Abstract
Although the remarkable versatility and efficacy of recombinant adeno-associated virus 2 (AAV2) vectors in transducing a wide variety of cells and tissues in vitro, and in numerous pre-clinical animal models of human diseases in vivo, have been well established, the published literature is replete with controversies with regard to the efficacy of AAV2 vectors in hematopoietic stem cell (HSC) transduction. A number of factors have contributed to these controversies, the molecular bases of which have begun to come to light in recent years. With the availability of several novel serotypes (AAV1 through AAV12), rational design of AAV capsid mutants, and strategies (self-complementary vector genomes, hematopoietic cell-specific promoters), it is indeed becoming feasible to achieve efficient transduction of HSC by AAV vectors in a murine serial bone marrow transplantation model in vivo, where stable integration of the proviral AAV genome does not lead to any overt hematological abnormalities. Thus, a better understanding of the AAV-HSC interactions, and the availability of a vast repertoire of novel serotype vectors, are likely to have significant implications in the use of AAV vectors in high-efficiency transduction of HSCs as well as in gene therapy applications involving the hematopoietic system.
Keywords: Adeno-associated virus, Viral vectors, Gene transfer, Gene expression, Hematopoietic stem cells
Gene therapy with vectors based on retroviruses and adenoviruses has been attempted in a number of clinical trials [Edelstein et al., 2007]. Initially, retroviral vectors yielded encouraging results, but the development of T cell lymphoma in non-human primates, and more recently, the development of T-cell leukemia in three children in a clinical trial for gene therapy of X-linked severe-combined immunodeficiency with these vectors has raised serious safety concerns [Deichmann et al., 2007; Hacein-Bey-Abina et al., 2003; Kohn et al., 2003]. Similarly, questions were raised with reference to efficacy of adenoviral vectors in gene therapy of cystic fibrosis, and subsequently, the death of a patient in a clinical trial for gene therapy of was attributed to the use of first-generation adenoviral vectors [Raper et al., 2003].
A third group of viruses, termed parvoviruses, have to date not been associated with any malignant disease. In fact, parvoviruses have been shown to possess anti-tumor properties [Asokan and Samulski, 2005; Li et al., 2005]. Parvoviruses are among the smallest of known viruses, which contain a single-stranded DNA genome, and infect all vertebrates. One parvovirus of human origin, the adeno-associated virus 2 (AAV2), has been studied extensively [Berns and Bohenzky, 1987; Muzyczka et al., 1984]. Although ∼90% of the human population is sero-positive for AAV2, no known disease has thus far been associated with AAV2 infection. AAV2 requires co-infection with a helper-virus, such as adenovirus, for its optimal replication, but in the absence of a helper-virus, the AAV2 genome integrates site-specifically into the human chromosome 19. The non-pathogenic nature of AAV2, coupled with the remarkable site-specificity of the proviral genome, were instrumental in the development of recombinant AAV2 vectors. Recombinant AAV2 vectors have been shown to efficiently transduce a variety of genes in a number of cell types in vitro, and their safety and efficacy in mediating sustained transgene expression has been documented in several small and large animal models in vivo [Griffey et al., 2006; Hermonat and Muzyczka, 1984; McCown et al., 1996; Rolling et al., 2006]. A number of Phase I/II clinical trials with AAV2 vectors are currently underway, and additional trials will soon be initiated [Aitken et al., 2001; Edelstein et al., 2007; Flotte et al., 1996; Flotte et al., 2004; Kay et al., 2000; Manno et al., 2003; Snyder and Francis, 2005; Wagner et al., 2002].
Controversies regarding hematopoietic stem cell transduction by AAV2 vectors
Recombinant AAV2 vectors have been shown to transduce certain cell types, such as brain and muscle, exceedingly well. However, controversies abound with reference to their efficacy in transducing hematopoietic stem cells (HSCs). For example, in 1994, we first reported successful transduction of CD34+ human primitive hematopoietic cells by recombinant AAV2 vectors at a relatively low vector:cell ratio of 1,000 [Zhou et al., 1994]. Subsequently, similar results were reported by a number of investigators [Nathwani et al., 2000; Santat et al., 2005]. However, others reported that AAV2-mediated transgene expression in HSCs occurred only when the vector particle:cell exceeded 106 [Hargrove et al., 1997; Malik et al., 1997], and one group concluded that human CD34+ cells were impervious to transduction by recombinant AAV2 vectors [Alexander et al., 1997]. This group also claimed that transgene expression observed by others was due to ‘pseudo-transduction’ mediated by contaminants in the vector stocks [Alexander et al., 1997].
In systematic studies undertaken in our laboratory spanning the past decade have unraveled the underlying bases of many, if not all, of the controversies related to HSC transduction by AAV2 vectors, and has been described previously [Ponnazhagan et al., 1997; Zhong et al., 2004; Zhong et al., 2006a], and reviewed recently [Srivastava, 2005; Zhong et al., 2006b]. In essence, the following obstacles have been identified that limit high-efficiency AAV2-mediated transduction of human HSCs: (i) variable levels of expression of the cell surface receptor and co-receptor for AAV2; and (ii) lack of viral second-strand DNA synthesis to yield transcriptionally-active vector genomes. The impaired viral second-strand DNA synthesis also impacts on the efficiency of the proviral genome integration with the host chromosomal DNA. For instance, two groups have reported that AAV2-mediated transgene expression in CD34+ cells is transient presumably because of inefficient integration. On the other hand, efficient integration of the proviral genome in human CD34+ cells and their progenitors has been reported by others using a variety of techniques such as polymerase chain reaction (PCR), Southern blotting, and fluorescence in situ hybridization (FISH). Santat et al. have documented successful transduction of primitive human HSCs capable of serial engraftment in immune-deficient mice, and Paz et al. recently demonstrated that recombinant AAV2 vectors integrated more efficiently in the more quiescent subpopulations of human CD34+ HSCs [Paz et al., 2007; Santat et al., 2005]. We have molecularly cloned and determined the nucleotide sequences spanning the junctions between vector genomes and host chromosomal DNA from long-term cultures of human CD34+ cells (data not shown), which provide further evidence for successful transduction of, and proviral integration into, primary human HSCs.
