Abstract
Peptidomometic analogues, H-Dmt-Tic-NH2-CH2-Ph or -Bid exhibit δ-opioid receptor activities. Substitution of Tic by the Aba-Gly scaffold coupled to the C-termini -CH2-Ph (1), -NH-Ph (2) and Gly*-Bid (3) shifted receptor affinity and selectivity to μ-opioid receptors (Kiμ = 0.46, 1.48 and 19.9 nM, respectively) with μ agonism. These represent templates for a new class of μ-opioid agonists. Further modification with negative or positive charges could yield altered properties suitable for therapeutic application for pain relief.
Introduction1
H-Dmt-Tic-OH,2, 3 which evolved from H-Tyr-Tic-OH,4 as a simplified form of TIP-(P),5 represents the minimal sequence that selectively interacts with δ opioid receptors as a potent δ-antagonist. This dipeptide underwent extensive modifications and structure-activity studies,6, 7 which included N-terminal modification with alkyl substitutions;8, 3 replacement of Tic by heteroaliphatic, heteroaromatic nuclei,9 or D-Phe;10 alteration of the C-terminus of Tic by substituents containing a hydrophobic groups;3 and addition of a third aromatic center with or without inserting interposing linkers.11–13 Many of the analogues had unique properties, including enhanced δ-opioid antagonism,3, 6–13 conversion from a δ-antagonist to a δ-agonist and viceversa,11, 14 mixed μ-agonism/δ-agonism, mixed μ-agonism/δ-antagonism,3, 8, 11 or development into an irreversible fluorescent δ-antagonist.13 From these and other studies it was concluded that small modifications can change drastically the pharmacological profile in molecules related to the Dmt-Tic pharmacophore.15, 16, 17
One liability of small peptides is cyclization to a dioxopiperazine 18 during the acidic steps involved in peptide synthesis and purification. 19–21 This phenomenon occurs not only with peptides containing Tic or other constrained residues at the C-terminus, 21 but also with other amino acids. 19, 20 Dioxopiperazine formation from Dmt-Tic peptides appeared to be essentially negligible under neutral conditions; 2, 21 however, even when present, cyclo(Dmt-Tic) exhibited δ opioid receptor affinity (Ki δ = 9.84 nM). 22 Different strategies were reported to stabilize opioid peptides containing Tic at position 2 against dioxopiperazine formation. Schiller et al., to avoid slow spontaneous Tyr-Tic dioxopiperazine formation from TIPP (H-Tyr-Tic-Phe-Phe-OH) when dissolved in DMSO or MeOH, synthesized the corresponding peptides containing a reduced peptide bond [CH2-NH (Ψ)] between Tic2 and Phe3 residues. 5, 23 Salvadori et al., published the synthesis of the N,N-dimethylated analogue of H-Dmt-Tic-OH as another method to avoid the dioxopiperazine formation; 8 in both cases the modified analogues resulted more active than the parent compounds.
Looking through the literature was recognized the 2-(4-amino-4,5-dihydro-3-oxo-1H-benzo[c]azepin-2(3H)-yl)acetic acid (abbreviated Aba-Gly) as a useful scaffold to prevent potential dioxopiperazine formation in opioid peptides containing the Dmt-Tic pharmacophore. 24 Moreover, Aba-Gly considered as a mimetic of the Phe-Gly or Tic-Gly dipeptide sequence, when inserted in the μ selective agonist dermorphin (H-Tyr-D-Ala-Phe-Gly-Tyr-Pro-Ser-NH2) and it’s N-terminal tetrapeptide, is able to shift affinity and selectivity from μ to δ receptors. 25 Starting from these considerations we decided to introduce the the Aba-Gly scaffold into peptides containing the Dmt-Tic pharmacophore. H-Dmt-Tic-Gly-NH-Ph (μ agonist/δ agonist), H-Dmt-Tic-Gly-NH-CH2-Ph (μ agonist/δ antagonist) and H-Dmt-Tic-NH-CH2-Bid (δ agonist) were selected as reference compounds. μ agonists/δ agonists could be interesting analgesics with low dependence on chronic use.26 Opioid ligands with a mixed μ agonist/δ antagonist activity profile may have diminished propensity to induce tolerance and therefore may have therapeutic advantages over μ agonist analgesics for long term treatment of pain.27, 28 Delta opioid receptor agonists as H-Dmt-Tic-Gly*-Bid [Gly*-Bid, -NH-CH2-1H-benzimidazole-2-yl] and H-Dmt-Tic-Asp*-Bid [Asp*-Bid, -NH-CH(CH2-COOH)-1H-benzimidazole-2-yl] are attractive potential analgesics, since these compounds exhibit strong antinociceptive activity with relatively few side effects.29 Delta opioid receptor agonists produce antidepressant-like and anxiolytic-like effects and regulate BDNF mRNA expression in rodents30, 31 The regulation of BDNF mRNA expression could be useful in multiple sclerosis and related deseases.32 δ-opioid receptor activation protects cortical neurons, giving hibernation and neuroprotection.33–35 Activation of delta- and kappa-opioid receptors affords cardioprotection.36
