y. 2009. Systems biology of autosomal dominant polycystic kidney disease (ADPKD): computational identification of gene expression pathways and integrated regulatory networks. Hum. Mol. Genet. 18:2328–2343 10.1093/hmg/ddp165 [DOI] [PubMed] [Google Scholar]
- Streets A.J., Wagner B.E., Harris P.C., Ward C.J., Ong A.C. 2009. Homophilic and heterophilic polycystin 1 interactions regulate E-cadherin recruitment and junction assembly in MDCK cells. J. Cell Sci. 122:1410–1417 10.1242/jcs.045021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takakura A., Contrino L., Beck A.W., Zhou J. 2008. Pkd1 inactivation induced in adulthood produces focal cystic disease. J. Am. Soc. Nephrol. 19:2351–2363 10.1681/ASN.2007101139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takakura A., Contrino L., Zhou X.Z., Bonventre J.V., Sun Y.P., Humphreys B.D., Zhou J. 2009. Renal injury is a third hit promoting rapid development of adult polycystic kidney disease. Hum. Mol. Genet. 18:2523–2531 10.1093/hmg/ddp147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torres V.E. 1998. New insights into polycystic kidney disease and its treatment. Curr. Opin. Nephrol. Hypertens. 7:159–169 [DOI] [PubMed] [Google Scholar]
- Torres V.E. 2008. Role of vasopressin antagonists. Clin. J. Am. Soc. Nephrol. 3:1212–1218 10.2215/CJN.05281107 [DOI] [PubMed] [Google Scholar]
- Torres V.E., Wang X., Qian Q., Somlo S., Harris P.C., Gattone V.H., II 2004. Effective treatment of an orthologous model of autosomal dominant polycystic kidney disease. Nat. Med. 10:363–364 10.1038/nm1004 [DOI] [PubMed] [Google Scholar]
- Torres V.E., Boletta A., Chapman A., Gattone V., Pei Y., Qian Q., Wallace D.P., Weimbs T., Wüthrich R.P. 2010. Prospects for mTOR inhibitor use in patients with polycystic kidney disease and hamartomatous diseases. Clin. J. Am. Soc. Nephrol. 5:1312–1329 10.2215/CJN.01360210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsiokas L., Kim E., Arnould T., Sukhatme V.P., Walz G. 1997. Homo- and heterodimeric interactions between the gene products of PKD1 and PKD2. Proc. Natl. Acad. Sci. USA. 94:6965–6970 10.1073/pnas.94.13.6965 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsiokas L., Arnould T., Zhu C., Kim E., Walz G., Sukhatme V.P. 1999. Specific association of the gene product of PKD2 with the TRPC1 channel. Proc. Natl. Acad. Sci. USA. 96:3934–3939 10.1073/pnas.96.7.3934 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsiokas L., Kim S., Ong E.C. 2007. Cell biology of polycystin-2. Cell. Signal. 19:444–453 10.1016/j.cellsig.2006.09.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Adelsberg J. 1999. Peptides from the PKD repeats of polycystin, the PKD1 gene product, modulate pattern formation in the developing kidney. Dev. Genet. 24:299–308 [DOI] [PubMed] [Google Scholar]
- Vassilev P.M., Guo L., Chen X.Z., Segal Y., Peng J.B., Basora N., Babakhanlou H., Cruger G., Kanazirska M., Ye Cp, et al. 2001. Polycystin-2 is a novel cation channel implicated in defective intracellular Ca(2+) homeostasis in polycystic kidney disease. Biochem. Biophys. Res. Commun. 282:341–350 10.1006/bbrc.2001.4554 [DOI] [PubMed] [Google Scholar]
- Wahl P.R., Serra A.L., Le Hir M., Molle K.D., Hall M.N., Wüthrich R.P. 2006. Inhibition of mTOR with sirolimus slows disease progression in Han:SPRD rats with autosomal dominant polycystic kidney disease (ADPKD). Nephrol. Dial. Transplant. 21:598–604 10.1093/ndt/gfi181 [DOI] [PubMed] [Google Scholar]
- Walz G., Budde K., Mannaa M., Nürnberger J., Wanner C., Sommerer C., Kunzendorf U., Banas B., Hörl W.H., Obermüller N., et al. 2010. Everolimus in patients with autosomal dominant polycystic kidney disease. N. Engl. J. Med. 363:830–840 10.1056/NEJMoa1003491 [DOI] [PubMed] [Google Scholar]