Transduction of murine HSCs by recombinant AAV vectors
It was nearly two decades ago that transduction of primary murine HSCs by AAV2 vectors was first reported by LaFace et al. [LaFace et al., 1988]. However, transgene expression occurred in only ∼1.5% of progenitor cell colonies presumably because of the use of relatively crude preparations of vector stocks, heterogeneous population of target cells, and sub-optimal promoter and transgene cassette. A decade and a half ago, our laboratory embarked on systematic studies to characterize the AAV2-murine HSC interaction in vitro and in vivo. During the course of these studies, we observed that the following obstacles limit high-efficiency AAV2-mediated transduction of murine HSCs: (i) sub-optimal levels of expression of the cell surface receptor and co-receptor for AAV2; and (ii) impaired intracellular trafficking and nuclear transport of AAV2 vectors; (iii) inefficient viral uncoating in the nucleus; and (iv) lack of viral second-strand DNA synthesis to yield transcriptionally-active vector genomes [Zhong et al., 2004; Srivastava, 2005; Zhong et al., 2006a; Zhong et al., 2006b].
In retrospect, it has become increasingly clear that murine HSC transduction has been attempted with AAV2 vectors, shown schematically in Fig. 1A, that are single-stranded, and transcriptionally-inactive, contain a sub-optimal promoter, such as the cytomegalovirus (CMV) immediate-early gene, which is known to be subjected to transcriptional silencing in murine tissues, and the vector serotype, for which there is sub-optimal expression of the receptor/co-receptor on HSC surface [Cordier et al., 2001; Zhong et al., 2004; Zhong et al., 2006b]. Thus, we decided to alter each of these three parameters and generated recombinant AAV vectors containing the enhanced green fluorescent protein (EGFP) reporter gene, shown schematically in Fig. 1B, where we used double-stranded AAV genome, which bypasses the need for viral second-strand DNA synthesis; used cellular (β-globin gene) and viral (parvovirus B19) promoters known to be active in hematopoietic progenitor cells; and some of the recently developed novel AAV serotypes (AAV1 through AAV12) [Gao et al., 2002], having previously shown that single-stranded (ss) AAV1 vectors transduce murine HSCs more efficiently than ssAAV2 vectors in vivo, and that self-complementary (sc) AAV1, AAV7, AAV8, and AAV10 vectors transduce murine HSCs more efficiently than scAAV2 vectors in vitro [Maina et al., 2008].
Figure 1.
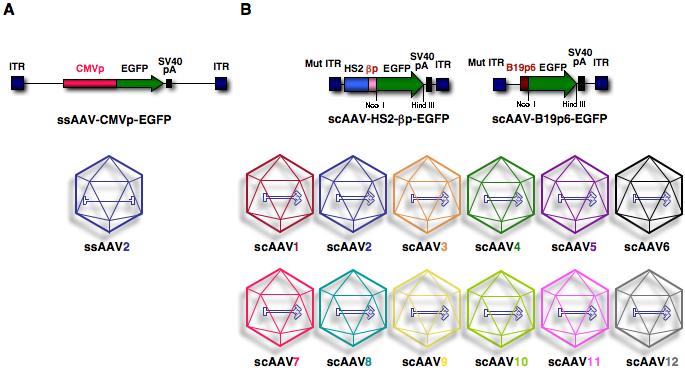
Schematic representation of conventional, single-stranded AAV2 and double-stranded, self-complementary AAV serotype vectors. ITR, inverted terminal repeat; CMVp, cytomegalovirus immediate-early promoter; EGFP, enhanced green fluorescent protein; SV40 pA, simian virus 40 polyadenylation signal; Mut ITR, mutant inverted terminal repeat; HS2, DNA-hypersensitive site 2 enhancer; βp, β-globin promoter; ssAAV, single-stranded AAV; scAAV, self-complementary AAV [Maina et al., 2008].
Bone marrow transplantation of murine HSCs transduced ex vivo with scAAV serotype vectors into lethally-irradiated syngeneic recipient mice revealed the following: (i) the transduction efficiency of AAV1 vectors was higher than that of AAV2 vectors, (ii) the transduction efficient of AAV7 vectors was comparable to that of AAV1 vectors, (iii) the B19p6 promoter was more efficient than the HS2-β-globin enhancer/promoter, and (iv) AAV8 and AAV10 vectors were not significantly more efficient than AAV2 vectors [Maina et al., 2008]. Lineage analysis of transgene expression mediated by scAAV serotype vectors in murine hematopoietic progenitor cells 6-months post-primary transplantation, shown in Table 1, clearly revealed that the use of the erythroid cell lineage-specific promoters led to the transgene expression which was restricted to erythroid cells regardless of the AAV serotype vector used. Secondary transplantation of bone marrow mononuclear cells from a scAAV7-B19p6-EGFP vector-transduced primary recipient mouse used to transplant four lethally-irradiated syngeneic mice revealed erythroid lineage-restricted expression in up to 30% of erythroid cells three months post-secondary transplantation. (Table 1).