Chemistry
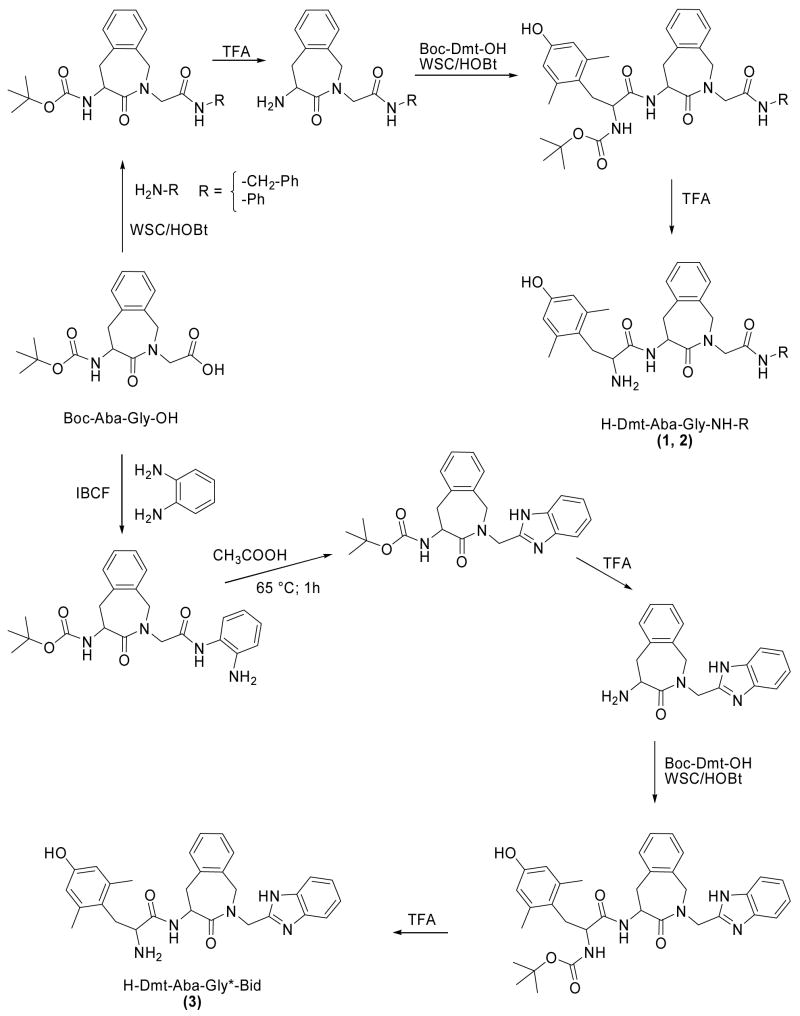
Compounds 1–3 were prepared stepwise by solution peptide synthetic methods as outlined in Scheme 1. Boc-Aba-Gly-OH 24 was condensed with benzylamine or aniline via WSC/HOBt. After N-terminal Boc deprotection with TFA, Aba-Gly amides were condensed with Boc-Tic-OH via WSC/HOBt. After N- terminal Boc deprotection (TFA), Boc-Dmt-OH was condensed via WSC/HOBt. Final N-terminal Boc deprotection with TFA gave compounds (1, 2). Compound (3) containing the C-terminal 1H-benzimidazol-2-yl (Bid) was synthesized in a similar manner. Mixed carbonic anhydride coupling of Boc-Aba-Gly-OH with o-phenylendiamine gave the corresponding crude intermediate monoamide, which were converted without purification to the desired heteroaromatic derivative by cyclization and dehydration in acetic acid, as outlined in Scheme 1. After Nα Boc deprotection with TFA, the corresponding derivative was condensed with Boc-Tic-OH and then with Boc-Dmt-OH via WSC/HOBt. Final N-terminal Boc deprotection with TFA gave compound (3). Final compounds (1–3) were purified by preparative HPLC.
Scheme 1.
Synthesis of compounds 1–3.
Results and Discussion
Receptor Affinity Analysis
Receptor binding and functional bioactivity are reported in Table 1. The new compounds (1–3) have affinity for δ-opioid receptors ranging from Kiδ = 11.0–427 nM, with a lack of affinity ranging from 355 to 13800 foldwith respect to the reference compounds. The μ receptors affinity of compounds 1–3 gave better results, in fact, they show Kiμ values in the nanomolar range, in quite good accord with the reference compounds, with a low preference for μ receptors Kiδ/Kiμ = 18.4–23.9. It is interesting to note that Aba-Gly is able to shift selectivity from μ to δ when inserted into the μ selective dermorphin and in it’s N-terminal tetrapeptide sequence. On the contrary, Aba-Gly when inserted in peptides or pseudopeptides containing the essentially δ selective Dmt-Tic pharmacophore, induces selectivity for μ receptors.
Table 1.
Receptor Binding and Functional Bioactivity.
| Receptor affinitya (nM) | Selectivity | Functional bioactivity (nM) | ||||||
|---|---|---|---|---|---|---|---|---|
| no. | Structure | δ/μ | μ/δ | MVD (IC50)b | MVD (Ke)c | GPI (IC50)b | ||
| H-Dmt-Tic-Gly-NH-CH2-Phd | 0.031 | 0.16 | – | 5.3 | 0.56 | 2.69 | ||
| H-Dmt-Tic-Gly-NH-Ph d | 0.042 | 0.16 | – | 3.6 | 3.02 | - | 2.57 | |
| H-Dmt-Tic-Gly*-Bidd | 0.035 | 0.50 | – | 14 | 0.13 | - | 26.9 | |
| H-Tyr-Pro-Phe-Phe-NH2e (EM-2) | 9,200 | 0.69 | 13,300 | - | 344 | - | 5.79 | |
| H-Tyr-Pro-Trp-Phe-NH2e (EM-1) | 1,500 | 0.36 | 4,200 | - | 36.3 | - | 10.1 | |
| 1 |
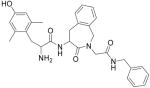
H-Dmt-Aba-Gly-NH-CH2-Ph |
11.0 ± 2.3 (3) | 0.46 ± 0.07 (3) | 23.9 | - | 830 ± 70 | 51 ± 5 | |
| 2 |
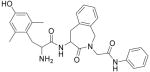
H-Dmt-Aba-Gly-NH-Ph |
27.2 ± 5.7 (3) | 1.48 ± 0.11 (3) | 18.4 | - | Partial Agonist (max 40%) IC50 = 650 nM | 95 ± 7.5 | |
| 3 |
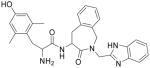
H-Dmt-Aba-Gly*-Bid |
427 ± 38 (3) | 19.9 ± 0.57 (3) | 21.5 | Partial Agonist (max 40%) IC50 = 2,700 nM | 231 ± 15 | ||
The Ki values (nM) were determined according to Chang and Prusoff 48 as detailed in the Experimental Section. The mean ± SE with n repetitions in parenthesis is based on independent duplicate binding assays with five to eight peptide doses using several different synaptosomal preparations.
Agonist activity was expressed as IC50 obtained from dose-response curves. These values represent the mean ± SE for at least five fresh tissue semples. Deltorphin C and dermorphin were the internal standards for MVD (δ-opioid receptor bioactivity) and GPI (μ-opioid receptor bioactivity) tissue preparation, respectively.
