Cep152, the orthologue of Drosophila Asterless, is a Plk4 target that functions with Plk4 in centriole assembly.
Abstract
Centrioles are microtubule-based structures that organize the centrosome and nucleate cilia. Centrioles duplicate once per cell cycle, and duplication requires Plk4, a member of the Polo-like kinase family; however, the mechanism linking Plk4 activity and centriole formation is unknown. In this study, we show in human and frog cells that Plk4 interacts with the centrosome protein Cep152, the orthologue of Drosophila melanogaster Asterless. The interaction requires the N-terminal 217 residues of Cep152 and the crypto Polo-box of Plk4. Cep152 and Plk4 colocalize at the centriole throughout the cell cycle. Overexpression of Cep152 (1–217) mislocalizes Plk4, but both Cep152 and Plk4 are able to localize to the centriole independently of the other. Depletion of Cep152 prevents both normal centriole duplication and Plk4-induced centriole amplification and results in a failure to localize Sas6 to the centriole, an early step in duplication. Cep152 can be phosphorylated by Plk4 in vitro, suggesting that Cep152 acts with Plk4 to initiate centriole formation.
Introduction
Centrioles organize two organelles, the centrosome and the cilium. Centrosomes are formed when centrioles recruit pericentriolar material, which contains microtubule-nucleating factors. Cilia form when centrioles interact with the plasma membrane and initiate a ciliary axoneme. Thus, controlling centriole number ensures that cells have the proper number of centrosomes and cilia. Maintaining two centrosomes per cell is important for proper cell division in early development and segregation of cell fate determinants (O’Connell et al., 2000; Stevens et al., 2007; Basto et al., 2008; Castellanos et al., 2008; Rodrigues-Martins et al., 2008). In addition, having extra centrosomes may contribute to genomic instability (Nigg, 2006; Ganem et al., 2009).
A G1 cell typically has a pair of centrioles that duplicate once per cell cycle, with a new centriole forming adjacent to each of the two existing centrioles. We will use the convention of referring to newly formed centrioles as daughter centrioles and the older centrioles as mother centrioles. Centriole formation begins at the G1/S transition and is regulated by the activity of Plk4, a divergent member of the Polo-like kinase family. Plk4 is required for centriole duplication, and Plk4 overexpression causes multiple centrioles to form adjacent to the two existing centrioles (Habedanck et al., 2005). In addition, Plk4 overexpression in unfertilized fly eggs initiates de novo centriole formation (Peel et al., 2007; Rodrigues-Martins et al., 2007). These results suggest that Plk4 is a key regulator of centriole formation.
Little is known about how Plk4 initiates centriole assembly. Several proteins in the centriole duplication pathway have been identified, including Sas6, CPAP, Cep135, and CP110 in mammalian cells (Leidel et al., 2005; Kleylein-Sohn et al., 2007; Strnad et al., 2007). Phosphorylation of Caenorhabditis elegans SAS-6 by the kinase ZYG-1, which functions similarly to Plk4 (O’Connell et al., 2001), is important for centriole duplication (Kitagawa et al., 2009). However, ZYG-1 is evolutionarily unrelated to Plk4 (Carvalho-Santos et al., 2010; Hodges et al., 2010), and Plk4 has not been shown to phosphorylate any centriole assembly proteins. An interaction has been identified between Plk4 and Slimb/β-TrCP (Cunha-Ferreira et al., 2009; Rogers et al., 2009; Holland et al., 2010), which is part of the Skp1-Cul1–F box ubiquitin ligase complex. Expression of nondegradable Plk4 mutants causes centriole amplification (Cunha-Ferreira et al., 2009; Rogers et al., 2009; Holland et al., 2010). Plk4 undergoes autophosphorylation (Sillibourne et al., 2010), and the interaction with β-TrCP depends on Plk4 autophosphorylation (Holland et al., 2010), indicating that Plk4 activity is self-regulating.
In this study, we took advantage of the unique properties of egg systems (Paweletz et al., 1984; Palazzo et al., 1992) to identify Plk4-interacting proteins from Xenopus laevis egg extract under conditions in which Plk4 can stimulate centriole formation. We found that Plk4 interacts with Cep152, a protein previously shown to localize to the centrosome (Andersen et al., 2003). The Drosophila melanogaster Cep152 orthologue Asterless (Varmark et al., 2007) is required for centriole duplication (Blachon et al., 2008; Dobbelaere et al., 2008), and depletion of the zebrafish Cep152 results in reduced cilia formation (Blachon et al., 2008). We find that Cep152 and Plk4 localize to a similar region of the centriole in human cells and that Cep152 depletion prevents both centriole duplication and Plk4 overexpression–induced centriole amplification. Lastly, Cep152 can be phosphorylated by Plk4, suggesting that the two proteins function together to initiate centriole formation.
Results and discussion
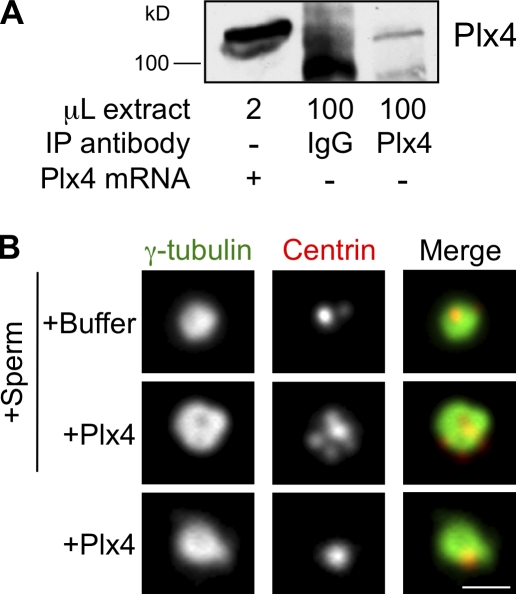
We developed a Xenopus egg extract system to characterize the action of Plk4 in centriole duplication. Xenopus embryos can make thousands of centrioles in the absence of transcription or translation (Gard et al., 1990), and egg extract can assemble centrosomes (Félix et al., 1994; Stearns and Kirschner, 1994) and duplicate centrioles (Hinchcliffe et al., 1999; Lacey et al., 1999). To study the function of Plk4 in centriole assembly, we made use of the ability of egg extracts to translate input mRNA (Murray and Kirschner, 1989). mRNA encoding the Xenopus Plk4 orthologue, Plx4, was added to translation-competent interphase Xenopus egg extract. A polyclonal antibody raised against Plx4 recognized the translated protein, whereas endogenous Plx4 was only detectable when immunoprecipitated from a larger volume of egg extract (Fig. 1 A).