Table 1.
| Primary Transplantation | T lymphocytes | B lymphocytes | Myeloid | Erythroblasts | Mature RBCs |
|---|---|---|---|---|---|
| Mock | 0.1 ± 0.02 | 0.1 ± 0.02 | 0.1 ± 0.01 | 3 ± 0.7 | 0.3 ± 0.01 |
| scAAV1-HS2-βp-EGFP | 0.1 ± 0.05 | 0.3 ± 0.07 | 0.2 ± 0.11 | 21 ± 12 | 0.3 ± 0.06 |
| scAAV1-B19p6-EGFP | 0.2 ± 0.13 | 0.5 ± 0.28 | 0.3 ± 0.21 | 38 ± 10 | 0.1 ± 0.08 |
| scAAV2-HS2-βp-EGFP | 0.2 ± 0.06 | 0.3 ± 0.02 | 0.2 ± 0.07 | 16 ± 0.7 | 0.3 ± 0.07 |
| scAAV2-B19p6-EGFP | 0.2 ± 0.03 | 0.3 ± 0.03 | 0.3 ± 0.03 | 19 ± 0.9 | 0.3 ± 0.07 |
| scAAV7-HS2-βp-EGFP | 0.2 ± 0.02 | 0.2 ± 0.03 | 0.1 ± 0.02 | 16 ± 4 | 0.9 ± 0.03 |
| scAAV7-B19p6-EGFP | 0.1 ± 0.06 | 0.2 ± 0.01 | 0.2 ± 0.10 | 32 ± 21 | 0.2 ± 0.04 |
| scAAV8-HS2-βp-EGFP | 0. 3 ± 0.07 | 0.3 ± 0.05 | 0.1 ± 0.04 | 19 ± 6 | 0.2 ± 0.01 |
| scAAV8-B19p6-EGFP | 0.3 ± 0.08 | 0.3 ± 0.04 | 0.1 ± 0.02 | 22 ± 8 | 0.4 ± 0.1 |
| scAAV10-HS2-βp-EGFP | 0.2 ± 0.08 | 0.2 ± 0.08 | 0.2 ± 0.04 | 13 ± 2 | 0.1 ± 0.02 |
| scAAV10-B19p6-EGFP | 0.2 ± 0.08 | 0.1± 0.14 | 0.1± 0.04 | 12 ± 4 | 0.3 ± 0.07 |
| Secondary Transplantation | T lymphocytes | B lymphocytes | Myeloid | Erythroblasts | Mature RBCs |
|---|---|---|---|---|---|
| Mock | 0.02 | 0.01 | 0.03 | 3.0 | 0.04 |
| scAAV7-B19p6-EGFP #1 | 0.05 | 0.05 | 0.04 | 22.0 | 0.08 |
| scAAV7-B19p6-EGFP #2 | 0.06 | 0.05 | 0.04 | 19.0 | 0.07 |
| scAAV7-B19p6-EGFP #3 | 0.04 | 0.07 | 0.05 | 32.0 | 0.08 |
| scAAV7-B19p6-EGFP #4 | 0.02 | 0.04 | 0.03 | 23.0 | 0.08 |
Bone marrow cells from primary transplant recipient mice 6-months post-transplantation were incubated with phycoerythrin (PE)-conjugated lineage specific rat anti-mouse mAbs (anti-B220 for B lymphocytes; anti-CD4 and anti-CD8a for T lymphocytes; anti-Gr-1 for myeloid cells; and anti-Ter119 for mature RBCs) separately and analyzed for EGFP expression by flow cytometry. Erythroblasts were stained with anti-c-kit-PE and anti-CD71-PE-Cy5 antibody. Bone marrow cells from a scAAV7-B19p6-EGFP vector-transduced primary recipient mouse were used to transplant 4 lethally-irradiated syngeneic mice (#1-#4). Three months post-secondary transplantation, bone marrow cells were analyzed as described above.
Southern blot analysis using EGFP DNA as a probe further corroborated previously published studies on stable integration of the proviral AAV genomes into host cell chromosomal DNA, and morphological analyses of peripheral blood and bone marrow cells following transplantation of HSCs following mock-transduction, or transduction with scAAV serotype vectors indicated no deleterious effects of transduced transgene expression [Maina et al., 2008]. The used of scAAV1 and scAAV7 serotype shuttle vectors for transduction of HSCs in a murine bone marrow transplant model further allowed examination of AAV proviral integration pattern in the mouse genome, as well as recovery and nucleotide sequence analyses of AAV-HSC DNA junction fragments. The proviral integration pattern was observed to be random, and recombination sites were localized to different chromosomes (Figure 2). None of the integration sites was found to be in a transcribed gene or near an oncogene, and all animals followed for up to one year exhibited no pathological abnormalities [Han et al., 2008]. Thus, AAV proviral integration-induced risk of oncogenesis was not found in our studies, which provide functional confirmation of stable transduction of self-renewing multipotential HSCs by scAAV vectors as well as show promise for the use of these vectors in the potential treatment of disorders of the hematopoietic system.
Figure 2.
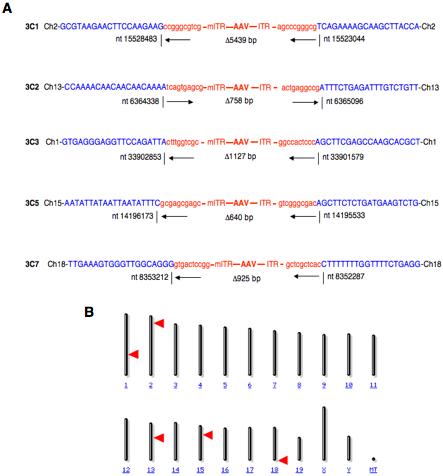
(A) Nucleotide sequences of AAV vector-mouse cellular DNA junctions. Parts of AAV-ITR sequences are indicated in red, and the flanking mouse genomic DNA sequences are in blue. The locations of each junction are indicated with nucleotide numbers obtained from the NCBI database. Deletion sizes are noted under each provirus, and mouse chromosome numbers are indicated at each end. (B) Chromosomal distribution of AAV integration sites. A schematic mouse chromosome ideogram obtained from the NCBI is shown with AAV vector integration sites. Red arrows denote the AAV targeted sites [Han et al., 2008].