The Ke (equilibrium dissociation constant) values of opioid antagonists against the agonists (deltorphin C and dermorphin) were determined by the method of Kosterlitz and Watt.51
Data taken from Balboni, G. et al.11
Functional Bioactivity
Compounds (1–3) were tested in the electrically stimulated MVD and GPI assays for intrinsic activity (Table 1). Our data reveal that all analogues were endowed of weakly agonists or partial agonist activity for δ receptors in the MVD assay. In accordance with Kiμ binding data, IC50 values in the GPI test follow the same trend: benzyl amide (51 nM) > anilide (95 nM) > Bid (231 nM). It is interesting to note that the IC50 value of compound (1) is comparable to endomorphin 1 and 2, potent and selective μ agonists. 37
Conclusions
Remembering the aim of this work, we evaluated the possibility to insert the Aba-Gly scaffold into some reference opioid peptides and pseudopeptides containing the Dmt-Tic pramacophore (δ agonists, μ agonist/δ agonist and μ agonist/δ antagonist). As expected, no dioxopiperazine formation was evidenced, probably deriving from the lack of cis isomerism in the amide bond between Tyr1 or Dmt1 and Tic2 or Pro2.10, 24, 25, 38–40. This is quite easy proved by evaporation of the solution deriving from HPLC purification. This solution is acidic enough (0.1% TFA) to catalyse the cyclization to dioxopiperazine under mild worming. All peptides containing Tic in position 2 potentially give this reaction in these conditions. More interestingly, compounds (1–3) could be considered as non peptide derivatives, or peptidomimetics, and therefore potentially devoid of any drawback linked to peptide structures. Considering opioid peptides and pseudopeptides containing the Dmt-Tic pharmacophore, the substitution of Tic residue usually gives derivatives less active especially for δ receptors. 41–43 Finally, H-Dmt-Aba-Gly-NH-CH2-Ph could be a useful and versatile starting point in the development of new opioid ligands. In fact, it is quite easy to prepare analogues of the Aba-Gly scaffold, (as an example Aba-D-Ala). 25, 44 More interesting data could be obtained from new scaffolds as: Aba-Asp or Aba-Glu (the negative charge can provide affinity and selectivity for δ receptors) 45 and Aba-Lys or Aba-Arg (the positive charge can provide affinity, selectivity and especially antagonism for μ receptors; unpublished data). Work is in progress regarding the synthesis of Aba-Lys.
Experimental
Chemistry
General Methods
Crude compounds were purified by preparative reversed-phase HPLC [Waters Delta Prep 4000 system with Waters Prep LC 40 mm Assembly column C18 (30 cm × 4 cm, 15 μm particle)] and eluted at a flow rate of 25 mL/min with mobile phase solvent A (10% acetonitrile + 0.1% TFA in H2O, v/v), and a linear gradient from 25 to 75% B (60%, acetonitrile + 0.1% TFA in H2O, v/v) in 25 min. Analytical HPLC analyses were performed with a Beckman System Gold (Beckman ultrasphere ODS column, 250 mm × 4.6 mm, 5 μm particle). Analytical determinations and capacity factor (K′) of the products used HPLC in solvents A and B programmed at flow rate of 1 mL/min with linear gradients from 0 to 100% B in 25 min. Analogues had less than 1% impurities at 220 and 254 nm. TLC was performed on precoated plates of silica gel F254 (Merck, Darmstadt, Germany): (A) 1-butanol/AcOH/H2O (3:1:1, v/v/v); (B) CH2Cl2/toluene/methanol (17:1:2). Ninhydrin (1% ethanol, Merck), fluorescamine (Hoffman-La Roche) and chlorine spray reagents. Melting points were determined on a Kofler apparatus and are uncorrected. Optical rotations were assessed at 10 mg/mL in methanol with a Perkin-Elmer 241 polarimeter in a 10 cm water-jacketed cell. Molecular weights of the compounds were determined by a MALDI-TOF analysis (Hewlett Packard G2025A LD-TOF system mass spectrometer) and α-cyano-4-hydroxycinnamic acid as a matrix. 1H NMR (δ) spectra were measured, when not specified, in DMSO-d6 solution using a Bruker AC-200 spectrometer, and peak positions are given in parts per million downfield from tetramethylsilane as internal standard. Elemental analysis of the new compounds is reported in Table 2.
Table 2.
Elemental analysis of compounds 1–3.a
| Comp. | Formula | MH+, m/z | C | H | N | |
|---|---|---|---|---|---|---|
| 1 | C32H35F3N4O6 | 516 | Calc | 61.14 | 5.61 | 8.91 |
| Found | 60.98 | 5.54 | 8.84 | |||
| 2 | C31H33F3N4O6 | 502 | Calc | 60.58 | 5.41 | 9.12 |
| Found | 60.87 | 5.57 | 8.94 | |||
| 3 | C33H33F6N5O7 | 499 | Calc | 54.62 | 4.58 | 9.65 |
| Found | 54.88 | 4.72 | 9.77 |
Only the analysis of the new compounds, detailed in the Experimental Section, are included.
Boc-Aba-Gly-NH-CH2-Ph
To a solution of Boc-Aba-Gly-OH 24 (0.1 g, 0.3 mmol) and benzylamine (0.03 mL, 0.3 mmol) in DMF (10 mL) at 0 °C, HOBt (0.05 g, 0.33 mmol), and WSC (0.06 g, 0.33 mmol) were added. The reaction mixture was stirred for 3 h at 0 °C and 24 h at room temperature. After DMF was evaporated, the residue was dissolved in EtOAc and washed with citric acid (10% in H2O), NaHCO3 (5% in H2O), and brine. The organic phase was dried (Na2SO4) and evaporated to dryness. The residue was precipitated from Et2O/Pe (1:9, v/v): yield 0.12 g (92%); Rf(B) 0.68; HPLC K′ 8.59; mp 78–80 °C; [α]20D +4.2; MH+ 425; 1H-NMR (DMSO-d6) δ 1.40 (m, 9H), 2.92–3.17 (m, 2H), 4.09–4.48 (m, 6H), 4.92 (m, 1H), 6.96–7.14 (m, 9H).
