Abstract
Certain disease states are characterized by disturbances in protein production, accumulation, or clearance. In the central nervous system (CNS), alterations in metabolism of proteins such as amyloid-beta (Aβ), alpha-synuclein, or tau may cause degenerative diseases such as Alzheimer’s disease (AD), Parkinson’s disease or fronto-temporal dementias respectively. In AD, dysregulation of Aβ metabolism is indicated by a massive buildup of this protein in the brains of those with AD. In rare, autosomal dominant forms of AD, mutations in the amyloid precursor protein or in components of the enzymes which produce Aβ (presenilin-1 and presenilin-2) appear to result in Aβ overproduction. However, whether dysregulation of Aβ metabolism (increased synthesis or clearance) causes the most common form of AD (sporadic >99%) is not known. Furthermore, there has not been a method available which could determine the synthesis or clearance rate of Aβ or any other protein produced in the CNS. This report describes a method to determine the production rate and clearance rate of proteins produced by the CNS in vivo in humans. We report the first measurements of the fractional production and clearance rates of Aβ in vivo in the human CNS to be 7.6%/hr and 8.2%/hr respectively. This method may be used to search for novel biomarkers of disease, assess underlying differences in protein metabolism that contribute to disease, and to evaluate treatments in terms of their pharmacodynamic properties on proposed disease causing pathways.
INTRODUCTION
Protein production and clearance
Protein production and clearance are important parameters that are tightly regulated and reflect normal physiology as well as disease states1–6. Previous studies of protein metabolism in humans have focused on whole body or peripheral body proteins, but not on proteins produced in the central nervous system (CNS). A technique to measure specific protein metabolism in the CNS could provide important insights in CNS protein physiology in health and disease. Certain disease states are characterized by disturbances in protein production, accumulation, or clearance. In the CNS, disturbances in metabolism of proteins such as the prion protein7, alpha-synuclein8, tau9, or amyloid-beta10 (Aβ), can contribute to, and in some cases, cause neurodegenerative diseases such as Creuzfeldt–Jakob disease, Parkinson’s disease, frontotemporal dementia, or Alzheimer’s disease (AD) respectively.
Aβ in AD
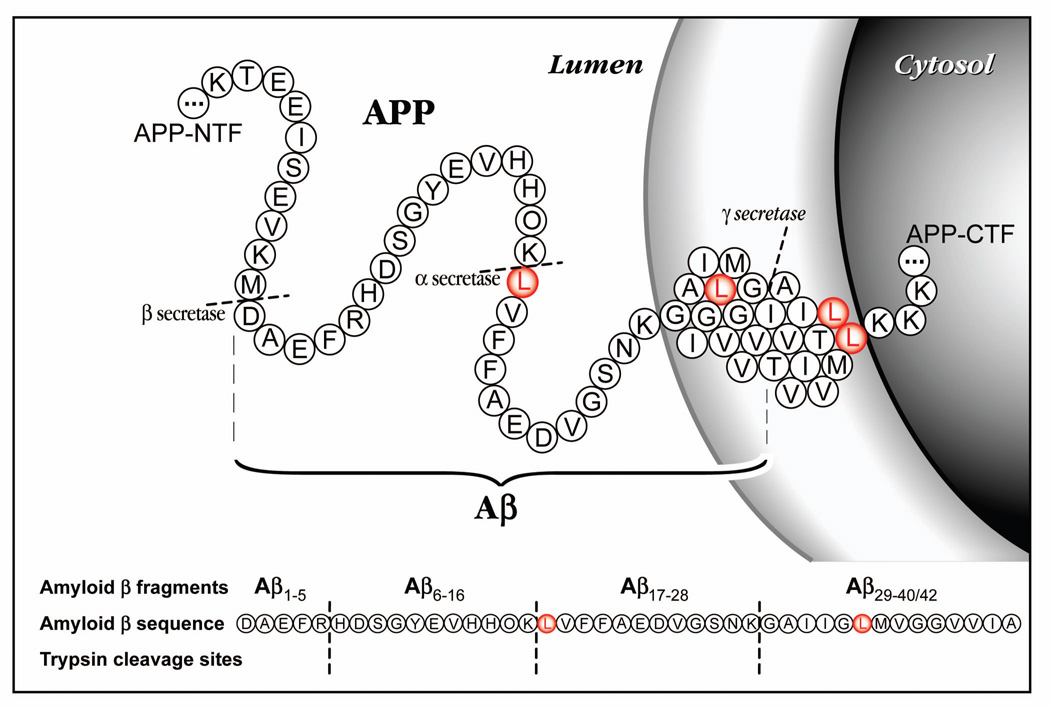
Biochemical, genetic, and animal model evidence implicates Aβ as a pathogenic peptide in AD. The neuropathologic and neurochemical hallmarks of AD include synaptic loss and selective neuronal death, a decrease in certain neurotransmitters, and the presence of abnormal proteinaceous deposits in neurons (neurofibrillary tangles), in the cerebral vasculature (amyloid angiopathy), and in the extracellular space (diffuse and neuritic plaques). The main constituent of plaques is Aβ, a 38–43 amino acid peptide cleaved from the amyloid precursor protein (APP)11,12 (fig. 1). Throughout life, soluble Aβ is secreted mostly by neurons, but also other cell types. In late-onset AD, the total amount of Aβ that accumulates in brain is ~100–200-fold higher in AD brain homogenates versus control brains13,14. Disturbance of Aβ production can lead to rare forms of AD in humans. Mutations in 3 different genes (APP, PS-1 and PS-2) which cause early-onset autosomal dominant AD, all result in overproduction of total Aβ or Aβ4211. In Down’s syndrome, three copies of APP result in increased Aβ production and 100% of these individuals develop AD pathology by age 3515. However, in late-onset AD (~99% of AD cases), there is not strong evidence for Aβ over-production. Therefore, the underlying cause for Aβ deposition (increased production vs. decreased clearance) is not known in the vast majority of AD.