Figure 1.
Plx4 overexpression drives centriole amplification in Xenopus egg extract. (A) Western blot of Xenopus egg extracts after addition of Plx4 mRNA or immunoprecipitation (IP) of endogenous Plx4 using anti-Plx4 or nonimmune IgG as a control. Immunoblotted with anti-Plx4. (B) Xenopus egg extract incubated with Plx4 or no mRNA with or without Xenopus sperm. Centrin and γ-tubulin mark the centrioles and pericentriolar material, respectively. Bar, 1 µm.
We first tested whether translated Plx4 could stimulate the formation of multiple centrioles around sperm centrioles added to the extract. Addition of Plx4 mRNA and sperm centrioles together to egg extract resulted in the formation of centrosomes bearing multiple centrin foci surrounding a single bright centrin focus, presumably representing the sperm centriole, within 2 h at 16°C (Fig. 1 B). We next tested whether translated Plx4 could stimulate de novo centriole formation. Addition of Plx4 mRNA alone to egg extract resulted in the formation of many centriole-like structures within 2 h at 16°C at a mean density of 2 × 104/µl extract (Fig. 1 B). We judged these structures to be centrioles based on three criteria: (1) they were able to organize centrosomes, as demonstrated by their ability to form microtubule asters in mitotic extract (Fig. S1 A), (2) they were labeled by antibodies against centrosomal markers including γ-tubulin, Plx4, and acetylated α-tubulin (Fig. S1 B), and (3) they were able to serve as sites for assembly of new centrin foci after extended incubation in interphase extract (Fig. S1 C). Thus, overexpression of Plx4 in Xenopus extracts promotes de novo centriole formation similar to that observed in Drosophila eggs (Peel et al., 2007; Rodrigues-Martins et al., 2007).
Based on the ability of Plx4 to generate centrioles in Xenopus egg extract, we sought to identify Plx4-interacting proteins from this source. For this purpose, we used a kinase-dead form of Plx4 (Plx4-D154A), as this protein might have a more sustained interaction with binding partners. Plx4-D154A was translated in egg extract and purified by affinity chromatography, and associated proteins were identified by mass spectrometry. Seven Xenopus proteins were identified: Cep152, Brg1, Pbrm1, Wdr33, Cad, Chd1, and Atxn2 (Fig. S1 D). Cep152 was identified by three unique peptides and was chosen for further analysis because it was previously associated with centrosome structure and function (Andersen et al., 2003; Varmark et al., 2007; Blachon et al., 2008; Dobbelaere et al., 2008).
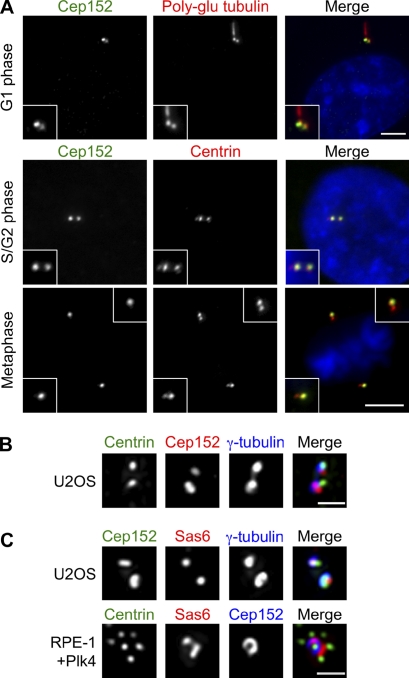
We examined the localization of endogenous Cep152 and a GFP-Cep152 fusion protein in human RPE-1 cells. Antibody against endogenous Cep152 revealed that it localized to the centrosome throughout the cell cycle (Fig. 2 A). A similar distribution was observed for transiently expressed GFP-Cep152 (unpublished data). In some cases, the apparent amount of Cep152 on the two centrosomes differed (Fig. 2 A); however, this was not correlated with centriole age or presence of a primary cilium, and likely represents experimental variation. Cep152 localization in G1 phase centrosomes of U2OS cells was examined by deconvolution microscopy and found to be distinct from that of centrin, a marker of the distal end of the centriole, and γ-tubulin, a marker of the pericentriolar material (Fig. 2 B). In duplicated centriole pairs, Cep152 signal partially overlapped with that of Sas6 (Fig. 2 C), which localizes to the proximal end of new centrioles (Kleylein-Sohn et al., 2007; Strnad et al., 2007). The observed pattern of localization is most consistent with Cep152 being concentrated at the proximal end of the mother centriole.
Figure 2.
Cep152 localizes to centrioles throughout the cell cycle. (A) RPE-1 cells at the indicated cell cycle stages were fixed and stained for Cep152, centrin, or polyglutamylated (poly-glu) tubulin, which marks centrioles and primary cilia, and DNA. Insets show enlarged centrosomes. Bars, 5 µm. (B) G1 centrosomes in a U2OS cell stained for Cep152, centrin, and γ-tubulin. (C, top) Centrosomes in a U2OS cell stained for Cep152, Sas6, and γ-tubulin. (bottom) Centrosome in a RPE-1 cell overexpressing Plk4 stained for centrin, Sas6, and Cep152. (B and C) Images are maximum projections of deconvoluted image stacks. Bars, 1 µm.
If Plk4 and Cep152 function together in centriole duplication, we would expect them to have a similar evolutionary distribution. Several of the core centriole components, including Sas6, CPAP/Sas-4, and Cep135/Bld10, are widely conserved structurally and functionally in organisms with centrioles (Leidel and Gönczy, 2003; Dammermann et al., 2004; Matsuura et al., 2004; Leidel et al., 2005; Basto et al., 2006; Kleylein-Sohn et al., 2007; Carvalho-Santos et al., 2010; Hodges et al., 2010). In contrast, Plk4 has a more limited distribution, being present in chordates, insects, and a few other invertebrate groups, but absent from C. elegans and most ciliated single-celled eukaryotes (Carvalho-Santos et al., 2010; Hodges et al., 2010). By searching for reciprocal BLAST hits, we found that Cep152 has the same distribution as Plk4, including absence from C. elegans, which is similar to what was reported for the Drosophila orthologue Asterless (Hodges et al., 2010). We used multiple sequence alignment of vertebrate and invertebrate orthologues of Cep152 with Drosophila Asterless to identify two conserved regions in Cep152, CR1 and CR2 (Fig. S2 A). CR1 is found in Cep152 in vertebrates and invertebrates, whereas CR2 is only present in vertebrates. In addition to these two regions, Cep152 orthologues share homology in their two structural maintenance of chromosomes (SMC)–like coiled-coil domains (Fig. 3 A, SMC-A and SMC-B).