Information Gaps to be Filled
In my view, the following three broad areas of further investigations deserve greater scrutiny:
(i) Development of hematopoietic stem/progenitor cell-specific AAV vectors
Our systematic studies during the past decade have not only unraveled several obstacles that limit efficient transduction of HSCs by recombinant AAV2 vectors, but have also reinforced the need to continue further studies focused on the basic understanding of all aspects of the life cycle of AAV2 vectors at the molecular level [Srivastava, 2001; Srivastava, 2004; Wu et al., 2007; Zhao et al., 2007; Zhong et al., 2006b]. Similar studies are also needed in order to identify and characterize the optimal AAV serotype vector(s) to achieve efficient transduction of HSC. In this context, it is important to note that endogenous AAV sequences are the third most prevalent in the human bone marrow [Gao et al., 2004]. Thus, it would be worthwhile to develop these serotypes into recombinant vectors, which may also be instrumental in achieving high-efficiency transduction of primary human hematopoietic stem and progenitor cells. In this context, it is noteworthy that in our recent studies [Zhong et al., 2008], we have developed novel AAV2 vectors containing mutations in the surface-exposed tyrosine residues that transduce HeLa cells ∼10-fold more efficiently in vitro (Figure 3 A-C), and murine hepatocytes nearly 30-fold more efficiently in vivo at a log lower vector dose (Figure 3 D,E). These vectors also allow the production of therapeutic levels of human Factor IX (F.IX) an ∼10-fold reduced vector dose (Figure 3 F). In preliminary studies, we have observed that primary murine and human hematopoietic stem/progenitor cells can also be transduced more efficiently by these tyrosine-mutant AAV2 vectors in vitro, and respectively in syngeneic and NOD/SCID mice in vivo (unpublished data). It is also noteworthy that with a few exceptions, these tyrosine residues are highly conserved in AAV serotypes 1 through 12, and we have also begun to generate the tyrosine-mutants of each of these serotypes. The availability of such a vast repertoire of novel tyrosine-mutant AAV serotype vectors should allow us to gain a better understanding of the role of tyrosine-phosphorylation of AAV capsids in various steps in the virus life cycle, which is likely to have important implications in the optimal use of recombinant AAV serotype tyrosine-mutant vectors in HSC transduction. It is also tempting to speculate that it may eventually be feasible to develop recombinant AAV serotype vectors that allow regulated transgene expression in each of the hematopoietic progenitor cell lineage.
Figure 3.
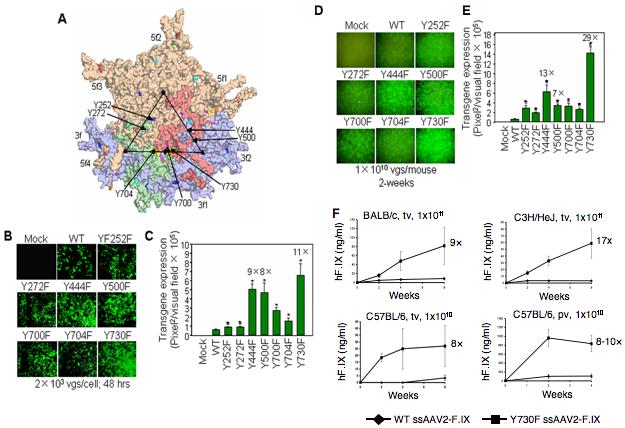
Novel tyrosine-mutant AAV2 vectors. (A) The position of the seven surface-exposed tyrosine residues on the AAV2 capsid surface, Y252, Y272, Y444, Y500, Y700, Y704, and Y730, are indicated by the arrows. Site-directed mutations of these 7 tyrosine residues to phenylalanine residues (tyrosine-phenylalanine, Y-F) were perfumed and tyrosine-mutant capsid scAAV2-EGFP vectors were generated. (B) AAV2-mediated transgene expression in HeLa cells following transduction with surface-exposed tyrosine-mutant capsid scAAV2-EGFP vectors. (C) Quantitative analyses of AAV2 transduction efficiency. *P<0.01 vs. WT scAAV2-EGFP. (D) AAV2-mediated transduction of hepatocytes from normal C57BL/6 mice injected via tail vein with tyrosine-mutant capsid scAAV2-EGFP vectors. (E) Quantitative analyses of AAV2 transduction efficiency. *P<0.01 vs. WT scAAV2-EGFP. (F) Comparative analyses of the WT or Y730F ssAAV2-ApoE/hAAT-hF.IX vector-mediated transduction efficiency in hepatocytes in mice in vivo. Human F.IX (hF.IX) expression in plasma as a function of time after injection of 1×1011 viral particles/animal in BALB/c and C3H/HeJ mice via tail vein (tv), and 1×1010 viral particles/animal in C57BL/6 mice via tail vein (tv) or portal vein (pv). Fold-increase of hF.IX peak levels of Y730F vectors compared to the WT capsid vectors is indicated for each panel [Zhong et al., 2008].