Figure 1.
The amino acid sequence of Aβ is depicted in the amyloid precursor protein (APP) in the cell with the leucines (L) labeled in red to indicate possible labeling sites. The sequence of Aβ is shown below with the trypsin digest sites indicated to demonstrate the fragments that were analyzed by mass spectrometry.
Measurement of Aβ synthesis and clearance in humans
No methods were previously available to quantify protein synthesis or clearance rates in the CNS of humans. Such a method would be valuable to assess not only Aβ synthesis or clearance rates in humans but also the metabolism of a variety other proteins relevant to diseases of the CNS. In order to address critical questions about underlying AD pathogenesis and Aβ metabolism, we developed a method for quantifying Aβ fractional synthesis rate (FSR) and fractional clearance rate (FCR) in vivo in the CNS of humans. The following results indicate that by administering a stable isotope labeled amino acid (13C6-leucine), sampling cerebrospinal fluid (CSF), and using high-resolution tandem mass spectrometry to quantify labeled Aβ, reproducible rates of Aβ synthesis and clearance can be quantified in humans.
METHODS
Participants and Sampling
All human studies were approved by the Washington University Human Studies Committee and the General Clinical Research Center (GCRC) Advisory Committee. Informed consent was obtained from all participants. All participants were screened to be in good general health and without neurologic disease. Seven men and three women (23–45 yrs old) participated. Each research participant was admitted to the GCRC at 7:00 AM after an overnight fast from 8PM the preceding evening. The GCRC Research Kitchen provided meals (60% carbohydrate, 20% fat, 20% protein, low leucine diet during labeled leucine infusion) at 9AM, 1PM, and 6PM and the participant had free access to water. All food and water consumption was recorded during the admission by nursing staff and the GCRC kitchen. One intravenous catheter was placed in an antecubital vein and used to administer the stable isotope labeled leucine solution. A second intravenous catheter was placed in the contra-lateral antecubital vein to obtain blood samples. A subarachnoid catheter was inserted at the L3–L4 interspace via a Touhy needle, so that CSF could be sampled without performing multiple lumbar punctures16. The intravenous catheters were placed by trained registered nurses and the lumbar catheter was placed by trained physicians with extensive experience in lumbar puncture. Blood samples were obtained hourly except from 11PM to 7AM, when they were obtained every other hour. CSF samples were obtained hourly throughout the study. The participants were encouraged to stay in bed except to use the restroom.
In Vivo labeling
13C6-leucine was dissolved in medical grade normal saline and then filtered through a 0.22 micron filter the day before each study. The labeled leucine was bolused and infused intravenously using a medical IV pump at a rate of 1.8, 2.0, or 2.5 mg/kg/hr.
Materials
13C6-leucine was obtained from Cambridge Isotope Laboratories, Inc. (Andover, MA). M266 antibody was generously provided by Eli Lilly17. CNBr-activated sepharose 4B beads were obtained from Amersham Biosciences. Formic acid (98%) and ammonium bicarbonate (ultra >99.5%) were obtained from Fluka. Sequence Grade Modified Trypsin was obtained from Promega (Madison, WI).
Amyloid-β immunoprecipitation
Antibody beads were prepared by covalently binding m266 antibody to CNBr sepharose beads per the manufacturers protocol at a concentration of 10 mg/ml m266 antibody. The antibody beads were stored at 4°C in a 50% slurry of PBS 0.02% azide. The immunoprecipitation mixture was 250 µl of 5x RIPA, 12.5 µl of 100x protease inhibitors, and 30 µl of antibody-bead slurry added to 1 ml of sample in an eppendorf tube which was rotated overnight at 4°C. The beads were rinsed once with 1x RIPA and twice with 25 mM ammonium bicarbonate. They were aspirated dry after the final rinse and Aβ was eluted off the antibody-bead complex using 30 µl of pure formic acid. After centrifuging the beads again, the formic acid supernatant was transferred to a new eppendorf tube and speed-vacuum dried. The formic acid was evaporated in a Savant speed-vac (model AES2010) for 15 minutes at low rate (ambient temperature) with radiant cover and full vacuum, followed by 30 minutes at medium rate (43°C) with radiant cover and full vacuum. The sample was reconstituted in 5µl of acetonitrile and 20µl of 25 mM ammonium bicarbonate, pH at 8.0. The sample was digested with 400 ng sequence grade trypsin and incubated at 37 °C for 16 hours.
Liquid Chromatography/Mass Spectrometry
A Waters (Milford, MA) capillary liquid chromatography system with auto injector was interfaced to a Thermo-Finnigan (San Jose, CA) LCQ-DECA equipped with an electrospray ionization source (LC-ESI-tandem MS). A 5µl aliquot of each sample was injected onto a Vydac C-18 capillary column (0.3 × 150mm MS 5µm). In positive-ion scanning mode, LC-ESI-MS analysis of trypsin-digested synthetic and immunoprecipitated Aβ yielded the expected parent ions at masses 1325.2 for Aβ17–28 and 1331.2 for 13C6-leucine labeled Aβ17–28. To obtain amino acid sequence and abundance data, these parent ions were subjected to collision induced dissociation (CID 28%), and tandem MS analysis of their doubly-charged species ([M+2H]+2; m/z 663.6 and 666.6) were scanned in selected reaction monitoring mode (SRM), so that the y- and b-series ions generated were used for isotope ratio quantitation.