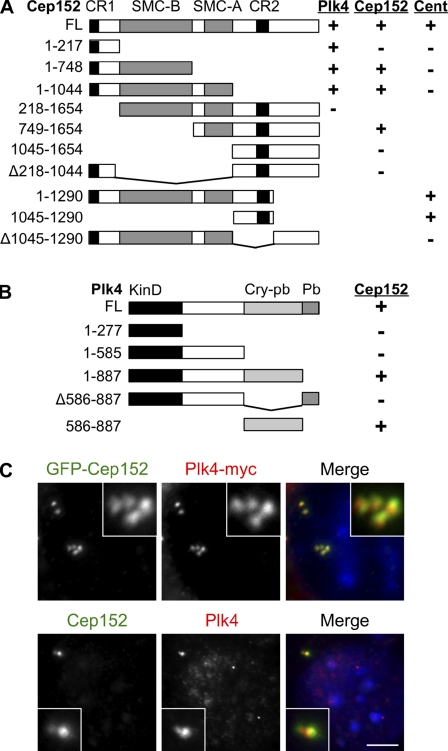
Figure 3.
Cep152 and Plk4 interaction and localization. (A) Schematic of Cep152 full length (FL) and deletion constructs showing the ability to interact with Plk4 and Cep152 and to localize to the centrosome (Cent). Interactions were determined by coimmunoprecipitation of GFP-Cep152 constructs from HEK293T lysates with Plk4-myc or Cep152-myc. Centrosome localization was tested by expression of fragments as GFP fusions in U2OS cells. Numbers indicate Cep152 amino acids. CR1 and CR2 indicate conserved domains in Cep152 orthologues; SMC-A and SMC-B indicate coiled-coil regions. (B) Schematic of Plk4 deletion constructs and summary of interactions with Cep152. Domains: Cry-pb, crypto Polo-box; Pb, Polo-box; KinD, kinase domain. Interactions were determined by coimmunoprecipitation of Plk4-myc fragments from HEK293T lysates with GFP-Cep152. (C, top) U2OS cells expressing GFP-Cep152 and Plk4-myc labeled with antibodies to GFP and myc. (bottom) Untransfected U2OS cells labeled with antibodies to Cep152 and Plk4. Insets show enlarged centrosomes. Bar, 5 µm.
To characterize the interaction of Cep152 with Plk4, we first tested whether the interaction could be observed in human cells as in frog egg extracts. Epitope-tagged human Plk4 and Cep152 expressed in human HEK293T cells were able to interact with the other in reciprocal immunoprecipitation experiments (Fig. S2 B). By expressing deletion constructs bearing the indicated parts of Cep152 and Plk4 as epitope-tagged proteins in HEK293T cells (Fig. 3, A and B), we found that the first 217 residues of Cep152, which includes CR1, are necessary and sufficient to bind Plk4 (Fig. 3 A) and that the crypto Polo-box region of Plk4 is necessary and sufficient to bind Cep152 (Fig. 3 B). Using two differently tagged versions of Cep152, we found that Cep152 is able to interact with itself and that at least one of the SMC-like domains is necessary for interaction with full-length Cep152 (Fig. 3 A). Lastly, we found that a region including CR2 was necessary and sufficient for localization of a GFP fusion protein to the centrosome (Figs. 3 A and S2 C). To determine whether the observed interaction of Plk4 and Cep152 is also likely to be occurring at the centrosome, we examined their localization in U2OS cells. Similar, overlapping patterns of localization were observed for both epitope-tagged versions of Plk4 and Cep152 and the endogenous proteins (Fig. 3 C).
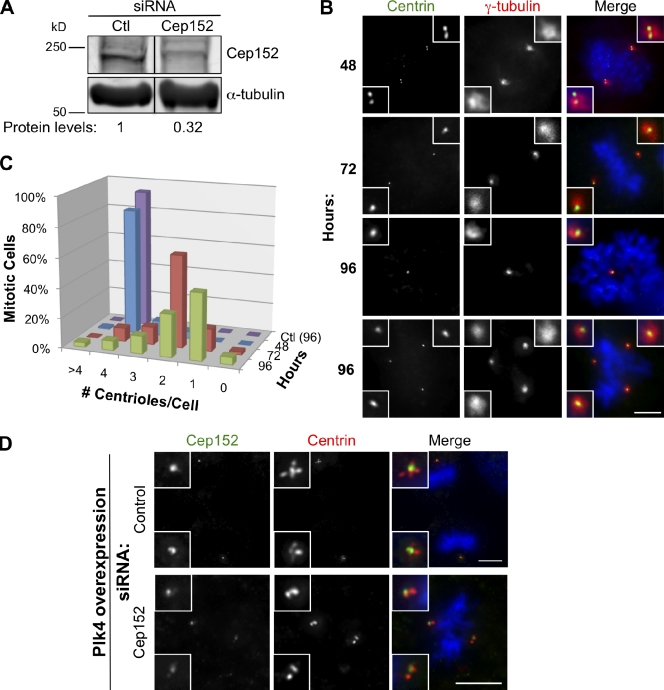
We next tested whether Cep152, like Plk4, is required for centriole duplication in mammalian cells. U2OS cells were transfected with siRNAs against Cep152, and Cep152 protein depletion was apparent starting at 48 h after transfection, reaching maximum extent at 72 h (Fig. 4 A). Centriole number was determined by centrin labeling in mitotic cells that lacked Cep152 labeling. In contrast to cells transfected with control siRNAs, we observed a stepwise decrease in centriole number over time in Cep152-depleted cells, which is consistent with a defect in centriole duplication followed by segregation in mitosis (Fig. 4, B and C). In cells that had two or more centrioles 96 h after RNAi treatment, these centrioles were single centrioles rather than pairs, suggesting that they too resulted from failure of centriole duplication followed by a failure of segregation at mitosis (Fig. 4 B). Similar results were observed with Cep152 depletion in HeLa cells (unpublished data). Both bipolar and monopolar spindles were observed in cells with a single centriole after Cep152 depletion (unpublished data), which is similar to what is observed upon depletion of Plk4 (Habedanck et al., 2005). The centriole duplication phenotype could be rescued by expressing RNAi-resistant GFP-Cep152, but not by GFP, indicating that the phenotype is specific to Cep152 depletion (Fig. S3).
Figure 4.