(ii) Integration of the AAV proviral genome
It is generally assumed that recombinant AAV vector genomes remain episomal, but those studies have been carried out in tissues that are post-mitotic [Duan et al., 1998; Inagaki et al., 2007; Nakai et al., 2003; Zhong et al., 2006a]. In HSCs, which must divide to give rise to progenitor cells, the proviral genome would be lost if not stably integrated into host cell chromosomal DNA. In our previous studies, we documented stable integration of the proviral genome into chromosomal DNA in pluripotent HSC transduced with ssAAV1 and AAV2 vectors in a small number of animals [Tan et al., 2001; Zhong et al., 2006a]. Therefore, further studies with a larger number of animals are needed to define this issue at the molecular level. In addition, the integration patterns of ssAAV vectors versus scAAV vectors should also be compared and contrasted. Detailed sequence analyses of integration junctions in various cell lineages will be helpful in not only determining clonality, but also in establishing whether the proviral integration occurs in transcriptionally-active versus the non-transcribed regions of the genome. In our recent studies using a shuttle vector [Han et al., 2008], we have attempted to address some of these issues, but further detailed investigations are clearly warranted.
(iii) Possible insertional mutagenesis induced by AAV vectors
In view of a recent report of the development of hepatocellular carcinoma in mice injected with a relatively high AAV2 vector dose neonatally [Donsante et al., 2007], it is crucial that the safety and efficacy of the AAV serotype vectors be rigorously examined following integration of the proviral genome into the host chromosomal DNA. It should be emphasized, however, that other investigators found no evidence for AAV-induced malignancy in large-scale studies involving adult mice or p53-deficient mice [Bell et al., 2006; Bell et al., 2005; Schuettrumpf et al., 2007]. These discrepancies with the post-mitotic tissues notwithstanding, the biological consequences of stable transduction and long-term transgene expression in hematopoietic progenitor cells should nonetheless be examined further.
Future Prospects
My prediction is that despite the lack of enthusiasm in the development of recombinant AAV2 vectors for HSC transduction in the past, a more complete understanding of the virus-host cell interactions, coupled with the availability of an ever-expanding vast repertoire of novel AAV serotype vectors, will lead to high-efficiency transduction of HSC in not too distant a future, which in turn, will provide a safer alternative to the more commonly used retroviral vectors in hematopoietic stem cell gene therapy in humans.
Acknowledgments
I thank Drs. Richard Jude Samulski and James M. Wilson for generously providing the packaging plasmids for AAV serotypes, and the members of my laboratory for helpful discussions. This research was supported in part by Public Health Service grants R01 EB-002073, R01 HL-065770, HL-076901, and P01 DK-058327 (Project 1) from the National Institutes of Health.
Contract grant sponsor: National Institutes of Health; Contract grant numbers: R01 EB-002073, R01 HL-065770, HL-076901, and P01 DK-058327 (Project 1)
References
- Aitken ML, Moss RB, Waltz DA, Dovey ME, Tonelli MR, McNamara SC, Gibson RL, Ramsey BW, Carter BJ, Reynolds TC. A phase I study of aerosolized administration of tgAAVCF to cystic fibrosis subjects with mild lung disease. Hum Gene Ther. 2001;12:1907–16. doi: 10.1089/104303401753153956. [DOI] [PubMed] [Google Scholar]
- Alexander IE, Russell DW, Miller AD. Transfer of contaminants in adeno-associated virus vector stocks can mimic transduction and lead to artifactual results. Hum Gene Ther. 1997;8:1911–20. doi: 10.1089/hum.1997.8.16-1911. [DOI] [PubMed] [Google Scholar]
- Asokan A, Samulski RJ. From crystal structure to clinic: highlights of the Tenth International Parvovirus Workshop. Mol Ther. 2005;11:656–60. doi: 10.1016/j.ymthe.2005.03.014. [DOI] [PubMed] [Google Scholar]
- Bell P, Moscioni AD, McCarter RJ, Wu D, Gao G, Hoang A, Sanmiguel JC, Sun X, Wivel NA, Raper SE, Furth EE, Batshaw ML, Wilson JM. Analysis of tumors arising in male B6C3F1 mice with and without AAV vector delivery to liver. Mol Ther. 2006;14:34–44. doi: 10.1016/j.ymthe.2006.03.008. [DOI] [PubMed] [Google Scholar]
- Bell P, Wang L, Lebherz C, Flieder DB, Bove MS, Wu D, Gao GP, Wilson JM, Wivel NA. No evidence for tumorigenesis of AAV vectors in a large-scale study in mice. Mol Ther. 2005;12:299–306. doi: 10.1016/j.ymthe.2005.03.020. [DOI] [PubMed] [Google Scholar]
- Berns KI, Bohenzky RA. Adeno-associated viruses: an update. Adv Virus Res. 1987;32:243–306. doi: 10.1016/s0065-3527(08)60479-0. [DOI] [PubMed] [Google Scholar]