TFA·H-Aba-Gly-NH-CH2-Ph
Boc-Aba-Gly-NH-CH2-Ph (0.12 g, 0.28 mmol) was treated with TFA (1 mL) for 0.5 h at room temperature. Et2O/Pe (1:1, v/v) were added to the solution until the product precipitated: yield 0.12 g (96%); Rf(A) 0.42; HPLC K′ 5.63; mp 93–95 °C; [α]20D +5.6; MH+ 324.
Boc-Dmt-Aba-Gly-NH-CH2-Ph
To a solution of Boc-Dmt-OH (0.05 g, 0.16 mmol) and TFA·H-Aba-Gly-NH-CH2-Ph (0.07 g, 0.16 mmol) in DMF (10 mL) at 0 °C, NMM (0.02 mL, 0.16 mmol), HOBt (0.03 g, 0.18 mmol), and WSC (0.04 g, 0.18 mmol) were added. The reaction mixture was stirred for 3 h at 0 °C and 24 h at room temperature. After DMF was evaporated, the residue was dissolved in EtOAc and washed with citric acid (10% in H2O), NaHCO3 (5% in H2O), and brine. The organic phase was dried (Na2SO4) and evaporated to dryness. The residue was precipitated from Et2O/Pe (1:9, v/v): yield 0.09 g (88%); Rf(B) 0.67; HPLC K′ 8.59; mp 105–107 °C; [α]20D −3.2; MH+ 616; 1H-NMR (DMSO-d6) δ 1.40 (s, 9H), 2.35 (s, 6H), 2.92–3.17 (m, 4H), 4.09–4.48 (m, 6H), 4.92 (m, 2H), 6.29 (s, 2H), 6.96–7.14 (m, 9H).
TFA·H-Dmt-Aba-Gly-NH-CH2-Ph (1)
Boc-Dmt-Aba-Gly-NH-CH2-Ph (0.09 g, 0.15 mmol) was treated with TFA (1 mL) for 0.5 h at room temperature. Et2O/Pe (1:1, v/v) were added to the solution until the product precipitated: yield 0.09 g (96%); Rf(A) 0.52; HPLC K′ 6.79; mp 120–122 °C; [α]20D −4.8; MH+ 516; 1H-NMR (DMSO-d6) δ 2.35 (s, 6H), 2.92–3.17 (m, 4H), 3.95–4.48 (m, 7H), 4.92 (m, 1H), 6.29 (s, 2H), 6.96–7.14 (m, 9H).
Boc-Aba-Gly-NH-Ph
This compound was obtained by condensation of Boc-Aba-Gly-OH 24 with aniline via WSC/HOBt as reported for Boc-Aba-Gly-NH-CH2-Ph: yield 0.1 g (90%); Rf(B) 0.61; HPLC K′ 8.35; mp 75–77 °C; [α]20D +4.9; MH+ 410; 1H-NMR (DMSO-d6) δ 1.40 (m, 9H), 2.92–3.17 (m, 2H), 4.14–4.48 (m, 4H), 4.92 (m, 1H), 6.96–7.64 (m, 9H).
TFA·H-Aba-Gly-NH-Ph
Boc-Aba-Gly-NH-Ph was treated with TFA as reported for TFA·H-Aba-Gly-NH-CH2-Ph: yield 0.09 g (97%); Rf(A) 0.37; HPLC K′ 5.36; mp 98–100 °C; [α]20D +6.4; MH+ 310.
Boc-Dmt-Aba-Gly-NH-Ph
This compound was obtained by condensation of Boc-Dmt-OH with TFA·H-Aba-Gly-NH-Ph via WSC/HOBt as reported for Boc-Dmt-Aba-Gly-NH-CH2-Ph: yield 0.08 g (89%); Rf(B) 0.59; HPLC K′ 8.21; mp 111–113 °C; [α]20D −4.8; MH+ 602; 1H-NMR (DMSO-d6) δ 1.40 (s, 9H), 2.35 (s, 6H), 2.92–3.17 (m, 4H), 4.14–4.48 (m, 4H), 4.92 (m, 2H), 6.29 (s, 2H), 6.96–7.64 (m, 9H).
TFA·H-Dmt-Aba-Gly-NH-Ph (2)
Boc-Dmt-Aba-Gly-NH-Ph was treated with TFA as reported for TFA·H-Dmt-Aba-Gly-NH-CH2-Ph: yield 0.07 g (95%); Rf(A) 0.46; HPLC K′ 6.33; mp 126–128 °C; [α]20D −5.3; MH+ 502; 1H-NMR (DMSO-d6) δ 2.35 (s, 6H), 2.92–3.17 (m, 4H), 3.95–4.48 (m, 5H), 4.92 (m, 1H), 6.29 (s, 2H), 6.96–7.64 (m, 9H).
tert-butyl 2-((1H-benzo[d]imidazol-2-yl)methyl)-2,3,4,5-tetrahydro-3-oxo-1H-benzo[c]azepin-4-ylcarbamate [Boc-Aba-Gly*-Bid]
A solution of Boc-Aba-Gly-OH 24 (0.1 g, 0.3 mmol) and NMM (0.03 mL, 0.3 mmol) in DMF (10 mL) was treated at −20 °C with IBCF, (0.04 mL, 0.3 mmol). After 10 min at −20 °C, o-phenylendiamine (0.03 g, 0.3 mmol) was added. The reaction mixture was allowed to stir while slowly warming to room temperature (1 h) and was then stirred for 3 h. The solvent was evaporated, and the residue was partitioned between EtOAc and H2O. The EtOAc layer was washed with 5% NaHCO3 and brine and dried over Na2SO4. The solution was filtered, the solvent was evaporated, and the residual solid was dissolved in glacial AcOH (10 mL). The solution was heated at 65 °C for 1 h. After the solvent was evaporated, the residue was crystallized from Et2O/Pe (1:9, v/v): yield 0.1 g (82%); Rf(B) 0.51; HPLC K′ 7.23; mp 85–87 °C; [α]20D +6.4; MH+ 407; 1H-NMR (DMSO-d6) δ 1.40 (m, 9H), 2.92–3.17 (m, 2H), 4.45–4.48 (m, 4H), 4.92 (m, 1H), 6.96–7.70 (m, 8H).