Calculation of labeled ratio
Percent labeled Aβ was calculated as the ratio of all labeled MS/MS ions from Aβ17–28 divided by all unlabeled MS/MS ions from Aβ17–28. A custom Microsoft Excel spreadsheet with macros was used to calculate the ratio. A detailed description of the MS/MS quantification of labeled versus unlabeled peptides is under preparation (R. Bateman, D.M. Holtzman, K.E. Yarasheski).
RESULTS
In Vivo labeling and quantitation of Aβ
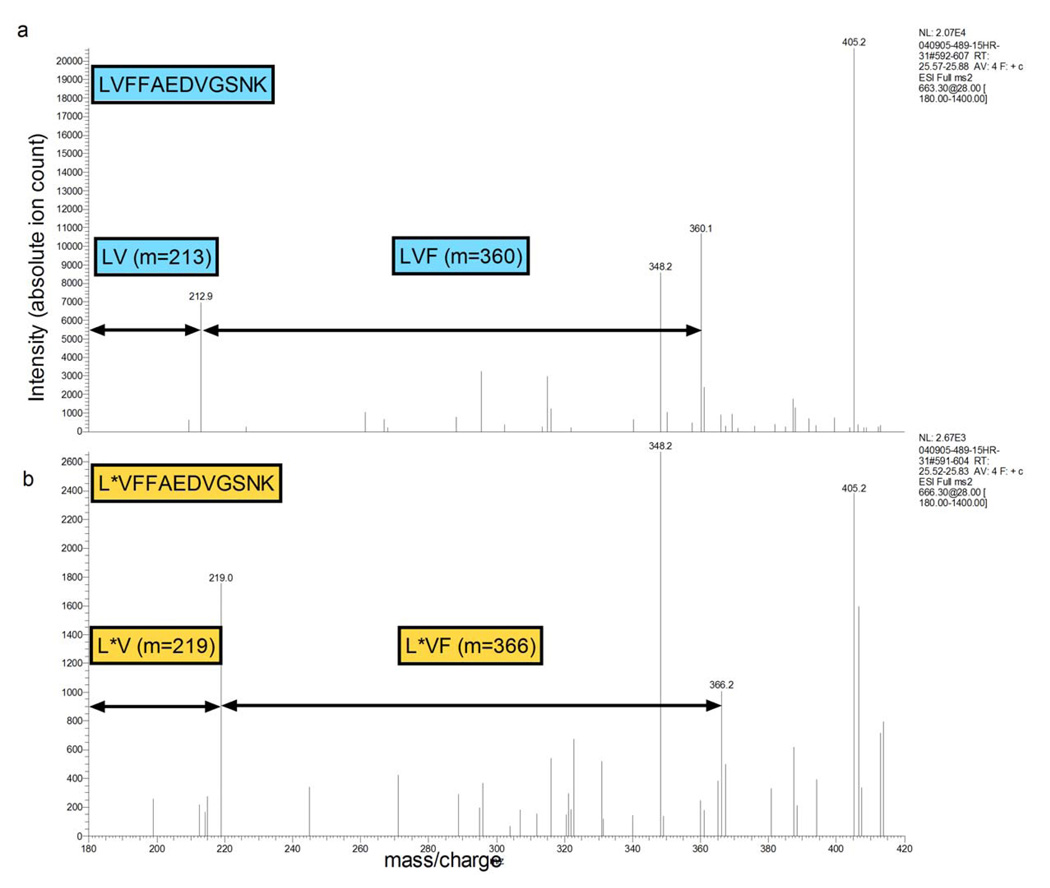
To determine if labeled Aβ could be produced and detected in vivo in a human, a single participant underwent a 24 hour infusion of labeled leucine followed by a lumbar puncture to obtain CSF. Aβ was immunoprecipitated from the CSF sample with the anti-Aβ antibody m266, trypsin digested and analyzed by liquid chromatography mass spectrometry (LC-MS). The m266 antibody is directed against the central domain of Aβ and binds to all Aβ species containing amino acids 13–28. The results demonstrated that unlabeled and labeled Aβ could be detected and measured in human CSF (fig. 2). A pharmacokinetic study was conducted to optimize the labeling and sampling times, so that detectable 13C6-leucine labeling of Aβ was achieved and maintained for an adequate period of time that permitted us to use steady-state equations to calculate Aβ synthesis and clearance rates. We tested a range of 13C6-leucine intravenous infusion dosages (1.8 to 2.5 mg/kg/hr), durations (6, 9, or 12 hours) and CSF/blood sampling times (from 12 to 36 hours duration). We found that labeled Aβ could be reliably quantified after 9 or 12 hours of label infusion, but not after 6 hours of label infusion. The synthesis portion of the labeling curve could be determined in the first 12 hours of sampling; however, the clearance portion of the labeling curve could only be determined with 36 hours of sampling. Based on these results, optimal labeling parameters for Aβ were defined to be 9 hours of IV infusion of the label and 36 hours of sample collection. These parameters allowed for assessment of both the fractional synthesis rate and fractional clearance rate portions of the labeling curve.
Figure 2.
Human CSF was collected after intravenous infusion of 13C6-leucine. Representative spectra of unlabeled (a) and labeled (b) Aβ17–28 are shown. The spectra were obtained using MS/MS analysis of unlabeled parent ion Aβ17–28 at m/z 663.3 or labeled parent ion Aβ17–28 at m/z 666.3. Note the MS/MS ions containing leucine (Aβ17) are mass shifted by 6 Daltons demonstrating the labeled leucine. The Aβ ions without leucine at position 17 are not labeled and are not mass shifted by 6 Daltons.
