Abstract
The atonal (ato) proneural gene specifies a stereotypic number of sensory organ precursors (SOP) within each body segment of the Drosophila ectoderm. Surprisingly, the broad expression of Ato within the ectoderm results in only a modest increase in SOP formation, suggesting many cells are incompetent to become SOPs. Here, we show that the SOP promoting activity of Ato can be greatly enhanced by three factors: the Senseless (Sens) zinc finger protein, the Abdominal-A (Abd-A) Hox factor, and the epidermal growth factor (EGF) pathway. First, we show that expression of either Ato alone or with Sens induces twice as many SOPs in the abdomen as in the thorax, and do so at the expense of an abdomen-specific cell fate: the larval oenocytes. Second, we demonstrate that Ato stimulates abdominal SOP formation by synergizing with Abd-A to promote EGF ligand (Spitz) secretion and secondary SOP recruitment. However, we also found that Ato and Sens selectively enhance abdominal SOP development in a Spitz-independent manner, suggesting additional genetic interactions between this proneural pathway and Abd-A. Altogether, these experiments reveal that genetic interactions between EGF-signaling, Abd-A, and Sens enhance the SOP-promoting activity of Ato to stimulate region-specific neurogenesis in the Drosophila abdomen.
Keywords: Atonal, Senseless, Proneural, Abdominal-A, Hox, EGF, sensory organ precursor, Rhomboid, oenocyte
INTRODUCTION
The Drosophila peripheral nervous system consists of a variety of sensory organs that detect stimuli such as light, sound, smell, taste, touch, and stretch (Jan and Jan, 1993; Lai and Orgogozo, 2004). While every sensory organ is highly specialized to perform a given function, each initially develops from precursor cells specified by a proneural gene. Proneural genes encode a family of related basic Helix-Loop-Helix (bHLH) transcription factors that are required for both the selection of the sensory organ precursor (SOP) as well as restricting its fate (Bertrand et al., 2002; Powell and Jarman, 2008). The atonal (ato) proneural gene, for example, specifies SOP cells that will form the internal proprioceptive stretch receptors known as chordotonal (ch) organs throughout the body segments as well as photosensitive and olfactory sense organs within the head segments (Gupta and Rodrigues, 1997; Jarman et al., 1993; Jarman et al., 1994; Jarman et al., 1995). In contrast, the achaete (ac) and scute (sc) family of proneural genes specifies SOP cells that will form the external sensory bristles (Jan and Jan, 1994; Villares and Cabrera, 1987). Thus, the type of sensory organ that forms is determined, at least in part, by the specific expression pattern of each proneural factor.
A great deal is known about how proneural genes select individual SOP cells. Initially, proneural genes are expressed in small cell clusters of ectoderm known as proneural fields in which each cell is competent to form an SOP cell (Parks et al., 1997; Simpson, 1997; Simpson and Carteret, 1990). However, many of these cells instead adopt an epidermal cell fate through a lateral inhibition mechanism utilizing the Notch-Delta signaling pathway (Lai, 2004). During SOP selection, the individual cells within the proneural field up-regulate proneural gene expression to activate Delta expression. The cell within the proneural field that produces the most Delta triggers high levels of Notch signaling in adjacent cells, which subsequently inhibits SOP fate and promotes epidermal cell development. Hence, the asymmetric activation of the Notch-Delta pathway results in a limited number of SOP cells forming from each proneural field.
Based on our understanding of SOP cell selection, the number of proneural fields as well as the size of the proneural field will dictate the number of sense organs that develop within each body region. Since Notch-Delta interactions require direct cell-cell contact, small proneural fields produce a single SOP whereas larger proneural fields can give rise to multiple SOPs (Lage et al., 1997). According to this model, the broad expression of a proneural gene should convert the ectoderm into a large proneural field and specify numerous SOP cells. Consistent with this idea, the broad expression of proneural genes can enhance the formation of sensory organs (Dominguez and Campuzano, 1993; Rodriguez et al., 1990). However, not all ectodermal cells are responsive to proneural factors as the ectopic expression of ato induces the formation of relatively few extra ch organs (Goulding et al., 2000; Jarman et al., 1993). These findings indicate many cells within the Drosophila ectoderm are incompetent to respond to ato to become a ch organ SOP cell. In this study, we investigate factors that enhance the proneural activity of ato within the developing Drosophila ectoderm.