2TFA·H-Aba-Gly*-Bid
Boc-Aba-Gly*-Bid was treated with TFA as reported for TFA·H-Aba-Gly-NH-CH2-Ph: yield 0.09 g (96%); Rf(A) 0.28; HPLC K′ 4.40; mp 102–104 °C; [α]20D +6.9; MH+ 307.
Boc-Dmt-Aba-Gly*-Bid
To a solution of Boc-Dmt-OH (0.12 g, 0.4 mmol) and 2TFA·H-Aba-Gly*-Bid (0.21 g, 0.4 mmol) in DMF (10 mL) at 0 °C, NMM (0.09 mL, 0.8 mmol), HOBt (0.07 g, 0.44 mmol), and WSC (0.08 g, 0.44 mmol) were added. The reaction mixture was stirred for 3 h at 0 °C and 24 h at room temperature. After DMF was evaporated, the residue was dissolved in EtOAc and washed with NaHCO3 (5% in H2O), and brine. The organic phase was dried (Na2SO4) and evaporated to dryness. The residue was precipitated from Et2O/Pe (1:9, v/v): yield 0.21 g (88%); Rf(B) 0.46; HPLC K′ 6.98; mp 115–117 °C; [α]20D+4.9; MH+ 599; 1H-NMR (DMSO-d6) δ 1.40 (m, 9H), 2.35 (s, 6H), 2.92–3.17 (m, 4H), 4.45–4.48 (m, 4H), 4.92 (m, 2H), 6.29 (s, 2H), 6.96–7.70 (m, 8H).
2TFA·H-Dmt-Aba-Gly*-Bid (3)
Boc-Dmt-Aba-Gly*-Bid was treated with TFA as reported for TFA·H-Dmt-Aba-Gly-NH-CH2-Ph: yield 0.15 g (90%); Rf(B) 0.46; HPLC K′ 4.95; mp 134–136 °C; [α]20D +5.5; MH+ 499; 1H-NMR (DMSO-d6) δ 2.35 (s, 6H), 2.92–3.17 (m, 4H), 3.95 (m, 1H), 4.45–4.48 (m, 4H), 4.92 (m, 1H), 6.29 (s, 2H), 6.96–7.70 (m, 8H).
Competitive Receptor Binding Assays
These assays were conducted as described in detail elsewhere using rat brain synaptosomes (P2 fraction).3, 12 45, 46 Membrane preparations were preincubated to eliminate endogenous opioid peptides and stored at −80 °C in buffered 20% glycerol.46, 47 Each analogue was analyzed in duplicate using five to eight dosages of peptide and independent repetitions with different synaptosomal preparations (n values are listed in Table 1 in parentheses and the results are listed as the mean ± SE). Unlabeled peptide (2 μM) was used to determine non-specific binding in the presence of 1.9 nM [3H]deltorphin C (45.0 Ci/mmol, Perkin-Elmer, Boston, MA; KD = 1.4 nM) for δ-opioid receptors, and for μ-opioid receptors, 3.5 nM [3H]DAMGO (50.0 Ci/mmol, Amersham Biosciences, Buckinghamshire, U.K.; KD = 1.5 nM). Glass fiber filters (Whatman GFC) were soaked in 0.1% polyethylenimine to enhance the signal/noise ratio of the bound radiolabeled-synaptosome complex, and the filters washed thrice in ice-cold buffered BSA.46 The affinity constants (Ki) were calculated according to Cheng and Prusoff.48
Functional Bioactivity in Isolated Organ Preparations
Preparations of myenteric plexus-longitudinal muscle obtained from male guinea pig ileum (GPI, enriched in μ-opioid receptors) and preparations of MVD (containing δ-opioid receptors) were used for field stimulation with bipolar rectangular pulses of supramaximal voltage.49 Agonists were evaluated for their ability to inhibit the electrically evoked twitch. The biological potency of the compounds was comparedagainst the activity of the μ-opioid receptor agonist dermorphin in GPI and with MVD for the δ-opioid receptor measured agonist deltorphin C. The results are expressed as the IC50 obtained from dose-response curves (Prism, GraphPad). To evaluate antagonism, compounds were added to the bath and allowed to interact with tissue receptor sites 5 min before adding the standard peptide. Competitive antagonist activities were evaluated for their ability to shift the deltorphin C (MVD) and dermorphin (GPI) log concentration-response curve to the right; pA2 values were determined using the Schild Plot.50 IC50 (nM, mean ± SE) as well as the pA2 were obtained from at least six experiments conducted with fresh tissues.
Supplementary Material
This material is available free of charge via the Internet at: http://pubs.acs.org.
Acknowledgments
This research was supported in part by the University of Cagliari (PRIN 2004), University of Ferrara, Italy, and the Intramural Research Program of the NIH, and NIEHS. The authors appreciate the professional services of the library staff of the NIEHS.
References
- 1.Abbreviations. In addition to the IUPAC-IUB Commission on Biochemical Nomenclature (J. Biol. Chem. 1985, 260, 14–42), this paper uses the following additional symbols and abbreviations: H-Aba-Gly-OH, 2-(4-amino-4,5-dihydro-3-oxo-1H-benzo[c]azepin-2(3H)-yl)acetic acid; Aba-Gly*-Bid, 2-((1H-benzo[d]imidazol-2-yl)methyl)-4-amino-1,2,4,5-tetrahydrobenzo[c]azepin-3-one; Ac, acetyl; Asp*-Bid, -NH-CH(CH2-COOH)-1H-benzimidazole-2-yl; Bid, 1H-benzimidazol-2-yl; Boc, tert-butyloxycarbonyl; DAMGO, [D-Ala2, N-Me-Phe4, Gly-ol5] enkephalin; DEL C, deltorphin C, (H-Tyr-D-Ala-Phe-Asp-Val-Val-Gly-NH2); DER, dermorphin (H-Tyr-D-Ala-Phe-Gly-Tyr-Pro-Ser-NH2); DMF, N, N-dimethylformamide; DMSO-d6, hexadeuteriodimethyl sulfoxide; Dmt, 2′,6′-dimethyl-L-tyrosine; Gly*-Bid, -NH-CH2-1H-benzimidazole-2-yl; GPI, guinea-pig ileum; HOBt, 1-hydroxybenzotriazole; HPLC, high performance liquid chromatography; MVD, mouse vas deferens; pA2, negative log of the molar concentration required to double the agonist concentration to achieve the original response; TFA, trifluoroacetic acid; Tic, 1,2,3,4-tetrahydroisoquinoline-3-carboxylic acid; TIP(P), H.-Tyr-Tic-Phe-(Phe)-OH; TLC, thin-layer chromatography; WSC, 1-ethyl-3-[3′-dimethyl)aminopropyl]-carbodiimide hydrochloride; Z, benzyloxycarbonyl; NMM, 4-methylmorpholine; MALDI-TOF, matrix assisted laser desorption ionization time-of-flight.