- Cordier L, Gao GP, Hack AA, McNally EM, Wilson JM, Chirmule N, Sweeney HL. Muscle-specific promoters may be necessary for adeno-associated virus-mediated gene transfer in the treatment of muscular dystrophies. Hum Gene Ther. 2001;12:205–15. doi: 10.1089/104303401750061267. [DOI] [PubMed] [Google Scholar]
- Deichmann A, Hacein-Bey-Abina S, Schmidt M, Garrigue A, Brugman MH, Hu J, Glimm H, Gyapay G, Prum B, Fraser CC, Fischer N, Schwarzwaelder K, Siegler ML, de Ridder D, Pike-Overzet K, Howe SJ, Thrasher AJ, Wagemaker G, Abel U, Staal FJ, Delabesse E, Villeval JL, Aronow B, Hue C, Prinz C, Wissler M, Klanke C, Weissenbach J, Alexander I, Fischer A, von Kalle C, Cavazzana-Calvo M. Vector integration is nonrandom and clustered and influences the fate of lymphopoiesis in SCID-X1 gene therapy. J Clin Invest. 2007;117:2225–32. doi: 10.1172/JCI31659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donsante A, Miller DG, Li Y, Vogler C, Brunt EM, Russell DW, Sands MS. AAV vector integration sites in mouse hepatocellular carcinoma. Science. 2007;317:477. doi: 10.1126/science.1142658. [DOI] [PubMed] [Google Scholar]
- Duan D, Sharma P, Yang J, Yue Y, Dudus L, Zhang Y, Fisher KJ, Engelhardt JF. Circular intermediates of recombinant adeno-associated virus have defined structural characteristics responsible for long-term episomal persistence in muscle tissue. J Virol. 1998;72:8568–77. doi: 10.1128/jvi.72.11.8568-8577.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edelstein ML, Abedi MR, Wixon J. Gene therapy clinical trials worldwide to 2007-an update. J Gene Med. 2007;9:833–42. doi: 10.1002/jgm.1100. [DOI] [PubMed] [Google Scholar]
- Flotte T, Carter B, Conrad C, Guggino W, Reynolds T, Rosenstein B, Taylor G, Walden S, Wetzel R. A phase I study of an adeno-associated virus-CFTR gene vector in adult CF patients with mild lung disease. Hum Gene Ther. 1996;7:1145–59. doi: 10.1089/hum.1996.7.9-1145. [DOI] [PubMed] [Google Scholar]
- Flotte TR, Brantly ML, Spencer LT, Byrne BJ, Spencer CT, Baker DJ, Humphries M. Phase I trial of intramuscular injection of a recombinant adeno-associated virus alpha 1-antitrypsin (rAAV2-CB-hAAT) gene vector to AAT-deficient adults. Hum Gene Ther. 2004;15:93–128. doi: 10.1089/10430340460732490. [DOI] [PubMed] [Google Scholar]
- Gao G, Vandenberghe LH, Alvira MR, Lu Y, Calcedo R, Zhou X, Wilson JM. Clades of Adeno-associated viruses are widely disseminated in human tissues. J Virol. 2004;78:6381–8. doi: 10.1128/JVI.78.12.6381-6388.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao GP, Alvira MR, Wang L, Calcedo R, Johnston J, Wilson JM. Novel adeno-associated viruses from rhesus monkeys as vectors for human gene therapy. Proc Natl Acad Sci U S A. 2002;99:11854–9. doi: 10.1073/pnas.182412299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffey MA, Wozniak D, Wong M, Bible E, Johnson K, Rothman SM, Wentz AE, Cooper JD, Sands MS. CNS-directed AAV2-mediated gene therapy ameliorates functional deficits in a murine model of infantile neuronal ceroid lipofuscinosis. Mol Ther. 2006;13:538–47. doi: 10.1016/j.ymthe.2005.11.008. [DOI] [PubMed] [Google Scholar]
- Hacein-Bey-Abina S, Von Kalle C, Schmidt M, McCormack MP, Wulffraat N, Leboulch P, Lim A, Osborne CS, Pawliuk R, Morillon E, Sorensen R, Forster A, Fraser P, Cohen JI, de Saint Basile G, Alexander I, Wintergerst U, Frebourg T, Aurias A, Stoppa-Lyonnet D, Romana S, Radford-Weiss I, Gross F, Valensi F, Delabesse E, Macintyre E, Sigaux F, Soulier J, Leiva LE, Wissler M, Prinz C, Rabbitts TH, Le Deist F, Fischer A, Cavazzana-Calvo M. LMO2-associated clonal T cell proliferation in two patients after gene therapy for SCID-X1. Science. 2003;302:415–9. doi: 10.1126/science.1088547. [DOI] [PubMed] [Google Scholar]
- Han Z, Zhong L, Maina N, Hu Z, Li X, Chouthai NS, Bischof D, Weigel-Van Aken KA, Slayton WB, Yoder MC, Srivastava A. Recombinant AAV proviral genomes are stably integrated in primary murine hematopoietic stem cells. Hum. Gene Ther. 2008;19:267–78. doi: 10.1089/hum.2007.161. [DOI] [PubMed] [Google Scholar]
- Hargrove PW, Vanin EF, Kurtzman GJ, Nienhuis AW. High-level globin gene expression mediated by a recombinant adeno-associated virus genome that contains the 3′ gamma globin gene regulatory element and integrates as tandem copies in erythroid cells. Blood. 1997;89:2167–75. [PubMed] [Google Scholar]
- Hermonat PL, Muzyczka N. Use of adeno-associated virus as a mammalian DNA cloning vector: transduction of neomycin resistance into mammalian tissue culture cells. Proc Natl Acad Sci U S A. 1984;81:6466–70. doi: 10.1073/pnas.81.20.6466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inagaki K, Ma C, Storm TA, Kay MA, Nakai H. The role of DNA-PKcs and Artemis in opening viral DNA hairpin termini in various tissues in mice. J Virol. 2007;81:11304–21. doi: 10.1128/JVI.01225-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kay MA, Manno CS, Ragni MV, Larson PJ, Couto LB, McClelland A, Glader B, Chew AJ, Tai SJ, Herzog RW, Arruda V, Johnson F, Scallan C, Skarsgard E, Flake AW, High KA. Evidence for gene transfer and expression of factor IX in haemophilia B patients treated with an AAV vector. Nat Genet. 2000;24:257–61. doi: 10.1038/73464. [DOI] [PubMed] [Google Scholar]