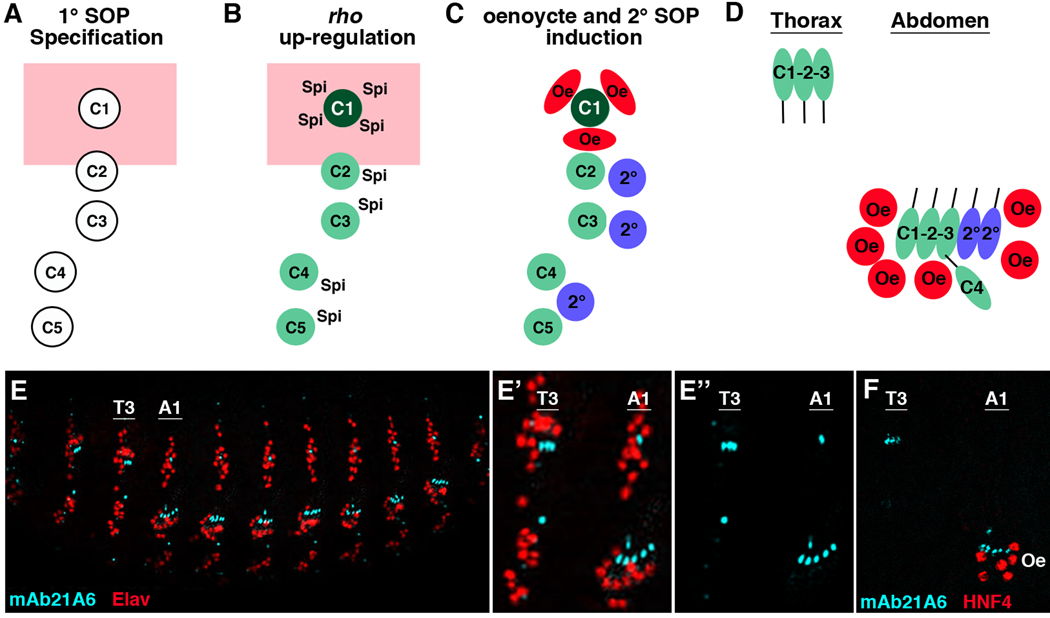
One mechanism that has been shown to stimulate the ability of ato to specify ch organ SOP cells is epidermal growth factor (EGF) signaling (Lage et al., 1997; Okabe and Okano, 1997). zur Lage et al. have shown that ato-expressing cells that receive the Spitz (Spi) EGF ligand can further up-regulate ato expression through an auto-regulatory enhancer that directly integrates both Ato and ETS (Pointed, an effector of EGF signaling) transcriptional inputs (zur Lage et al., 2004). Hence, EGF signaling enhances Ato expression resulting in the formation of additional ch organ SOPs. This model has direct physiological relevance as a subset of ato-expressing ch organ SOP cells have been shown to stimulate the secretion of the Spi ligand to induce the recruitment of additional SOP cells (Lage et al., 1997; Okabe and Okano, 1997). In the Drosophila abdomen, for example, five primary (1°) ch organ SOP cells activate the expression of the Rhomboid (Rho) protease to trigger Spi secretion and induce the formation of three secondary (2°) ch organ SOPs (Figure 1A–C). Thus, ato induces two types of ch organ SOP cells: 1° SOPs that form independent of EGF signaling, and 2° SOPs that are dependent upon EGF signaling.
Figure 1. Induction of oenoyctes and secondary ch organ SOP cells by EGF signaling.