In Vivo labeling protocol
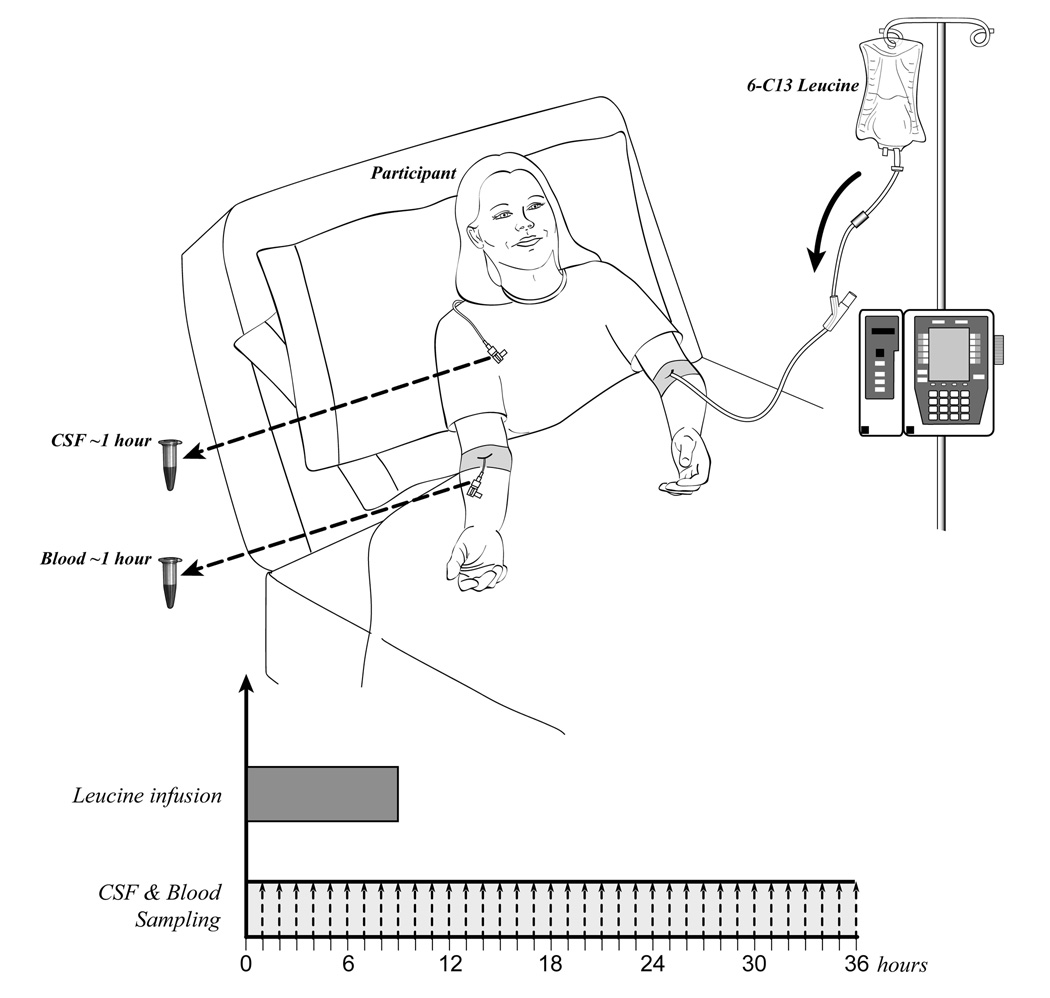
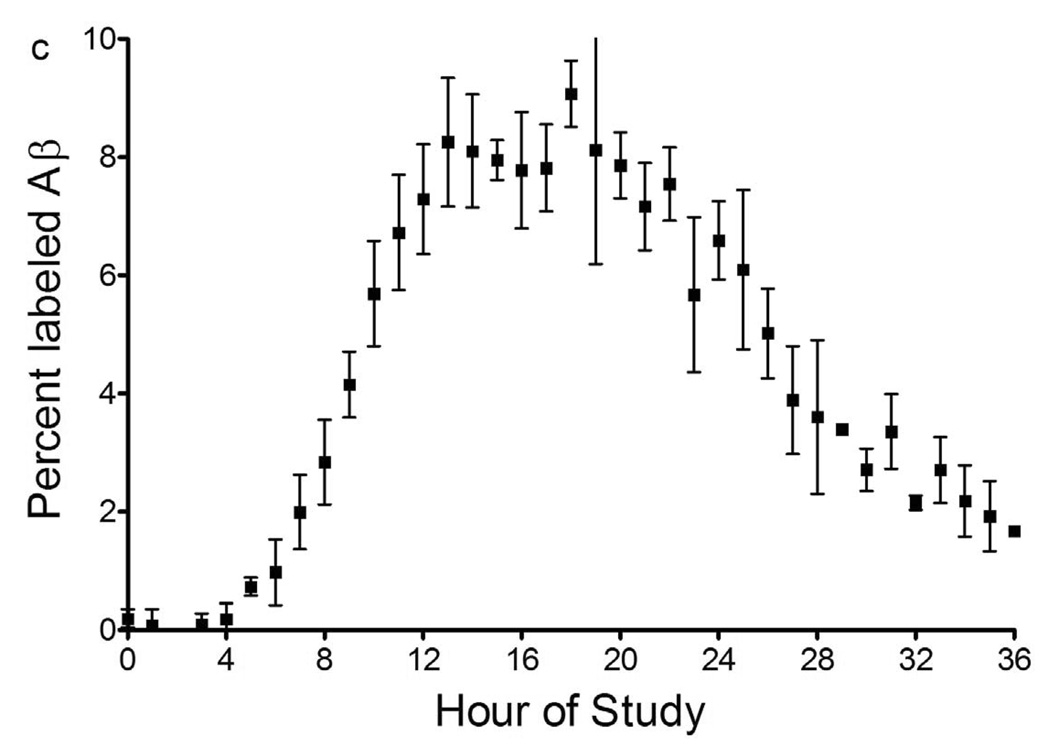
In the last three participants, 13C6-labeled leucine was administered with an initial bolus of 2 mg/kg over 10 minutes to reach a steady state of labeled leucine, followed by 9 hours of continuous intravenous infusion at a rate of 2 mg/kg/hr. Blood and CSF were sampled for 36 hours in the last 3 participants. Serial samples of 12 ml blood and 6 ml CSF were taken at one or two hour time intervals (fig. 3a). CSF has a production rate of ~20 ml per hour18 in a normal sized adult and replenishes itself throughout the procedure. The total amount of blood collected over the 36 hour study is 240 ml.
