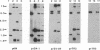Abstract
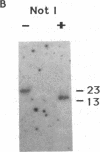
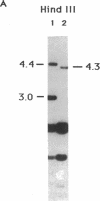
A circular plasmid containing a complete Tetrahymena thermophila rRNA gene (rDNA), with a tandem repeat of a 1.9-kilobase-pair segment encompassing the replication origin and the rRNA promoter, and a polylinker in the 3' nontranscribed spacer, was used to transform T. thermophila by microinjection. Most (20/21) stable transformants contained only recombinant linear palindromic rDNA molecules carrying rDNA sequences from both the donor plasmid and the recipient cell, as shown previously. However, in one transformant, the circular plasmid initially outreplicated the endogenous rDNA and was the major rDNA form for up to 65 generations. Stable circular replicons have not been reported previously in Tetrahymena. A single point mutation (+G) was identified in the repeated promoter of the plasmid maintained in this transformant. After recovery from the Tetrahymena transformant and recloning in Escherichia coli, the mutated circular plasmid again transformed Tetrahymena with stable maintenance of the circular rDNA plasmid. Transformants containing circular replicons were also obtained by using a similar plasmid from which the repeated promoter, but not the repeated replication origin, had been removed by BAL-31 deletion. We therefore propose that repeated rRNA promoters are deleterious in vivo in Tetrahymena, which normally lacks them. Transformants were obtained in 2-5 days compared with the 7-14 days required for transformation with unmutated rDNA plasmids by recombination. Similar results were obtained when a 550-base-pair segment containing the telomerase RNA gene of T. thermophila was inserted in the polylinker of the plasmid. We suggest that this plasmid is a useful vector system for transformation of Tetrahymena.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bateman E., Paule M. R. Promoter occlusion during ribosomal RNA transcription. Cell. 1988 Sep 23;54(7):985–992. doi: 10.1016/0092-8674(88)90113-4. [DOI] [PubMed] [Google Scholar]
- Blackburn E. H., Karrer K. M. Genomic reorganization in ciliated protozoans. Annu Rev Genet. 1986;20:501–521. doi: 10.1146/annurev.ge.20.120186.002441. [DOI] [PubMed] [Google Scholar]
- Bruns P. J., Katzen A. L., Martin L., Blackburn E. H. A drug-resistant mutation in the ribosomal DNA of Tetrahymena. Proc Natl Acad Sci U S A. 1985 May;82(9):2844–2846. doi: 10.1073/pnas.82.9.2844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Challoner P. B., Amin A. A., Pearlman R. E., Blackburn E. H. Conserved arrangements of repeated DNA sequences in nontranscribed spacers of ciliate ribosomal RNA genes: evidence for molecular coevolution. Nucleic Acids Res. 1985 Apr 11;13(7):2661–2680. doi: 10.1093/nar/13.7.2661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilley D., Preer J. R., Jr, Aufderheide K. J., Polisky B. Autonomous replication and addition of telomerelike sequences to DNA microinjected into Paramecium tetraurelia macronuclei. Mol Cell Biol. 1988 Nov;8(11):4765–4772. doi: 10.1128/mcb.8.11.4765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godiska R., Aufderheide K. J., Gilley D., Hendrie P., Fitzwater T., Preer L. B., Polisky B., Preer J. R., Jr Transformation of Paramecium by microinjection of a cloned serotype gene. Proc Natl Acad Sci U S A. 1987 Nov;84(21):7590–7594. doi: 10.1073/pnas.84.21.7590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greider C. W., Blackburn E. H. A telomeric sequence in the RNA of Tetrahymena telomerase required for telomere repeat synthesis. Nature. 1989 Jan 26;337(6205):331–337. doi: 10.1038/337331a0. [DOI] [PubMed] [Google Scholar]
- Grummt I., Kuhn A., Bartsch I., Rosenbauer H. A transcription terminator located upstream of the mouse rDNA initiation site affects rRNA synthesis. Cell. 1986 Dec 26;47(6):901–911. doi: 10.1016/0092-8674(86)90805-6. [DOI] [PubMed] [Google Scholar]