Cep152 is required for centriole duplication and amplification. (A) Western blotting of U2OS cell lysates 72 h after transfection with control (Ctl) siRNAs or Cep152 siRNAs immunoblotted for Cep152 or α-tubulin as a loading control. The indicated Cep152 protein levels are normalized to α-tubulin levels. Black lines indicate that intervening lanes have been spliced out. (B) U2OS cells were fixed at the indicated times after Cep152 siRNA transfection, and centriole number was determined by labeling for centrin and γ-tubulin. Insets show enlarged centrosomes. (C) Quantification of B. Centriole number in cells transfected with control siRNAs for 96 h was determined as described above. n = 200 cells over three experiments for each time point. (D) RPE-1 cells bearing Tet-inducible Plk4 were depleted of Cep152 by siRNA for 72 h then treated with doxycycline for 18 h to induce Plk4 expression. Centriole number was determined by labeling for centrin, and depleted cells were identified by reduced Cep152 staining. (B and D) Insets show enlarged centrosomes. Bars, 5 µm.
We next tested whether Cep152 is also required for Plk4-induced centriole amplification. Plk4 overexpression was induced in RPE-1 cells that had been depleted of Cep152, and centriole number was determined by centrin labeling. Cep152 depletion was less efficient in these cells compared with U2OS cells (Figs. 4 D and 5 B), and centriole duplication occurred normally, allowing us to specifically assay the requirement of Cep152 in centriole amplification. 18 h after induction of Plk4 overexpression, 99% of control mitotic cells had the expected amplified centriole “rosettes” at the spindle poles (Habedanck et al., 2005), whereas 64% of Plk4-overexpressing, Cep152-depleted cells had only two centrioles at each pole (Fig. 4 D). These results indicate that Cep152 is required both for normal centriole duplication and for Plk4-induced centriole amplification.
Figure 5.
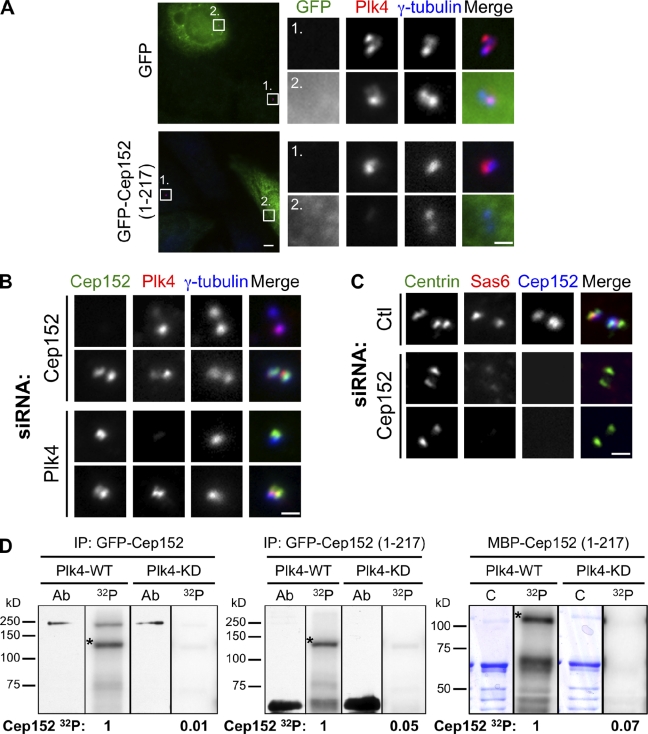
Functional interaction of Cep152 and Plk4. (A) Plk4 is lost from the centrosome in cells expressing GFP-Cep152 (1–217). U2OS cells transfected with GFP or GFP-Cep152 (1–217) were fixed 24 h after transfection and labeled with antibodies to GFP, Plk4, and γ-tubulin. Numbered boxes identify enlarged images shown on right. Bars (boxes) 1 µm; (fields) 5 µm. (B) U2OS cells were depleted of Plk4 or Cep152 by siRNA transfection, fixed 72 h after transfection, and labeled with antibodies to Cep152, Plk4, and γ-tubulin. Image pairs represent centrosomes in cells from the same coverslip taken with the same camera settings. (C) U2OS cells transfected for 72 h with either control siRNA (Ctl) or Cep152 siRNAs were arrested in S phase by incubation in aphidicolin for 24 h and fixed and labeled with antibodies to centrin, Sas6, and Cep152. 25% of Cep152-depleted cells had reduced Sas6 labeling on both centrioles (middle), 65% had no Sas6 labeling (bottom), and 10% had centrioles that differed in the amount of Sas6 (n = 150 cells). Exposure and gain conditions were constant for Sas6 image acquisition. (B and C) Bars, 1 µm. (D) Cep152 can be phosphorylated by Plk4 in vitro. Purified GST-Plk4, either wild type (WT) or kinase dead (KD), was incubated in the presence of [32P]ATP (32P) with full-length GFP-Cep152 (left) or GFP-Cep152 (1–217; middle) isolated from HEK293T cells by anti-GFP immunoprecipitation (IP) or with MBP-Cep152 (1–217; right) purified from E. coli. Proteins were visualized by Western blotting (Ab) of 0.1× kinase assay input using antibodies to GFP (left and middle) or by Coomassie stain (C) of the gel (right). Bands marked with an asterisk indicate autophosphorylated Plk4. Cep152 32P incorporation was normalized within each gel group. Black lines indicate that gel lanes imaged by different methods were spliced together.
Based on the aforementioned results, we envisioned several possibilities for the functional relationship between Cep152 and Plk4. The first is that one protein serves to localize the other to the centrosome and that localization is required for centriole duplication. Plk4 has been shown to localize to the centrosome via the crypto Polo-box domain (Habedanck et al., 2005). Because Cep152 binds the Plk4 crypto Polo-box (Fig. 3 B), expression of a Cep152 mutant containing the Plk4-binding domain but lacking the centrosome localization domain (Cep152 [1–217]) might mislocalize Plk4. Consistent with this, we found that expression of Cep152 (1–217) resulted in loss of centrosomal Plk4 labeling (Fig. 5 A) and subsequent progressive loss of centrioles, which is similar to that observed for Cep152 depletion (not depicted). Next, we directly tested whether Cep152 is required for Plk4 localization in cells depleted of Cep152. Plk4 localization to the centrosome was unchanged in U2OS cells depleted of Cep152; similarly, Cep152 localization to the centrosome was unchanged in cells depleted of Plk4 (Fig. 5 B). These results suggest that a Cep152 fragment that binds Plk4 is sufficient to outcompete one or more other factors that localize Plk4 to the centrosome but that neither protein is necessary for localization of the other.