Figure 3.
(a) Diagram of participant with an intravenous catheter in either antecubital vein and a lumbar catheter in the L3-4 intrathecal space. Participants were admitted to the Washington University GCRC at 7 AM and 2 IVs and one lumbar catheter were placed. In one IV, 13C6-labeled leucine was infused at a rate of 1.8 to 2.5 mg/kg/hr for 9 or 12 hours, after an initial bolus of 2 mg/kg. Each hour, 12 ml of plasma and 6 ml of CSF were obtained through the other IV and the lumbar catheter respectively. Each sample was then processed as described above with immunoprecipitation of Aβ, trypsin digest, and LC-ESI-MS analysis to determine the percent labeled Aβ at each hour time point.
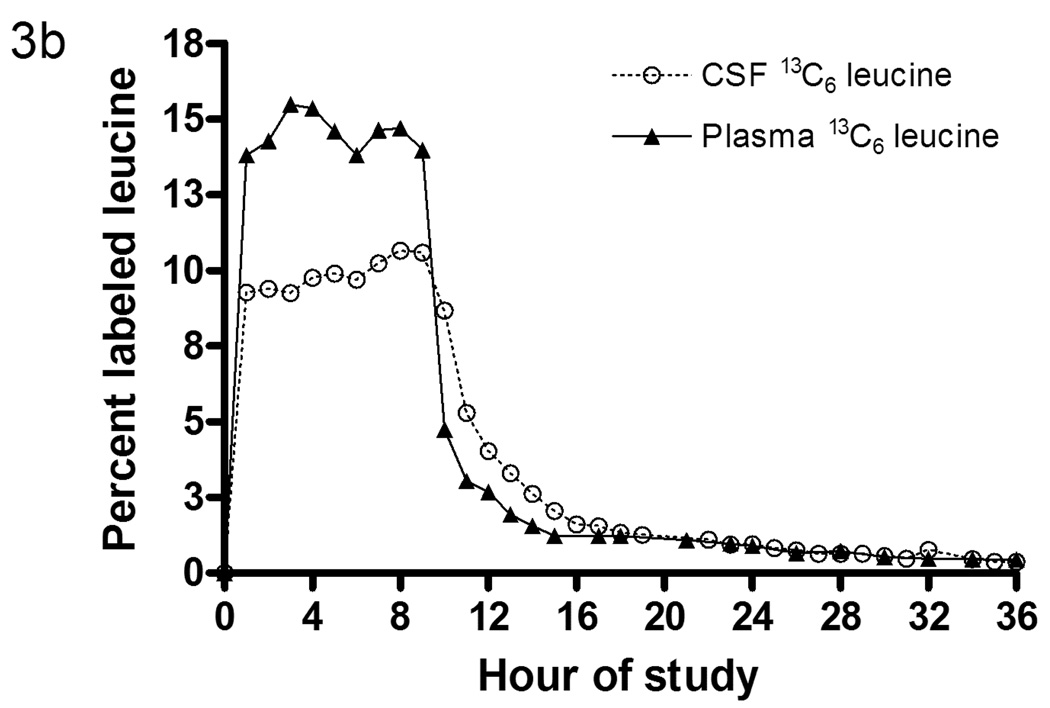
(b) Labeled leucine in CSF and blood from a participant during a 36 hour study. Note that CSF and plasma labeled leucine reaches near steady state within an hour after the initial bolus of 2mg/kg. Also note the exponential decay in labeled leucine clearance after the infusion of leucine into the bloodstream is stopped at 9 hours. The plasma labeled leucine is ~4% higher than the CSF labeled leucine during infusion.
(c) Average labeled CSF Aβ over 36 hours from 6 participants. The labeled Aβ curves were averaged and the mean for each time point shown +/− SEM. Each participant was labeled for 9 or 12 hours, while sampling occurred hourly from 0 to 12, 24, or 36 hours. There is no detectable incorporation of label in the first 4 hours, followed by an increase in percent labeled Aβ which plateaus near steady state labeled leucine levels (~10%), before decreasing over the last 12 hours of the study.
There were a total of 10 participants enrolled in the study, with 8 completing the pre-defined protocols and 2 studies stopped before completion due to post-lumbar puncture headache associated with the study. Two of the 8 completed studies had a 6 hour labeled leucine infusion, and labeled Aβ levels in these 2 participants were too low to accurately measure and were not used for analysis. The findings from the remaining 6 studies are reported here.
Labeled leucine quantitation
Plasma and CSF samples were analyzed to determine the amount of labeled leucine present in each fluid (fig. 3b). The tracer to tracee ratios (TTR) for plasma and CSF 13C6-leucine were quantified using capillary gas chromatography-mass spectrometry (GC-MS)19,20. 13C6-leucine reached steady state levels in both plasma and CSF within an hour of 14% and 10% respectively. This confirmed that leucine is rapidly transported across the blood-brain-barrier via known neutral amino acid transporter systems21.