- Iida C. T., Kownin P., Paule M. R. Ribosomal RNA transcription: proteins and DNA sequences involved in preinitiation complex formation. Proc Natl Acad Sci U S A. 1985 Mar;82(6):1668–1672. doi: 10.1073/pnas.82.6.1668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kownin P., Bateman E., Paule M. R. Effects of single-base substitutions within the Acanthamoeba castellanii rRNA promoter on transcription and on binding of transcription initiation factor and RNA polymerase I. Mol Cell Biol. 1988 Feb;8(2):747–753. doi: 10.1128/mcb.8.2.747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kownin P., Bateman E., Paule M. R. Eukaryotic RNA polymerase I promoter binding is directed by protein contacts with transcription initiation factor and is DNA sequence-independent. Cell. 1987 Aug 28;50(5):693–699. doi: 10.1016/0092-8674(87)90327-8. [DOI] [PubMed] [Google Scholar]
- Labhart P., Reeder R. H. Ribosomal precursor 3' end formation requires a conserved element upstream of the promoter. Cell. 1987 Jul 3;50(1):51–57. doi: 10.1016/0092-8674(87)90661-1. [DOI] [PubMed] [Google Scholar]
- Larson D. D., Blackburn E. H., Yaeger P. C., Orias E. Control of rDNA replication in Tetrahymena involves a cis-acting upstream repeat of a promoter element. Cell. 1986 Oct 24;47(2):229–240. doi: 10.1016/0092-8674(86)90445-9. [DOI] [PubMed] [Google Scholar]
- Larson D. D., Spangler E. A., Blackburn E. H. Dynamics of telomere length variation in Tetrahymena thermophila. Cell. 1987 Jul 31;50(3):477–483. doi: 10.1016/0092-8674(87)90501-0. [DOI] [PubMed] [Google Scholar]
- Løvlie A., Haller B. L., Orias E. Molecular evidence for somatic recombination in the ribosomal DNA of Tetrahymena thermophila. Proc Natl Acad Sci U S A. 1988 Jul;85(14):5156–5160. doi: 10.1073/pnas.85.14.5156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McStay B., Reeder R. H. A termination site for Xenopus RNA polymerase I also acts as an element of an adjacent promoter. Cell. 1986 Dec 26;47(6):913–920. doi: 10.1016/0092-8674(86)90806-8. [DOI] [PubMed] [Google Scholar]
- Orias E., Larson D., Hu Y. F., Yu G. L., Karttunen J., Løvlie A., Haller B., Blackburn E. H. Replacement of the macronuclear ribosomal RNA genes of a mutant Tetrahymena using electroporation. Gene. 1988 Oct 30;70(2):295–301. doi: 10.1016/0378-1119(88)90201-6. [DOI] [PubMed] [Google Scholar]
- Spangler E. A., Blackburn E. H. The nucleotide sequence of the 17S ribosomal RNA gene of Tetrahymena thermophila and the identification of point mutations resulting in resistance to the antibiotics paromomycin and hygromycin. J Biol Chem. 1985 May 25;260(10):6334–6340. [PubMed] [Google Scholar]
- Tondravi M. M., Yao M. C. Transformation of Tetrahymena thermophila by microinjection of ribosomal RNA genes. Proc Natl Acad Sci U S A. 1986 Jun;83(12):4369–4373. doi: 10.1073/pnas.83.12.4369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tower J., Culotta V. C., Sollner-Webb B. Factors and nucleotide sequences that direct ribosomal DNA transcription and their relationship to the stable transcription complex. Mol Cell Biol. 1986 Oct;6(10):3451–3462. doi: 10.1128/mcb.6.10.3451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yaeger P. C., Orias E., Shaiu W. L., Larson D. D., Blackburn E. H. The replication advantage of a free linear rRNA gene is restored by somatic recombination in Tetrahymena thermophila. Mol Cell Biol. 1989 Feb;9(2):452–460. doi: 10.1128/mcb.9.2.452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao M. C., Yao C. H. Accurate processing and amplification of cloned germ line copies of ribosomal DNA injected into developing nuclei of Tetrahymena thermophila. Mol Cell Biol. 1989 Mar;9(3):1092–1099. doi: 10.1128/mcb.9.3.1092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu G. L., Hasson M., Blackburn E. H. Circular ribosomal DNA plasmids transform Tetrahymena thermophila by homologous recombination with endogenous macronuclear ribosomal DNA. Proc Natl Acad Sci U S A. 1988 Jul;85(14):5151–5155. doi: 10.1073/pnas.85.14.5151. [DOI] [PMC free article] [PubMed] [Google Scholar]