- 2.Salvadori S, Attila M, Balboni G, Bianchi C, Bryant SD, Crescenzi O, Guerrini R, Picone D, Tancredi T, Temussi PA, Lazarus LH. δ Opioidmimetic antagonists: prototypes for designing a new generation of ultraselective opioid peptides. Mol Med. 1995;1:678–689. [PMC free article] [PubMed] [Google Scholar]
- 3.Salvadori S, Guerrini R, Balboni G, Bianchi C, Bryant SD, Cooper PS, Lazarus LH. Further studies on the Dmt-Tic pharmacophore: hydrophobic substituents at the C-terminus endow δ antagonists to manifest μ agonism or δ antagonism. J Med Chem. 1999;42:5010–5019. doi: 10.1021/jm990165m. [DOI] [PubMed] [Google Scholar]
- 4.Temussi PA, Salvadori S, Amodeo P, Bianchi C, Guerrini R, Tomatis R, Lazarus LH, Tancredi T. Selective opioid dipeptides. Biochem Biophys Res Commun. 1994;198:933–939. doi: 10.1006/bbrc.1994.1133. [DOI] [PubMed] [Google Scholar]
- 5.Schiller PW, Nguyen TMD, Weltrowska G, Wilkes BC, Marsden BJ, Lemieux C, Chung NN. Differential stereochemical requirements of μ vs δ opioid receptors for ligand binding and signal transduction: development of a class of potent and highly δ-selective peptide antagonists. Proc Natl Acad Sci US A. 1992;89:11871–11875. doi: 10.1073/pnas.89.24.11871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lazarus LH, Bryant SD, Cooper PS, Guerrini R, Balboni G, Salvadori S. Design of δ-opioid peptide antagonists for emerging drug applications. Drug Discovery Today. 1998;3:284–294. [Google Scholar]
- 7.Bryant SD, Jinsmaa Y, Salvadori S, Okada Y, Lazarus LH. Dmt and opioid peptides: A potent alliance. Biopolymers (Pept Sci) 2003;71:86–102. doi: 10.1002/bip.10399. [DOI] [PubMed] [Google Scholar]
- 8.Salvadori S, Balboni G, Guerrini R, Tomatis R, Bianchi C, Bryant SD, Cooper PS, Lazarus LH. Evolution of the Dmt-Tic pharmacophore: N-Terminal methylated derivatives with extraordinary δ opioid antagonist activity. J Med Chem. 1997;40:3100–3108. doi: 10.1021/jm9607663. [DOI] [PubMed] [Google Scholar]
- 9.Balboni G, Salvadori S, Guerrini R, Bianchi C, Santagata V, Calliendo G, Bryant SD, Lazarus LH. Opioid pseudopeptides containing heteroaromatic or heteroaliphatic nuclei. Peptides. 2000;21:1663–1671. doi: 10.1016/s0196-9781(00)00315-6. [DOI] [PubMed] [Google Scholar]
- 10.Capasso A, Amodeo P, Balboni G, Guerrini R, Lazarus LH, Temussi PA, Salvadori S. Design of μ selective opioid dipeptide antagonists. FEBS Lett. 1997;417:141–144. doi: 10.1016/s0014-5793(97)01271-4. [DOI] [PubMed] [Google Scholar]
- 11.Balboni G, Guerrini R, Salvadori S, Bianchi C, Rizzi D, Bryant SD, Lazarus LH. Evaluation of the Dmt-Tic pharmacophore: Conversion of a potent δ-opioid receptor antagonist into a potent δ-agonist and ligands with mixed properties. J Med Chem. 2002;45:713–720. doi: 10.1021/jm010449i. [DOI] [PubMed] [Google Scholar]
- 12.Balboni G, Salvadori S, Guerrini R, Negri L, Giannini E, Bryant SD, Jinsmaa Y, Lazarus LH. Synthesis and opioid activity of N, N-dimethyl-Dmt-Tic-NH-CH(R)-R′ analogues: Acquisition of potent ä antagonism. Bioorg Med Chem. 2003;11:5435–5441. doi: 10.1016/j.bmc.2003.09.039. [DOI] [PubMed] [Google Scholar]
- 13.Balboni G, Salvadori S, Dal Piaz A, Bortolotti F, Argazzi R, Negri L, Lattanzi R, Bryant SD, Jinsmaa Y, Lazarus LH. Highly selective fluorescent analogue of the potent δ-opioid receptor antagonist Dmt-Tic. J Med Chem. 2004;47:6541–6546. doi: 10.1021/jm040128h. [DOI] [PubMed] [Google Scholar]
- 14.Balboni G, Guerrini R, Salvadori S, Negri L, Giannini E, Bryant SD, Jinsmaa Y, Lazarus LH. Conversion of the potent δ-opioid agonist H-Dmt-Tic-NH-CH2-Bid into δ-opioid antagonists by N1-benzimidazole alkylation. J Med Chem. 2005;48:8112–8114. doi: 10.1021/jm058259l. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pagé D, Nguyen N, Bernard S, Coupal M, Gosselin M, Lepage J, Adam L, Brown W. New scaffolds in the development of mu opioid-receptor ligands. Bioorg Med Chem Lett. 2003;13:1585–1589. doi: 10.1016/s0960-894x(03)00194-x. [DOI] [PubMed] [Google Scholar]
- 16.Van den Eynde I, Laus G, Schiller PW, Kosson P, Chung NN, Lipkowski AW, Tourwé D. A new stuctural motif for μ-opioid antagonists. J Med Chem. 2005;48:3644–3648. doi: 10.1021/jm0491795. [DOI] [PubMed] [Google Scholar]
- 17.Schiller PW. Opioid peptide-derived analgesics. AAPS Journal. 2005;7:E560–E565. doi: 10.1208/aapsj070356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Marsden BJ, Nguyen TMD, Schiller PW. Spontaneous degradation via diketopiperazine formation of peptides containing a tetrahydroiosquinoline-3-carboxylic acid residue in the 2-position of the peptide sequence. Int J Pept Protein Res. 1993;41:313–316. doi: 10.1111/j.1399-3011.1993.tb00340.x. [DOI] [PubMed] [Google Scholar]