Labeled Aβ dynamics
For each hourly sample of CSF collected, the percent of labeled Aβ was determined by immunoprecipitation-MS/MS as described above (fig. 2). The MS/MS ions from labeled Aβ17–28 and unlabeled Aβ17–28 were summed and divided to produce a ratio of labeled Aβ to unlabeled Aβ. The mean labeled Aβ ratio and standard error (n=6) of each time point are shown in figure 3c. There was no measurable labeled Aβ for the first 4 hours, followed by an increase from 5 to 13 hours. There was no significant change from 13 to 24 hours. The labeled Aβ decreased from 24 to 36 hours.
Calculation of FSR and FCR
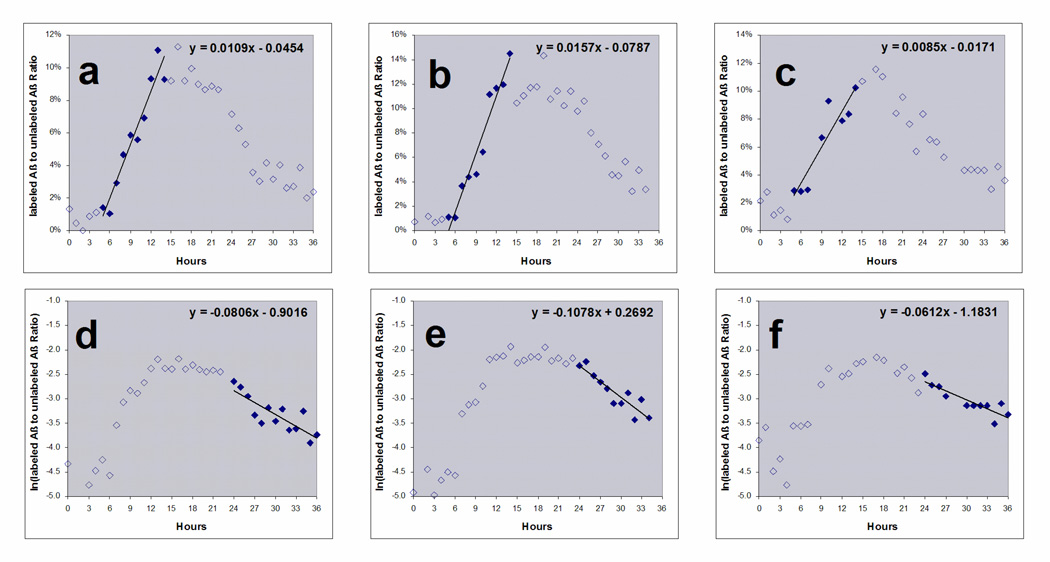
Fractional Synthesis Rate (FSR) was calculated using the standard formula: the slope of increase of labeled Aβ divided by the percentage of labeled leucine22 and reported in units of percent per hour. FSR, in percent per hour, was operationally defined as the slope of the linear regression from 5 to 13 hours divided by the average of CSF 13C6-labeled leucine level during infusion (fig. 4a–c).
Figure 4.
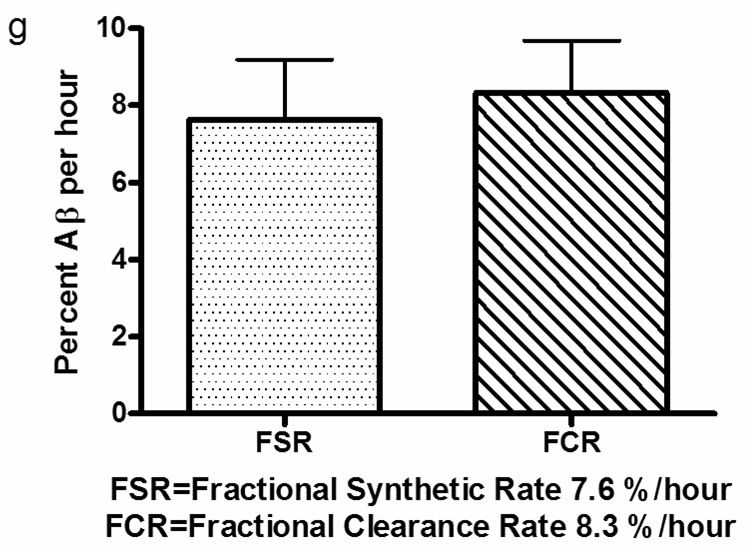
FSR and FCR of labeled Aβ curves from 3 participants with 9 hour label infusion and 36 hour sampling. FSR (a–c) is calculated by the slope of increasing labeled Aβ divided by the predicted steady state value. The predicted steady state value was estimated as the average CSF labeled leucine measured during labeling. The slope was defined to start after 4 to 6 hours lag time when there was no increase in labeled Aβ and ending 9 hours later (solid diamonds). The slope of FCR (d–f) is calculated by the slope of the natural logarithm of percent labeled Aβ from hours 24 to 36. (g) Average Aβ FSR & FCR: The average Aβ FSR of 6 participants and the average Aβ FCR of 3 participants is shown with standard deviation.
Fractional Clearance Rate (FCR) was calculated by fitting the slope of the natural logarithm of the clearance portion of the labeled Aβ curve. This was operationally defined as the natural log of the labeled Aβ from 24 to 36 hours (fig 4d–f). The average FSR of Aβ for these 6 healthy young participants was 7.6%/hr and the average FCR was 8.3%/hr (fig. 4g). These values were not statistically different from each other.