Plk4 is required for the localization of Sas6 and other centriole assembly proteins during centriole assembly (Kleylein-Sohn et al., 2007; Strnad et al., 2007). To determine whether Cep152 is similarly required for early centriole assembly, we assayed Sas6 localization in cells depleted of Cep152 (Fig. 5 C). Control U2OS cells had one focus of Sas6 at each centriole doublet in S phase–arrested and mitotic cells (Fig. 5 C, S phase cell). In contrast, Cep152-depleted cells that had failed to duplicate centrioles lacked Sas6 on mitotic centrioles and had either no Sas6 (65%; n = 150 cells) or reduced Sas6 (25%) on S phase centrioles (Fig. 5 C). We observed the same phenotype for Plk4 depletion (unpublished data), which is similar to the previous description of the relationship between Plk4 and Sas6 localization (Kleylein-Sohn et al., 2007; Strnad et al., 2007). These results suggest that both Cep152 and Plk4 act early in the centriole assembly pathway.
We next investigated whether Plk4 can phosphorylate Cep152. GFP-tagged Cep152 isolated from human cell lysates was incubated with purified wild-type GST-Plk4 (Plk4-WT) or the kinase-dead mutant GST-Plk4 D154A (Plk4-KD) and assayed for 32P incorporation (Fig. 5 D). Plk4-WT but not Plk4-KD was able to phosphorylate itself, as previously reported (Holland et al., 2010; Sillibourne et al., 2010). Both full-length GFP-Cep152 (Fig. 5 D, left) and the GFP-Cep152 (1–217) fragment that binds to Plk4 (Fig. 5 D, middle) were phosphorylated when incubated with Plk4-WT but not with Plk4-KD. To test whether this reflected direct phosphorylation of Cep152 by Plk4, we purified Cep152 (1–217) fused to maltose-binding protein (MBP) and incubated it with GST-Plk4. MBP-Cep152 (1–217) was phosphorylated by Plk4-WT but not by Plk4-KD (Fig. 5 D, right). In all reactions, the phosphorylation was specific to the Cep152 portion of the fusion proteins, as neither GFP nor MBP alone were phosphorylated (unpublished data). Thus, Plk4 can interact directly with Cep152 and phosphorylate it in vitro. In addition, at least one phosphorylated region corresponds to the N-terminal Cep152 fragment that is required for interaction with Plk4.
We propose that Plk4 and Cep152 act together early in centriole duplication. We have shown that Cep152 is a centriolar protein that interacts with Plk4 and can be phosphorylated by it in vitro. Cep152 is required for centriole duplication, Plk4 overexpression–induced centriole amplification and, as for Plk4, for localization of Sas6 to centrioles, which is an early step in their assembly. The hypothesis that Plk4 and Cep152 act together in centriole duplication is also reinforced by their pattern of evolutionary conservation. The identification of Cep152 as a Plk4-associated factor provides a new tool for understanding the function of Plk4 in centriole duplication and how this process is spatially and temporally regulated.
Materials and methods
Cell culture and transfection
HEK293T and U2OS cells were grown in DME + 10% fetal bovine serum (Invitrogen). RPE-1 cells were cultured in DME/F12 + 10% fetal bovine serum (Invitrogen). RPE-1 Tet-O:Plk4 GFP-hCent2 cells were provided by B. Tsou (Memorial Sloan-Kettering Center, New York, NY). Plk4 expression was induced by addition of 1 µg/ml doxycycline (Takara Bio Inc.) for 18 h. U2OS cells were arrested in S phase by incubation in 2 µg/ml aphidicolin (Sigma-Aldrich) for 24 h. Plasmids were transfected using Lipofectamine 2000 according to the manufacturer’s instructions (Invitrogen).
Plasmids
Full-length cDNAs of Cep152 (GenBank/EMBL/DDBJ accession no. NM_001194998), Plk4 (accession no. NM_014264), and Plx4 (accession no. NM_001089677) were obtained from Thermo Fisher Scientific. The ORFs of Cep152 and Plk4 were amplified by PCR and cloned into pEGFP-C1 (Takara Bio Inc.). Plx4, Plk4, and Cep152 were cloned into a pEGFP-N1 (Takara Bio Inc.) derivative with a Myc-6xHIS cassette in place of GFP and a CMVT7 promoter, pCMVT7-mh. Plx4-D154A was made by site-directed mutagenesis (QuickChange II; Agilent Technologies).
Deletion constructs of Cep152 and Plk4 were made by PCR amplification of the indicated regions and ligation into pEGFP-C1 or pCMVT7-mh, respectively. Internal deletion constructs for Cep152 and Plk4 were created by triple ligation of two PCR fragments joined by an XmaI site into the same vectors. RNAi-resistant GFP-Cep152RR was made by site-directed mutagenesis of pEGFP-C1-Cep152. A total of 13 base pairs were replaced in the two regions of Cep152 hybridizing to the siRNAs without altering the amino acid sequence.
The MBP-Cep152 (1–217) fusion was made by PCR amplification of amino acids 1–217 of Cep152 and ligation into pMal-c2X (New England Biolabs, Inc.). An expression construct of GST fused to Plx4 amino acids 860–942 was made by PCR amplification and ligation into pGEX-4T-1 (GE Healthcare) for antibody production. For GFP mRNA transcription, the pEGFP-N1 cytomegalovirus promoter was replaced with the CMVT7 promoter from pCMVT7-mh. Expression constructs of GFP fused to 6×His and GST used to generate the polyclonal anti-GFP antibody were provided by M. Nachury (Stanford University, Stanford, CA).
Antibodies
Anti-Plx4 antibody was obtained by immunizing rabbits with GST fused to the C-terminal 85 amino acids of Plx4 (Cocalico Biologicals). Plx4-specific antibodies were purified against GST-C-Plx4 immobilized on resin (Ultralink Biosupport; Thermo Fisher Scientific) after initially passing the serum over a GST column. The Plx4 antibody recognized human Plk4 specifically as determined by antibody-blocking experiments in U2OS cells and was used at 1.5 µg/ml for immunofluorescence and 0.15 µg/ml for Western blotting. Rabbit anti-Cep152 was obtained from Bethyl Laboratories, Inc. (peptide antigen, residues 825–875) and diluted 1:1,000 before use. Anti-GFP antibody was obtained by immunizing rabbits (Cocalico Biologicals) with GST-GFP purified from Escherichia coli. The antibody was affinity purified against GFP-6×His bound to Ni-NTA Sepharose beads (QIAGEN) and used at 1.5 µg/ml for immunofluorescence and 0.15 µg/ml on Western blots. The polyclonal anti-centrin antibody used for immunofluorescence of centrosomes in Xenopus egg extract was made against Xenopus centrin cloned from a stage 17 cDNA library (NCBI Protein database accession no. AAA79194) and expressed as a His-tagged protein in E. coli. Antibody was affinity purified against the antigen and used at 0.5 µg/ml.