- 19.Mazur RH, Schlatter JM. A novel peptide cleavage reaction. J Org Chem. 1963;28:1025–1029. [Google Scholar]
- 20.Wagner FW, Kapleau BR, Shepherd SL. Amino acid composition and sequence of urinary peptides containing hydroxyproline. Biochem Med. 1975;13:343–352. doi: 10.1016/0006-2944(75)90173-8. [DOI] [PubMed] [Google Scholar]
- 21.Capasso S, Sica F, Mazzarella L, Balboni G, Guerrini R, Salvadori S. Acid catalysis in the formation of dioxopiperazines from peptides containing tetrahydroisoquinoline-3-carboxylic acid at position 2. Int J Pept Protein Res. 1995;45:567–573. doi: 10.1111/j.1399-3011.1995.tb01321.x. [DOI] [PubMed] [Google Scholar]
- 22.Balboni G, Guerrini R, Salvadori S, Tomatis R, Bryant SD, Bianchi C, Attila M, Lazarus LH. Opioid diketopiperazines: Synthesis and activity of a unique class of δ opioid antagonists. Biol Chem Hoppe Seyler. 1997;378:19–29. doi: 10.1515/bchm.1997.378.1.19. [DOI] [PubMed] [Google Scholar]
- 23.Schiller PW, Weltrowska G, Nguyen TMD, Wilkes BC, Chung NN, Lemieux C. TIPP[Ψ]: A highly potent and stable pseudopeptide δ opioid receptor antagonist with extraordinary δ selectivity. J Med Chem. 1993;36:3182–3187. doi: 10.1021/jm00073a020. [DOI] [PubMed] [Google Scholar]
- 24.Tourwé D, Verschueren K, Frycia A, Davis P, Porreca F, Hruby VJ, Toth G, Jaspers H, Verheyden P, Van Binst G. Conformational restriction of Tyr and Phe side chains in opioid peptides: information about preferred and bioactive side-chain topology. Biopolymers. 1995;38:1–12. doi: 10.1002/(sici)1097-0282(199601)38:1<1::aid-bip1>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- 25.Ballet S, Frycia A, Piron J, Chung NN, Schiller PW, Kosson P, Lipkowski AW, Tourwé D. Synthesis and biological evaluation of constrained analogues of the opioid peptide H-Tyr-D-Ala-Phe-Gly-NH2 using the 4-amino-2-benzazepin-3-one scaffold. J Peptide Res. 2005;66:222–230. doi: 10.1111/j.1399-3011.2005.00291.x. [DOI] [PubMed] [Google Scholar]
- 26.Mollica A, Davis P, Ma SW, Porreca F, Lai J, Hruby VJ. Synthesis and biological activity of the first cyclic biphalin analogues. Bioorg Med Chem Lett. 2006;16:367–372. doi: 10.1016/j.bmcl.2005.09.080. [DOI] [PubMed] [Google Scholar]
- 27.Schiller PW, Fundytus ME, Merovitz L, Weltrowska G, Nguyen TMD, Lemieux C, Chung NN, Coderre TJ. The opioid μ agonist/δ antagonist DIPP-NH2[Ψ] produces a potent analgesic effect, no physical dependence and less tolerance than morphine in rats. J Med Chem. 1999;42:33520–3526. doi: 10.1021/jm980724+. [DOI] [PubMed] [Google Scholar]
- 28.Ananthan S, Khare NK, Saini SK, Seitz LE, Bartlett JL, Davis P, Dersch CM, Porreca F, Rothman RB, Bilsky EJ. Identification of opioid ligands possessing mixed μ agonist/δ antagonist activity among pyridomorphinans derived from naloxone, oxymorphone and hydromorphone. J Med Chem. 2004;47:1400–1412. doi: 10.1021/jm030311v. [DOI] [PubMed] [Google Scholar]
- 29.Varga EV, Navratilova E, Stropova D, Jambrosic J, Roeske WR, Yamamura HI. Agonist-specific regulation of the δ-opioid receptor. Life Science. 2004;76:599–612. doi: 10.1016/j.lfs.2004.07.020. [DOI] [PubMed] [Google Scholar]
- 30.Torregrossa MM, Jutkiewicz EM, Mosberg HI, Balboni G, Watson SJ, Woods JH. Peptidic delta opioid receptor agonists produce antidepressant-like effects in the forced swim test and regulate BDNF mRNA expression in rats. Brain Res. 2006;1069:172–181. doi: 10.1016/j.brainres.2005.11.005. and references cited herein. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Saitoh A, Kimura Y, Suzuki T, Kawai K, Nagase H, Kamei J. Potential anxiolytic and antidepressant-like activities of SNC80, a selective delta-opioid agonist, in behavioral models in rodents. J Pharmaco l Sci. 2004;95:374–380. doi: 10.1254/jphs.fpj04014x. [DOI] [PubMed] [Google Scholar]
- 32.Aharoni R, Eilam R, Domev H, Labunskay G, Sela M, Arnon R. The immunomodulator glatiramer acetate augments the expression of neurotrophic factors in brains of experimental autoimmune encephalomyelitis mice. Proc Natl Acad Sci U S A. 2005;102:19045–19050. doi: 10.1073/pnas.0509438102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhang J, Gibney GT, Zhao P, Xia Y. Neuroprotective role of delta-opioid receptors in cortical neurons. Am J Physiol Cell Physiol. 2002;282:C1225–1234. doi: 10.1152/ajpcell.00226.2001. [DOI] [PubMed] [Google Scholar]
- 34.Borlongan CV, Wang Y, Su TP. Delta opioid peptide (D-Ala2, D-Leu5) enkephalin: linking hibernation and neuroprotection. Front Biosci. 2004;9:3392–3398. doi: 10.2741/1490. [DOI] [PubMed] [Google Scholar]