- Wang X., Gattone V., II, Harris P.C., Torres V.E. 2005. Effectiveness of vasopressin V2 receptor antagonists OPC-31260 and OPC-41061 on polycystic kidney disease development in the PCK rat. J. Am. Soc. Nephrol. 16:846–851 10.1681/ASN.2004121090 [DOI] [PubMed] [Google Scholar]
- Wang X., Ward C.J., Harris P.C., Torres V.E. 2010. Cyclic nucleotide signaling in polycystic kidney disease. Kidney Int. 77:129–140 10.1038/ki.2009.438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward C.J., Turley H., Ong A.C., Comley M., Biddolph S., Chetty R., Ratcliffe P.J., Gattner K., Harris P.C. 1996. Polycystin, the polycystic kidney disease 1 protein, is expressed by epithelial cells in fetal, adult, and polycystic kidney. Proc. Natl. Acad. Sci. USA. 93:1524–1528 10.1073/pnas.93.4.1524 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei W., Hackmann K., Xu H., Germino G., Qian F. 2007. Characterization of cis-autoproteolysis of polycystin-1, the product of human polycystic kidney disease 1 gene. J. Biol. Chem. 282:21729–21737 10.1074/jbc.M703218200 [DOI] [PubMed] [Google Scholar]
- Wildman S.S., Hooper K.M., Turner C.M., Sham J.S., Lakatta E.G., King B.F., Unwin R.J., Sutters M. 2003. The isolated polycystin-1 cytoplasmic COOH terminus prolongs ATP-stimulated Cl- conductance through increased Ca2+ entry. Am. J. Physiol. Renal Physiol. 285:F1168–F1178 [DOI] [PubMed] [Google Scholar]
- Wilson P.D. 2004. Polycystic kidney disease. N. Engl. J. Med. 350:151–164 10.1056/NEJMra022161 [DOI] [PubMed] [Google Scholar]
- Xu C., Rossetti S., Jiang L., Harris P.C., Brown-Glaberman U., Wandinger-Ness A., Bacallao R., Alper S.L. 2007. Human ADPKD primary cyst epithelial cells with a novel, single codon deletion in the PKD1 gene exhibit defective ciliary polycystin localization and loss of flow-induced Ca2+ signaling. Am. J. Physiol. Renal Physiol. 292:F930–F945 10.1152/ajprenal.00285.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang B., Sonawane N.D., Zhao D., Somlo S., Verkman A.S. 2008. Small-molecule CFTR inhibitors slow cyst growth in polycystic kidney disease. J. Am. Soc. Nephrol. 19:1300–1310 10.1681/ASN.2007070828 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoder B.K., Hou X., Guay-Woodford L.M. 2002. The polycystic kidney disease proteins, polycystin-1, polycystin-2, polaris, and cystin, are co-localized in renal cilia. J. Am. Soc. Nephrol. 13:2508–2516 10.1097/01.ASN.0000029587.47950.25 [DOI] [PubMed] [Google Scholar]
- Yu S., Hackmann K., Gao J., Gao J., He X., Piontek K., García-González M.A., García González M.A., Menezes L.F., Xu H., et al. 2007. Essential role of cleavage of Polycystin-1 at G protein-coupled receptor proteolytic site for kidney tubular structure. Proc. Natl. Acad. Sci. USA. 104:18688–18693 10.1073/pnas.0708217104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu Y., Ulbrich M.H., Li M.H., Buraei Z., Chen X.Z., Ong A.C., Tong L., Isacoff E.Y., Yang J. 2009. Structural and molecular basis of the assembly of the TRPP2/PKD1 complex. Proc. Natl. Acad. Sci. USA. 106:11558–11563 10.1073/pnas.0903684106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zafar I., Belibi F.A., He Z., Edelstein C.L. 2009. Long-term rapamycin therapy in the Han:SPRD rat model of polycystic kidney disease (PKD). Nephrol. Dial. Transplant. 24:2349–2353 10.1093/ndt/gfp129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou J. 2009. Polycystins and primary cilia: primers for cell cycle progression. Annu. Rev. Physiol. 71:83–113 10.1146/annurev.physiol.70.113006.100621 [DOI] [PubMed] [Google Scholar]