Other antibodies used for immunofluorescence in this study were mouse anti–γ-tubulin (GTU-88; Sigma-Aldrich) at 1:1,000, mouse anti–α-tubulin (DM1α; Sigma-Aldrich) at 1:2,000, mouse anti–acetylated α-tubulin (6-11B-1; Abcam) at 1:500, mouse anti-GFP (3e6; Invitrogen) at 1:500, mouse anti–polyglutamylated tubulin (GT335; provided by C. Janke, Centre de Recherches de Biochemie Macromoléculaire, Montpellier, France) at 1:1,000, mouse anti–centrin-2 (20H5; provided by J. Salisbury, Mayo Clinic, Rochester, MN) at 1:2,000, anti-Sas6 (sc-81431; Santa Cruz Biotechnology, Inc.) at 1:200, and mouse anti-myc (9e10) at 1:500 for immunofluorescence and 1:5,000 on Western blots. Purified rabbit IgG was purchased from Jackson ImmunoResearch Laboratories, Inc.
Xenopus embryos and egg extracts
mRNAs of Plx4 and GFP were transcribed using the mMessage mMachine T7 kit (Applied Biosystems). Cytostatic factor (CSF) egg extracts were made as described previously (Murray, 1991) and supplemented with 50 mM sucrose. Plx4 mRNA was added to CSF extracts and incubated 1 h before calcium addition to release it into interphase and 2 h after calcium addition at 16°C. Addition of mRNA to mitotic extracts permitted sufficient Plx4 translation to drive de novo structure formation when the extract was released into interphase before the extract reentered mitosis. Centrin-positive structures formed ∼2 h after calcium were added to the CSF extract to release it into interphase; most formed within 30 min of their initial appearance. These structures only form in interphase extract; they do not form in CSF extracts incubated with Plx4 mRNA even after longer incubation time.
Centrosomes were visualized by incubating extracts on ice for 20 min to depolymerize microtubules and diluting them in 1× BRB80, 2% Triton X-100, 4% paraformaldehyde, and 0.25% glutaraldehyde for 3 min at room temperature. Samples were centrifuged through a 30% glycerol cushion onto a coverslip at 5,000 g and 4°C for 15 min. Coverslips were postfixed in methanol. For aster visualization, an equal volume of CSF extract was added to the interphase extract for an additional 30 min before centrifugation and the ice incubation was omitted. The number of centrioles per field was counted for at least 25 fields per coverslip and averaged to determine the mean centrioles per field. Average centrioles/field was multiplied by fields/coverslip and divided by the μl extract centrifuged onto the coverslip to obtain centrioles/μl extract.
Mass spectrometry
Plx4-D154A with a myc-6×His C-terminal tag was transcribed and translated in egg extract as described in the Xenopus embryos and egg extracts section. The extract was diluted 10 times in binding buffer (50 mM Tris, pH 7.6, 150 mM NaCl, 0.25% Triton X-100, 1 mM DTT, and protease inhibitors) and incubated with Ni-NTA agarose beads (QIAGEN). Proteins were eluted with reduced glutathione (GSH; Sigma-Aldrich) and were repurified with anti-Plx4 antibody cross-linked to protein A beads (Affi-Prep; Bio-Rad Laboratories). Proteins were eluted by incubation in sample buffer and run on an SDS-PAGE gel.
Silver-stained protein bands present in the Plx4-D154A sample but not in control (at ∼125, 170, and 190 kD) were excised and analyzed by mass spectrometry at the Stanford Vincent Coates Foundation Mass Spectrometry Laboratory (http://mass-spec.stanford.edu). Bands were digested with trypsin (Promega). A LCQ Deca XP Plus mass spectrometer was used (Thermo Fisher Scientific). The database was composed of Xenopus and Xenopus tropicalis protein sequences archived in the National Center for Biotechnology Information (NCBI). Proteins were identified on the basis of at least two peptide matches with a probability >95%. LOC548794 was identified as Wdr33 in Homologene (NCBI).
Immunoprecipitations
HEK293T cells were transiently transfected, incubated for 24 h, washed with PBS, and lysed in NP-40 buffer. Insoluble material was pelleted, and soluble material was incubated with rabbit anti-GFP antibodies and then with protein A beads (Affi-Prep). Beads were washed in NP-40 buffer, eluted in sample buffer, and run on SDS-PAGE gels.
Immunofluorescence
Cells were grown on coverslips and fixed in methanol for indirect immunofluorescence. After rehydration in PBS, cells were blocked in 3% BSA (Sigma-Aldrich) in PBS + 0.1% Triton X-100. Coverslips were incubated in primary antibodies diluted in blocking solution as previously indicated (see Antibodies), and Alexa Fluor 488–, 594–, or 350–conjugated secondary antibodies were diluted 1:200 in blocking solution (Invitrogen). Double labeling with monoclonal mouse antibodies was performed using secondary antibodies that recognize specific isotypes of mouse IgG (Invitrogen). For double labeling with rabbit anti-Plx4 and rabbit anti-Cep152, a monovalent goat anti–rabbit Fab fragment (Jackson ImmunoResearch Laboratories, Inc.) was used as a blocking agent at 20 µg/ml. Coverslips of cells or extract samples were imaged using OpenLab software (version 4.0.4; PerkinElmer) on a microscope (Axiovert 200M; Carl Zeiss, Inc.) with Plan Neofluar 100× 1.3 NA objectives. Images were captured using a cooled charge-coupled device camera (Orca ER; Hamamatsu Photonics) and were processed using Photoshop (Adobe).
For colocalizing Cep152, Sas6, centrin, and γ-tubulin, a z stack of 11–13 digital optical sections at 0.3-µm intervals was obtained using an epifluorescence inverted microscope (IX70; Olympus) in an imaging station (DeltaVision; Applied Precision) maintained by the Beckman Center Cell Sciences Imaging Facility with a 100× 1.35 NA objective (Olympus). Images were acquired with a charge-coupled device camera (CH350; Photometrics). Images were deconvolved using the constrained iterative algorithm and point-spread functions supplied with the DeltaVision system. Deconvoluted stacks were merged into a single image using maximum intensity projections.