- 35.Vecchio L, Soldani C, Bottone MG, Malatesta M, Martin TE, Rothblum LI, Pellicciari C, Biggiogera M. DADLE induces a reversible hibernation-like state in HeLa cells. Histochem Cell Biol. 2006;125:193–201. doi: 10.1007/s00418-005-0085-x. [DOI] [PubMed] [Google Scholar]
- 36.Peart JN, Gross GJ. Exogenous activation of delta- and kappa-opioid receptors affords cardioprotection in isolated murine heart. Basic Res Cardiol. 2004;99:29–37. doi: 10.1007/s00395-003-0430-y. [DOI] [PubMed] [Google Scholar]
- 37.Zadina JE, Hackler L, Ge LJ, Kastin AJ. A potent and selective endogenous agonist for the mu opiate receptor. Nature. 1997;386:499–502. doi: 10.1038/386499a0. [DOI] [PubMed] [Google Scholar]
- 38.Thomas KM, Naduthambi D, Zondlo NJ. Electronic control of amide cis-trans isomerism via the aromatic-prolyl interaction. J Am Chem Soc. 2006;128:2216–2217. doi: 10.1021/ja057901y. [DOI] [PubMed] [Google Scholar]
- 39.Li T, Fujita Y, Tsuda Y, Miyazaki A, Ambo A, Sasaki Y, Jinsmaa Y, Bryant SD, Lazarus LH, Okada Y. Development of potent μ-opioid receptor ligands using tyrosine analogues of endomorphin-2. J Med Chem. 2005;48:586–592. doi: 10.1021/jm049384k. [DOI] [PubMed] [Google Scholar]
- 40.Okada Y, Fujita Y, Motoyama T, Tsuda Y, Yokoi T, Li T, Sasaki Y, Ambo A, Jinsmaa Y, Bryant SD, Lazarus LH. Structural studies of [2′,6′-dimethyl-L-tyrosine1]endomorphin-2 analogues: enhanced activity and cis orientation of the Dmt-Pro amide bond. Bioorg Med Chem. 2003;11:1983–1994. doi: 10.1016/s0968-0896(03)00068-3. [DOI] [PubMed] [Google Scholar]
- 41.Santagada V, Balboni G, Caliendo G, Guerrini R, Salvadori S, Bianchi C, Bryant SD, Lazarus LH. Assesment of substitution in the second pharmacophore of Dmt-Tic analogues. Bioorg Med Chem Lett. 2000;10:2745–2748. doi: 10.1016/s0960-894x(00)00569-2. [DOI] [PubMed] [Google Scholar]
- 42.Pagé D, Naismith A, Schmidt R, Coupal M, Labarre M, Gosselin M, Bellemare D, Payza K, Brown W. Novel C-terminus modifications of the Dmt-Tic motif: a new class of dipeptide analogues showing altered pharmacological profiles toward the opioid receptors. J Med Chem. 2001;44:2387–2390. doi: 10.1021/jm015532k. [DOI] [PubMed] [Google Scholar]
- 43.Fujita Y, Tsuda Y, Motoyama T, Li T, Miyazaki A, Yokoi T, Sasaki Y, Ambo A, Niizuma H, Jinsmaa Y, Bryant SD, Lazarus LH, Okada Y. Studies on the structure-activity relationship of 2′,6′-dimethyl-L-tyrosine (Dmt) derivatives: bioactivity profile of H-Dmt-NH-CH3. Bioorg Med Chem Lett. 2005;15:599–602. doi: 10.1016/j.bmcl.2004.11.040. [DOI] [PubMed] [Google Scholar]
- 44.Van Rompaey K, Van den Eynde I, De Kimpe N, Tourwé D. A versatile synthesis of 2-substituted 4-amino-1,2,4,5-tetrahydro-2-benzazepine-3-ones. Tetrahedron. 2003;59:4421–4432. [Google Scholar]
- 45.Balboni G, Salvadori S, Guerrini R, Negri L, Giannini E, Jinsmaa Y, Bryant SD, Lazarus LH. Potent δ-opioid receptor agonists containing the Dmt-Tic pharmacophore. J Med Chem. 2002;45:5556–5563. doi: 10.1021/jm020336e. [DOI] [PubMed] [Google Scholar]
- 46.Lazarus LH, Salvadori S, Santagada V, Tomatis R, Wilson WE. Function of negative charge in the “address domain” of deltorphins. J Med Chem. 1991;34:1350–1359. doi: 10.1021/jm00108a017. [DOI] [PubMed] [Google Scholar]
- 47.Lazarus LH, Wilson WE, De Castiglione R, Guglietta A. Dermorphin gene sequence peptide with high affinity and selectivity for δ-opioid receptors. J Biol Chem. 1989;264:3047–3050. [PubMed] [Google Scholar]
- 48.Cheng YC, Prusoff WH. Relationships between the inhibition constant (Ki) and the concentration of inhibition which cause 50% inhibition (I50) of an enzymatic reaction. Biochem Pharmacol. 1973;22:3099–3108. doi: 10.1016/0006-2952(73)90196-2. [DOI] [PubMed] [Google Scholar]
- 49.Melchiorri P, Negri L, Falconieri-Erspamer G, Severini C, Corsi R, Soaje M, Erspamer V, Barra D. Structure-activity relationships of the δ-opioid-selective agonists, deltorphins. Eur J Pharmacol. 1991;195:201–207. doi: 10.1016/0014-2999(91)90536-y. [DOI] [PubMed] [Google Scholar]
- 50.Tallarida RJ, Murray RB. Manual of Pharmacological Calculation. 2. Springer-Verlag; New York: 1986. [Google Scholar]
- 51.Kosterlitz HW, Watt AJ. Kinetic parameters of narcotic agonists and antagonists, with particular reference to N-allylnoroxymorphone (naloxone) Br J Pharmacol. 1968;33:266–276. doi: 10.1111/j.1476-5381.1968.tb00988.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
This material is available free of charge via the Internet at: http://pubs.acs.org.