DISCUSSION
First results of Aβ synthesis and clearance rates in humans
Summary of technique
We report a novel method to determine the production and clearance rates of CNS proteins. The technique offers an accurate and reproducible way to determine the labeled amount of a protein that is in low picomolar abundance in human CSF. This technique may be adapted to measure proteins that are made in the CNS, present in the CSF or blood, and can be collected and measured by mass spectrometry, since all proteins are labeled simultaneously. The method may be used to determine changes that may be present in a disease state (e.g. AD vs. control), to find possible biomarkers, and to test proposed disease modifying therapies. This general approach may also be applied to other macromolecules produced in the CNS, including lipids, carbohydrates, and inflammatory markers by infusing a labeled precursor that crosses the blood-brain-barrier and isolating the labeled and unlabeled products. In addition, the method may offer information on the compartmentalization of proteins if labeled species are measured in separate compartments such as blood and CSF.
There exist established stable isotope tracer methods for quantifying synthesis and clearance rates of abundant (µg-mg) quantities of peripheral (liver, muscle, lung) human proteins, e.g., albumin, apolipoprotein B, myosin, and surfactant6,23–27. In general, these methods measure the abundance of the labeled and unlabeled proteins by using gas chromatography mass spectrometry of the derivitized amino acids. This approach is sensitive and accurate, but requires relatively large amounts of purified protein, and as such, is not ideal for low abundance proteins like Aβ (low picomolar quantities). Recent advances in electrospray ionization mass spectrometers and MS/MS quantitation provided the opportunity to further refine this approach so that sensitive, accurate, and reproducible quantitation of stable isotope labeling (±1%) in very low abundance proteins/peptides (pM) can be achieved. Our method exploits this technology to quantify in vivo 13C-incorporation into Aβ that has been immunoprecipitated from human CSF, digested with trypsin, and used to quantify in vivo Aβ protein synthesis and clearance rates.
Labeling
During labeling, all proteins that are being produced are labeled simultaneously, which offers the possibility of measuring multiple protein production and clearances rates on the same sample. In addition other isotopomer labels may be used, including other amino acids, simple carbohydrates, or acetate to determine kinetics of proteins, carbohydrates, or fatty acids respectively.
We chose to use a steady-state labeling infusion; however, others have used a bolus/chase design peripherally28. As demonstrated in figure 3b, plasma and CSF labeled leucine were similar in shape, with both having a steady state of labeling for 9 hours and rapid clearance of the label. However, the plasma leucine was labeled at 14% while CSF was labeled at 10% during label infusion. We chose to use the plateau of labeled leucine in the CSF in our estimates of FSR. Brain labeled leucine content is likely between the measured plasma levels and the CSF levels at each time point. However, CSF labeled leucine may better reflect the precursor enrichment in the brain.
Aβ production and clearance
Our results indicate Aβ is rapidly produced and cleared in the CNS in humans. To our knowledge, this represents the first estimate of the synthesis and clearance rate of a protein produced in the CNS in humans. The clearance rate of Aβ in humans measured by this technique is similar to, but slower than that measured in mouse brain by in vivo microdialysis29. Possible reasons for the differences include species difference in metabolism rate or measurement technique. In our current experiments, we have determined the synthesis and clearance of all Aβ species as we immoprecipitated Aβ with an antibody to the central domain of the molecule. To measure synthesis and clearance of specific Aβ species such as Aβ40 or Aβ42, immunoprecipitation of these species with C-terminal specific antibodies would allow such assessment. This method may be used to determine changes in underlying pathophysiology associated with AD, as a biomarker for diagnosis, or as a way to test new therapeutics (e.g. beta or gamma-secretase inhibitors) in terms of the pharmacodynamics on the proposed actions of drugs or other treatments.
ACKNOWLEDGMENTS
American Academy of Neurology Foundation, NIH grants AG05681, GCRC (MO1 RR00036), Mass Spectrometry Resource (NIH RR000954), Clinical Nutrition Research Unit (NIH DK056341), and Eli Lilly
COMPETING INTERESTS STATEMENT
The authors declare that they have no competing financial interests.
Literature Cited
- 1.Wolfe RR. Regulation of skeletal muscle protein metabolism in catabolic states. Curr Opin Clin Nutr Metab Care. 2005;8:61–65. doi: 10.1097/00075197-200501000-00009. [DOI] [PubMed] [Google Scholar]
- 2.Wolfe RR. Regulation of muscle protein by amino acids. J Nutr. 2002;132:3219S–3224S. doi: 10.1093/jn/131.10.3219S. [DOI] [PubMed] [Google Scholar]
- 3.San Pietro A, Rittenberg D. A study of the rate of protein synthesis in humans. II. Measurement of the metabolic pool and the rate of protein synthesis. J Biol Chem. 1953;201:457–473. [PubMed] [Google Scholar]
- 4.Balagopal P, Rooyackers OE, Adey DB, Ades PA, Nair KS. Effects of aging on in vivo synthesis of skeletal muscle myosin heavy-chain and sarcoplasmic protein in humans. Am J Physiol. 1997;273:E790–E800. doi: 10.1152/ajpendo.1997.273.4.E790. [DOI] [PubMed] [Google Scholar]