RNAi
Plk4 was depleted using an siRNA with the sequence 5′-CTGGTAGTACTAGTTCACCTA-3′ as previously described (Habedanck et al., 2005). Cep152 was depleted using a pool of two siRNA duplexes: 5′-GCGGATCCAACTGGAAATCTA-3′, as previously described (Kleylein-Sohn et al., 2007), and 5′-GCATTGAGGTTGAGACTAA-3′. All siRNAs were synthesized by Thermo Fisher Scientific. siRNAs were transfected into U2OS and RPE-1 cells using Lipofectamine RNAiMax following the manufacturer’s instructions (Invitrogen). For Cep152 depletion of 72 to 96 h, siRNAs were serially transfected every 24 h two and three times, respectively. An siRNA against luciferase was used as a control (Thermo Fisher Scientific). Western blot bands were quantified using an imager (Typhoon9210; GE Healthcare) and ImageQuant software (version 2003.01; GE Healthcare).
Kinase assay
Purified GST-Plk4 and GST-Plk4-D154A were made and kinase assays were performed as previously described (Holland et al., 2010). GFP, GFP-Cep152, and GFP-Cep152 (1–217) were immunoprecipitated from HEK293T cells as described above and added to the kinase reaction on the beads. MBP and MBP-Cep152 (1–217) were purified from E. coli using amylose resin (New England Biolabs, Inc.). Kinase reactions were stopped in sample buffer and run on SDS-PAGE gels. Dried gels were exposed to a storage phosphor screen (GE Healthcare), 32P incorporation was imaged using a Typhoon9210, and bands were quantified with ImageQuant. Samples for Western blots were eluted off the beads with sample buffer and run on SDS-PAGE gels.
Online supplemental material
Fig. S1 shows that Plx4-induced de novo objects have the same properties as centrioles and centrosomes. Fig. S2 shows that Plk4 and Cep152 interact and the conservation of Cep152. Fig. S3 shows rescue of the Cep152 depletion phenotype by RNAi-resistant Cep152. Online supplemental material is available at http://www.jcb.org/cgi/content/full/jcb.201006049/DC1.
Acknowledgments
We thank B. Tsou, J. Salisbury, C. Janke, and M. Nachury for reagents, C. Adams in the Stanford University Mass Spectrometry Group, members of the laboratory of K. Shen for assistance with confocal microscopy, and Y.L. Lee for the rabbit anti-GFP antibody.
This work was supported by the National Institutes of Health (grant GM52022 to T. Stearns).
Footnotes
Abbreviations used in this paper:
- CSF
- cytostatic factor
- MBP
- maltose-binding protein
- SMC
- structural maintenance of chromosomes
References
- Andersen J.S., Wilkinson C.J., Mayor T., Mortensen P., Nigg E.A., Mann M. 2003. Proteomic characterization of the human centrosome by protein correlation profiling. Nature. 426:570–574 10.1038/nature02166 [DOI] [PubMed] [Google Scholar]
- Basto R., Lau J., Vinogradova T., Gardiol A., Woods C.G., Khodjakov A., Raff J.W. 2006. Flies without centrioles. Cell. 125:1375–1386 10.1016/j.cell.2006.05.025 [DOI] [PubMed] [Google Scholar]
- Basto R., Brunk K., Vinadogrova T., Peel N., Franz A., Khodjakov A., Raff J.W. 2008. Centrosome amplification can initiate tumorigenesis in flies. Cell. 133:1032–1042 10.1016/j.cell.2008.05.039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blachon S., Gopalakrishnan J., Omori Y., Polyanovsky A., Church A., Nicastro D., Malicki J., Avidor-Reiss T. 2008. Drosophila asterless and vertebrate Cep152 Are orthologs essential for centriole duplication. Genetics. 180:2081–2094 10.1534/genetics.108.095141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carvalho-Santos Z., Machado P., Branco P., Tavares-Cadete F., Rodrigues-Martins A., Pereira-Leal J.B., Bettencourt-Dias M. 2010. Stepwise evolution of the centriole-assembly pathway. J. Cell Sci. 123:1414–1426 10.1242/jcs.064931 [DOI] [PubMed] [Google Scholar]
- Castellanos E., Dominguez P., Gonzalez C. 2008. Centrosome dysfunction in Drosophila neural stem cells causes tumors that are not due to genome instability. Curr. Biol. 18:1209–1214 10.1016/j.cub.2008.07.029 [DOI] [PubMed] [Google Scholar]
- Cunha-Ferreira I., Rodrigues-Martins A., Bento I., Riparbelli M., Zhang W., Laue E., Callaini G., Glover D.M., Bettencourt-Dias M. 2009. The SCF/Slimb ubiquitin ligase limits centrosome amplification through degradation of SAK/PLK4. Curr. Biol. 19:43–49 [DOI] [PubMed] [Google Scholar]
- Dammermann A., Müller-Reichert T., Pelletier L., Habermann B., Desai A., Oegema K. 2004. Centriole assembly requires both centriolar and pericentriolar material proteins. Dev. Cell. 7:815–829 10.1016/j.devcel.2004.10.015 [DOI] [PubMed] [Google Scholar]
- Dobbelaere J., Josué F., Suijkerbuijk S., Baum B., Tapon N., Raff J. 2008. A genome-wide RNAi screen to dissect centriole duplication and centrosome maturation in Drosophila. PLoS Biol. 6:e224 10.1371/journal.pbio.0060224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Félix M.A., Antony C., Wright M., Maro B. 1994. Centrosome assembly in vitro: role of gamma-tubulin recruitment in Xenopus sperm aster formation. J. Cell Biol. 124:19–31 10.1083/jcb.124.1.19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganem N.J., Godinho S.A., Pellman D. 2009. A mechanism linking extra centrosomes to chromosomal instability. Nature. 460:278–282 10.1038/nature08136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gard D.L., Hafezi S., Zhang T., Doxsey S.J. 1990. Centrosome duplication continues in cycloheximide-treated Xenopus blastulae in the absence of a detectable cell cycle. J. Cell Biol. 110:2033–2042 10.1083/jcb.110.6.2033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Habedanck R., Stierhof Y.D., Wilkinson C.J., Nigg E.A. 2005. The Polo kinase Plk4 functions in centriole duplication. Nat. Cell Biol. 7:1140–1146 10.1038/ncb1320 [DOI] [PubMed] [Google Scholar]
- Hinchcliffe E.H., Li C., Thompson E.A., Maller J.L., Sluder G. 1999. Requirement of Cdk2-cyclin E activity for repeated centrosome reproduction in Xenopus egg extracts. Science. 283:851–854 10.1126/science.283.5403.851 [DOI] [PubMed] [Google Scholar]