- Kohn DB, Sadelain M, Dunbar C, Bodine D, Kiem HP, Candotti F, Tisdale J, Riviere I, Blau CA, Richard RE, Sorrentino B, Nolta J, Malech H, Brenner M, Cornetta K, Cavagnaro J, High K, Glorioso J. American Society of Gene Therapy (ASGT) ad hoc subcommittee on retroviral-mediated gene transfer to hematopoietic stem cells. Mol Ther. 2003;8:180–7. doi: 10.1016/s1525-0016(03)00212-0. [DOI] [PubMed] [Google Scholar]
- LaFace D, Hermonat P, Wakeland E, Peck A. Gene transfer into hematopoietic progenitor cells mediated by an adeno-associated virus vector. Virology. 1988;162:483–6. doi: 10.1016/0042-6822(88)90491-6. [DOI] [PubMed] [Google Scholar]
- Li C, Bowles DE, van Dyke T, Samulski RJ. Adeno-associated virus vectors: potential applications for cancer gene therapy. Cancer Gene Ther. 2005;12:913–25. doi: 10.1038/sj.cgt.7700876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maina N, Han Z, Zhao W, Li X, Hu Z, Zhong L, Bischof D, Weigel-Van Aken KA, Slayton WB, Yoder MC, Srivastava A. Recombinant scAAV serotype vector-mediated transduction of hematopoietic stem cells and lineage-restricted long-term transgene expression in progenitor cells in a murine serial bone marrow transplantation model. Hum. Gene Ther. 2008 doi: 10.1089/hum.2007.143. in press. [DOI] [PubMed] [Google Scholar]
- Malik P, McQuiston SA, Yu XJ, Pepper KA, Krall WJ, Podsakoff GM, Kurtzman GJ, Kohn DB. Recombinant adeno-associated virus mediates a high level of gene transfer but less efficient integration in the K562 human hematopoietic cell line. J Virol. 1997;71:1776–83. doi: 10.1128/jvi.71.3.1776-1783.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manno CS, Chew AJ, Hutchison S, Larson PJ, Herzog RW, Arruda VR, Tai SJ, Ragni MV, Thompson A, Ozelo M, Couto LB, Leonard DG, Johnson FA, McClelland A, Scallan C, Skarsgard E, Flake AW, Kay MA, High KA, Glader B. AAV-mediated factor IX gene transfer to skeletal muscle in patients with severe hemophilia B. Blood. 2003;101:2963–72. doi: 10.1182/blood-2002-10-3296. [DOI] [PubMed] [Google Scholar]
- McCown TJ, Xiao X, Li J, Breese GR, Samulski RJ. Differential and persistent expression patterns of CNS gene transfer by an adeno-associated virus (AAV) vector. Brain Res. 1996;713:99–107. doi: 10.1016/0006-8993(95)01488-8. [DOI] [PubMed] [Google Scholar]
- Muzyczka N, Samulski RJ, Hermonat P, Srivastava A, Berns KI. The genetics of adeno-associated virus. Adv Exp Med Biol. 1984;179:151–61. doi: 10.1007/978-1-4684-8730-5_15. [DOI] [PubMed] [Google Scholar]
- Nakai H, Montini E, Fuess S, Storm TA, Grompe M, Kay MA. AAV serotype 2 vectors preferentially integrate into active genes in mice. Nat Genet. 2003;34:297–302. doi: 10.1038/ng1179. [DOI] [PubMed] [Google Scholar]
- Nathwani AC, Hanawa H, Vandergriff J, Kelly P, Vanin EF, Nienhuis AW. Efficient gene transfer into human cord blood CD34+ cells and the CD34+CD38- subset using highly purified recombinant adeno-associated viral vector preparations that are free of helper virus and wild-type AAV. Gene Ther. 2000;7:183–95. doi: 10.1038/sj.gt.3301068. [DOI] [PubMed] [Google Scholar]
- Paz H, Wong CA, Li W, Santat L, Wong KK, Chatterjee S. Quiescent subpopulations of human CD34-positive hematopoietic stem cells are preferred targets for stable recombinant adeno-associated virus type 2 transduction. Hum Gene Ther. 2007;18:614–26. doi: 10.1089/hum.2006.188. [DOI] [PubMed] [Google Scholar]
- Ponnazhagan S, Mukherjee P, Wang XS, Qing K, Kube DM, Mah C, Kurpad C, Yoder MC, Srour EF, Srivastava A. Adeno-associated virus type 2-mediated transduction in primary human bone marrow-derived CD34+ hematopoietic progenitor cells: donor variation and correlation of transgene expression with cellular differentiation. J Virol. 1997;71:8262–7. doi: 10.1128/jvi.71.11.8262-8267.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raper SE, Chirmule N, Lee FS, Wivel NA, Bagg A, Gao GP, Wilson JM, Batshaw ML. Fatal systemic inflammatory response syndrome in a ornithine transcarbamylase deficient patient following adenoviral gene transfer. Mol Genet Metab. 2003;80:148–58. doi: 10.1016/j.ymgme.2003.08.016. [DOI] [PubMed] [Google Scholar]
- Rolling F, Le Meur G, Stieger K, Smith AJ, Weber M, Deschamps JY, Nivard D, Mendes-Madeira A, Provost N, Pereon Y, Cherel Y, Ali RR, Hamel C, Moullier P, Rolling F. Gene therapeutic prospects in early onset of severe retinal dystrophy: restoration of vision in RPE65 Briard dogs using an AAV serotype 4 vector that specifically targets the retinal pigmented epithelium. Bull Mem Acad R Med Belg. 2006;161:497–508. discussion 508-9. [PubMed] [Google Scholar]