- 5.Yarasheski KE. Exercise, aging, and muscle protein metabolism. J Gerontol A Biol Sci Med Sci. 2003;58:M918–M922. doi: 10.1093/gerona/58.10.m918. [DOI] [PubMed] [Google Scholar]
- 6.Elias N, Patterson BW, Schonfeld G. In vivo metabolism of ApoB, ApoA-I, and VLDL triglycerides in a form of hypobetalipoproteinemia not linked to the ApoB gene. Arterioscler Thromb Vasc Biol. 2000;20:1309–1315. doi: 10.1161/01.atv.20.5.1309. [DOI] [PubMed] [Google Scholar]
- 7.Owen F, et al. Insertion in prion protein gene in familial Creutzfeldt-Jakob disease. Lancet. 1989;1:51–52. doi: 10.1016/s0140-6736(89)91713-3. [DOI] [PubMed] [Google Scholar]
- 8.Polymeropoulos MH, et al. Mutation in the alpha-synuclein gene identified in families with Parkinson's disease. Science. 1997;276:2045–2047. doi: 10.1126/science.276.5321.2045. [DOI] [PubMed] [Google Scholar]
- 9.Hutton M, et al. Association of missense and 5'-splice-site mutations in tau with the inherited dementia FTDP-17. Nature. 1998;393:702–705. doi: 10.1038/31508. [DOI] [PubMed] [Google Scholar]
- 10.Podlisny MB, Lee G, Selkoe DJ. Gene dosage of the amyloid beta precursor protein in Alzheimer's disease. Science. 1987;238:669–671. doi: 10.1126/science.2960019. [DOI] [PubMed] [Google Scholar]
- 11.Golde TE, Eckman CB, Younkin SG. Biochemical detection of Abeta isoforms: implications for pathogenesis, diagnosis, and treatment of Alzheimer's disease. Biochim Biophys Acta. 2000;1502:172–187. doi: 10.1016/s0925-4439(00)00043-0. [DOI] [PubMed] [Google Scholar]
- 12.Selkoe DJ. Alzheimer's disease: genes, proteins, and therapy. Physiol. Rev. 2001;81:741–766. doi: 10.1152/physrev.2001.81.2.741. [DOI] [PubMed] [Google Scholar]
- 13.Kuo YM, et al. Water-soluble Abeta (N-40, N-42) oligomers in normal and Alzheimer disease brains. J Biol Chem. 1996;271:4077–4081. doi: 10.1074/jbc.271.8.4077. [DOI] [PubMed] [Google Scholar]
- 14.Holtzman DM, et al. Society for Neuroscience Abstracts. Washington, DC: 2002. [Google Scholar]
- 15.Wisniewski KE, Wisniewski HM, Wen GY. Occurrence of neuropathological changes and dementia of Alzheimer's disease in Down's syndrome. Ann Neurol. 1985;17:278–282. doi: 10.1002/ana.410170310. [DOI] [PubMed] [Google Scholar]
- 16.Williams MA. Spinal catheter insertion via seated lumbar puncture using a massage chair. Neurology. 2002;58:1859–1860. doi: 10.1212/wnl.58.12.1859. [DOI] [PubMed] [Google Scholar]
- 17.DeMattos RB, et al. Peripheral anti-A beta antibody alters CNS and plasma A beta clearance and decreases brain A beta burden in a mouse model of Alzheimer's disease. Proc Natl Acad Sci U S A. 2001;98:8850–8855. doi: 10.1073/pnas.151261398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fishman RA. Cerebrospinal fluid in diseases of the nervous system. Philadelphia: Saunders; 1992. [Google Scholar]
- 19.Yarasheski KE, Smith SR, Powderly WG. Reducing plasma HIV RNA improves muscle amino acid metabolism. Am J Physiol Endocrinol Metab. 2005;288:E278–E284. doi: 10.1152/ajpendo.00359.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yarasheski KE, et al. Increased plasma gln and Leu Ra and inappropriately low muscle protein synthesis rate in AIDS wasting. Am J Physiol. 1998;275:E577–E583. doi: 10.1152/ajpendo.1998.275.4.E577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Smith QR, Momma S, Aoyagi M, Rapoport SI. Kinetics of neutral amino acid transport across the blood-brain barrier. J Neurochem. 1987;49:1651–1658. doi: 10.1111/j.1471-4159.1987.tb01039.x. [DOI] [PubMed] [Google Scholar]
- 22.Wolfe RR, Chinkes DL, Wolfe RR. Isotope tracers in metabolic research: principles and practice of kinetic analysis. Hoboken, N.J.: Wiley-Liss; 2005. [Google Scholar]
- 23.Patterson BW. Use of stable isotopically labeled tracers for studies of metabolic kinetics: an overview. Metabolism. 1997;46:322–329. doi: 10.1016/s0026-0495(97)90260-2. [DOI] [PubMed] [Google Scholar]
- 24.Yarasheski KE, Smith K, Rennie MJ, Bier DM. Measurement of muscle protein fractional synthetic rate by capillary gas chromatography/combustion isotope ratio mass spectrometry. Biol Mass Spectrom. 1992;21:486–490. doi: 10.1002/bms.1200211004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hasten DL, Morris GS, Ramanadham S, Yarasheski KE. Isolation of human skeletal muscle myosin heavy chain and actin for measurement of fractional synthesis rates. Am J Physiol. 1998;275:E1092–E1099. doi: 10.1152/ajpendo.1998.275.6.E1092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Merchak A, Patterson BW, Yarasheski KE, Hamvas A. Use of stable isotope labeling technique and mass isotopomer distribution analysis of [(13)C]palmitate isolated from surfactant disaturated phospholipids to study surfactant in vivo kinetics in a premature infant. J Mass Spectrom. 2000;35:734–738. doi: 10.1002/1096-9888(200006)35:6<734::AID-JMS2>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- 27.Schulte JN, Yarasheski KE. Effects of resistance training on the rate of muscle protein synthesis in frail elderly people. Int J Sport Nutr Exerc Metab. 2001;11 Suppl:S111–S118. doi: 10.1123/ijsnem.11.s1.s111. [DOI] [PubMed] [Google Scholar]
- 28.Garlick PJ, McNurlan MA, Preedy VR. A rapid and convenient technique for measuring the rate of protein synthesis in tissues by injection of [3H]phenylalanine. Biochem J. 1980;192:719–723. doi: 10.1042/bj1920719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cirrito JR, et al. In vivo assessment of brain interstitial fluid with microdialysis reveals plaque-associated changes in amyloid-beta metabolism and half-life. J Neurosci. 2003;23:8844–8853. doi: 10.1523/JNEUROSCI.23-26-08844.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]