- Hodges M.E., Scheumann N., Wickstead B., Langdale J.A., Gull K. 2010. Reconstructing the evolutionary history of the centriole from protein components. J. Cell Sci. 123:1407–1413 10.1242/jcs.064873 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holland A.J., Lan W., Niessen S., Hoover H., Cleveland D.W. 2010. Polo-like kinase 4 kinase activity limits centrosome overduplication by autoregulating its own stability. J. Cell Biol. 188:191–198 10.1083/jcb.200911102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitagawa D., Busso C., Flückiger I., Gönczy P. 2009. Phosphorylation of SAS-6 by ZYG-1 is critical for centriole formation in C. elegans embryos. Dev. Cell. 17:900–907 10.1016/j.devcel.2009.11.002 [DOI] [PubMed] [Google Scholar]
- Kleylein-Sohn J., Westendorf J., Le Clech M., Habedanck R., Stierhof Y.D., Nigg E.A. 2007. Plk4-induced centriole biogenesis in human cells. Dev. Cell. 13:190–202 10.1016/j.devcel.2007.07.002 [DOI] [PubMed] [Google Scholar]
- Lacey K.R., Jackson P.K., Stearns T. 1999. Cyclin-dependent kinase control of centrosome duplication. Proc. Natl. Acad. Sci. USA. 96:2817–2822 10.1073/pnas.96.6.2817 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leidel S., Gönczy P. 2003. SAS-4 is essential for centrosome duplication in C elegans and is recruited to daughter centrioles once per cell cycle. Dev. Cell. 4:431–439 10.1016/S1534-5807(03)00062-5 [DOI] [PubMed] [Google Scholar]
- Leidel S., Delattre M., Cerutti L., Baumer K., Gönczy P. 2005. SAS-6 defines a protein family required for centrosome duplication in C. elegans and in human cells. Nat. Cell Biol. 7:115–125 10.1038/ncb1220 [DOI] [PubMed] [Google Scholar]
- Matsuura K., Lefebvre P.A., Kamiya R., Hirono M. 2004. Bld10p, a novel protein essential for basal body assembly in Chlamydomonas: localization to the cartwheel, the first ninefold symmetrical structure appearing during assembly. J. Cell Biol. 165:663–671 10.1083/jcb.200402022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray A.W. 1991. Cell cycle extracts. Methods Cell Biol. 36:581–605 10.1016/S0091-679X(08)60298-8 [DOI] [PubMed] [Google Scholar]
- Murray A.W., Kirschner M.W. 1989. Cyclin synthesis drives the early embryonic cell cycle. Nature. 339:275–280 10.1038/339275a0 [DOI] [PubMed] [Google Scholar]
- Nigg E.A. 2006. Origins and consequences of centrosome aberrations in human cancers. Int. J. Cancer. 119:2717–2723 10.1002/ijc.22245 [DOI] [PubMed] [Google Scholar]
- O’Connell K.F., Maxwell K.N., White J.G. 2000. The spd-2 gene is required for polarization of the anteroposterior axis and formation of the sperm asters in the Caenorhabditis elegans zygote. Dev. Biol. 222:55–70 10.1006/dbio.2000.9714 [DOI] [PubMed] [Google Scholar]
- O’Connell K.F., Caron C., Kopish K.R., Hurd D.D., Kemphues K.J., Li Y., White J.G. 2001. The C. elegans zyg-1 gene encodes a regulator of centrosome duplication with distinct maternal and paternal roles in the embryo. Cell. 105:547–558 10.1016/S0092-8674(01)00338-5 [DOI] [PubMed] [Google Scholar]
- Palazzo R.E., Vaisberg E., Cole R.W., Rieder C.L. 1992. Centriole duplication in lysates of Spisula solidissima oocytes. Science. 256:219–221 10.1126/science.1566068 [DOI] [PubMed] [Google Scholar]
- Paweletz N., Mazia D., Finze E.M. 1984. The centrosome cycle in the mitotic cycle of sea urchin eggs. Exp. Cell Res. 152:47–65 10.1016/0014-4827(84)90229-5 [DOI] [PubMed] [Google Scholar]
- Peel N., Stevens N.R., Basto R., Raff J.W. 2007. Overexpressing centriole-replication proteins in vivo induces centriole overduplication and de novo formation. Curr. Biol. 17:834–843 10.1016/j.cub.2007.04.036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodrigues-Martins A., Riparbelli M., Callaini G., Glover D.M., Bettencourt-Dias M. 2007. Revisiting the role of the mother centriole in centriole biogenesis. Science. 316:1046–1050 10.1126/science.1142950 [DOI] [PubMed] [Google Scholar]
- Rodrigues-Martins A., Riparbelli M., Callaini G., Glover D.M., Bettencourt-Dias M. 2008. From centriole biogenesis to cellular function: centrioles are essential for cell division at critical developmental stages. Cell Cycle. 7:11–16 10.4161/cc.7.1.5226 [DOI] [PubMed] [Google Scholar]
- Rogers G.C., Rusan N.M., Roberts D.M., Peifer M., Rogers S.L. 2009. The SCFSlimb ubiquitin ligase regulates Plk4/Sak levels to block centriole reduplication. J. Cell Biol. 184:225–239 10.1083/jcb.200808049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sillibourne J.E., Tack F., Vloemans N., Boeckx A., Thambirajah S., Bonnet P., Ramaekers F.C., Bornens M., Grand-Perret T. 2010. Autophosphorylation of polo-like kinase 4 and its role in centriole duplication. Mol. Biol. Cell. 21:547–561 10.1091/mbc.E09-06-0505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stearns T., Kirschner M. 1994. In vitro reconstitution of centrosome assembly and function: the central role of gamma-tubulin. Cell. 76:623–637 10.1016/0092-8674(94)90503-7 [DOI] [PubMed] [Google Scholar]
- Stevens N.R., Raposo A.A., Basto R., St Johnston D., Raff J.W. 2007. From stem cell to embryo without centrioles. Curr. Biol. 17:1498–1503 10.1016/j.cub.2007.07.060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strnad P., Leidel S., Vinogradova T., Euteneuer U., Khodjakov A., Gönczy P. 2007. Regulated HsSAS-6 levels ensure formation of a single procentriole per centriole during the centrosome duplication cycle. Dev. Cell. 13:203–213 10.1016/j.devcel.2007.07.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varmark H., Llamazares S., Rebollo E., Lange B., Reina J., Schwarz H., Gonzalez C. 2007. Asterless is a centriolar protein required for centrosome function and embryo development in Drosophila. Curr. Biol. 17:1735–1745 10.1016/j.cub.2007.09.031 [DOI] [PubMed] [Google Scholar]