- Santat L, Paz H, Wong C, Li L, Macer J, Forman S, Wong KK, Chatterjee S. Recombinant AAV2 transduction of primitive human hematopoietic stem cells capable of serial engraftment in immune-deficient mice. Proc Natl Acad Sci U S A. 2005;102:11053–8. doi: 10.1073/pnas.0502902102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuettrumpf J, Baila S, Khazi F, Liu J, Bunte R, Arruda VR. AAV vectors do not increase the risk of tumor formation in p53 deficient models. Mol Ther. 2007;15(Suppl):S1. [Google Scholar]
- Snyder RO, Francis J. Adeno-associated viral vectors for clinical gene transfer studies. Curr Gene Ther. 2005;5:311–21. doi: 10.2174/1566523054065066. [DOI] [PubMed] [Google Scholar]
- Srivastava A. Gene therapy with viral vectors: the hope, the problems, and the solution. J Hematother Stem Cell Res. 2001;10:321–2. doi: 10.1089/15258160151135123. [DOI] [PubMed] [Google Scholar]
- Srivastava A. Gene delivery to human and murine primitive hematopoietic stem and progenitor cells by AAV2 vectors. Methods Mol Biol. 2004;246:245–54. doi: 10.1385/1-59259-650-9:245. [DOI] [PubMed] [Google Scholar]
- Srivastava A. Hematopoietic stem cell transduction by recombinant adeno-associated virus vectors: problems and solutions. Hum Gene Ther. 2005;16:792–8. doi: 10.1089/hum.2005.16.792. [DOI] [PubMed] [Google Scholar]
- Tan M, Qing K, Zhou S, Yoder MC, Srivastava A. Adeno-associated virus 2-mediated transduction and erythroid lineage-restricted long-term expression of the human beta-globin gene in hematopoietic cells from homozygous beta-thalassemic mice. Mol Ther. 2001;3:940–6. doi: 10.1006/mthe.2001.0346. [DOI] [PubMed] [Google Scholar]
- Wagner JA, Nepomuceno IB, Messner AH, Moran ML, Batson EP, Dimiceli S, Brown BW, Desch JK, Norbash AM, Conrad CK, Guggino WB, Flotte TR, Wine JJ, Carter BJ, Reynolds TC, Moss RB, Gardner P. A phase II, double-blind, randomized, placebo-controlled clinical trial of tgAAVCF using maxillary sinus delivery in patients with cystic fibrosis with antrostomies. Hum Gene Ther. 2002;13:1349–59. doi: 10.1089/104303402760128577. [DOI] [PubMed] [Google Scholar]
- Wu J, Zhao W, Zhong L, Han Z, Li B, Ma W, Weigel-Kelley KA, Warrington KH, Srivastava A. Self-complementary recombinant adeno-associated viral vectors: packaging capacity and the role of rep proteins in vector purity. Hum Gene Ther. 2007;18:171–82. doi: 10.1089/hum.2006.088. [DOI] [PubMed] [Google Scholar]
- Zhao W, Wu J, Zhong L, Srivastava A. Adeno-associated virus 2-mediated gene transfer: role of a cellular serine/threonine protein phosphatase in augmenting transduction efficiency. Gene Ther. 2007;14:545–50. doi: 10.1038/sj.gt.3302886. [DOI] [PubMed] [Google Scholar]
- Zhong L, Li B, Mah CS, Govindasamy L, Agbandje-McKenna M, Cooper MA, Herzog RW, Zolotukhin I, Warrington KH, Jr, Weigel-Van Aken KA, Hobbs JA, Zolotukhin S, Muzyczka N, Srivastava A. Next generation of adeno-associated virus 2 vectors: Point mutations in tyrosines lead to high-efficiency transduction at reduced doses. Proc. Natl. Acad. Sci., USA. 2008 doi: 10.1073/pnas.0802866105. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong L, Li W, Li Y, Zhao W, Wu J, Li B, Maina N, Bischof D, Qing K, Weigel-Kelley KA, Zolotukhin I, Warrington KH, Jr., Li X, Slayton WB, Yoder MC, Srivastava A. Evaluation of primitive murine hematopoietic stem and progenitor cell transduction in vitro and in vivo by recombinant adeno-associated virus vector serotypes 1 through 5. Hum Gene Ther. 2006a;17:321–33. doi: 10.1089/hum.2006.17.321. [DOI] [PubMed] [Google Scholar]
- Zhong L, Li W, Yang Z, Qing K, Tan M, Hansen J, Li Y, Chen L, Chan RJ, Bischof D, Maina N, Weigel-Kelley KA, Zhao W, Larsen SH, Yoder MC, Shou W, Srivastava A. Impaired nuclear transport and uncoating limit recombinant adeno-associated virus 2 vector-mediated transduction of primary murine hematopoietic cells. Hum Gene Ther. 2004;15:1207–18. doi: 10.1089/hum.2004.15.1207. [DOI] [PubMed] [Google Scholar]
- Zhong L, Zhao W, Wu J, Maina N, Han Z, Srivastava A. Adeno-associated virus-mediated gene transfer in hematopoietic stem/ progenitor cells as a therapeutic tool. Curr Gene Ther. 2006b;6:683–98. doi: 10.2174/156652306779010660. [DOI] [PubMed] [Google Scholar]
- Zhou SZ, Cooper S, Kang LY, Ruggieri L, Heimfeld S, Srivastava A, Broxmeyer HE. Adeno-associated virus 2-mediated high efficiency gene transfer into immature and mature subsets of hematopoietic progenitor cells in human umbilical cord blood. J Exp Med. 1994;179:1867–75. doi: 10.1084/jem.179.6.1867. [DOI] [PMC free article] [PubMed] [Google Scholar]