(A–C) Diagram of a typical abdominal segment (A1–A7) showing the development of the C1-C5 primary (1°) SOP cells, the secondary (2°) SOP cells, and the oenocytes. Dorsal is at top and the Spalt expression domain is shown in pink. (A) First, a set of 1° ch organ SOP cells are specified by ato during early embryogenesis (late stage 10/early stage 11). (B) Shortly after specification, the abdominal 1° SOP cells up-regulate rho and secrete the Spitz (Spi) EGF ligand. The relative expression levels of rho are denoted in green with the dorsal-most SOP cell (C1) expressing the highest amount of rho. (C) The activation of EGF signaling in neighboring cells induces a cluster of oenocytes (Oe, red) within the Spalt expression domain and three 2° ch organ SOP cells ventral to Spalt expression. (D) Close-up schematic of a stage 16 thoracic and abdominal segment comparing ch organ development and oenocyte formation within the dorsal/lateral ectoderm. Note, the T2/T3 thoracic segments contain three 1° SOPs (C1–C3) that form a dorsal ch organ consisting of three scolopodia (dch3). In contrast, the abdominal segments recruit two 2° SOPs to form a lateral ch organ with five scolopodia (lch5) as well as an lch1 organ (derived from the C4 SOP in A–C). In addition, only the abdominal segments recruit oenocytes that form in clusters in close proximity to the lch5 organ. For simplicity, only the neurons of each scolopodia are shown and the C5 neuron and other 2° scolopodia that form the ventral ch organs (VchA and VchB) are not shown. (E) Lateral view of a stage 16 Drosophila embryo immunostained using the mAb21A6 antibody (blue) that marks the scolopodial sensory cilia and a general nuclear neuronal marker (Elav, red). (E’-E”) Close-up view of the T3 thoracic and A1 abdominal segments highlighting the dch3 and lch5 scolopodial sensory cilia. (F) Close-up view of the T3 thoracic and A1 abdominal segment of a stage 16 Drosophila embryo immunostained using mAb21A6 (blue) and a HNF4 antibody (red) that marks oenocytes. Note that oenocytes only form within the abdominal segments.
While both the thoracic and abdominal segments of the developing Drosophila embryo make 1° ch organ SOP cells, only the abdominal 1° SOPs that express the abdominal-A (abd-A) Hox factor stimulate sufficient rho expression to induce 2° ch organ SOP cells (Brodu et al., 2002; Heuer and Kaufman, 1992; Wong and Merritt, 2002). Moreover, not all Spi-receiving cells adopt a 2° ch organ SOP fate, as EGF signaling initiated by the 1° ch organ SOP cells also induces the formation of the larval oenocytes (Figure 1). Larval oenocytes are an abdomen-specific cell type that form in clusters of three to nine cells and are essential for lipid metabolism and larval growth (Brodu et al., 2002, 2004; Gutierrez et al., 2007). In contrast, even though a similar set of 1° ch organ SOP cells forms in the thorax, these SOPs do not up-regulate rho to recruit 2° SOPs or oenocytes resulting in segmental differences in sensory organ structure and embryonic patterning (Figure 1D–F).
The decision to form an abdominal 2° SOP or larval oenocyte and the number of each cell type generated is determined by the levels of EGF ligand received and whether the receiving cell expresses the Spalt transcription factors (Spalt-major (Salm) and Spalt-related (Salr)) (Elstob et al., 2001; Rusten et al., 2001). Oenocytes are induced within the Spalt-positive dorsal ectoderm of each abdominal segment by the dorsal-most 1° ch organ SOP cell (the C1 cell) that expresses the highest level of rho (Figure 1) (Lage et al., 1997). In contrast, the three 2° SOP cells form from cells within the Spalt-negative ectoderm that lie in close proximity to the ventrally located 1° SOPs (C2-C5) that express lower levels of rho (Lage et al., 1997). When EGF-mediated signaling is activated throughout the ectoderm, numerous oenocytes are specified whereas only one or two extra 2° ch organ SOPs develop per segment (Elstob et al., 2001; Lage et al., 1997; Okabe and Okano, 1997; Rusten et al., 2001). Thus, many cells within the ectoderm are capable of responding to EGF signaling to form oenocytes, but relatively few can form SOPs.
Here, we further investigate the genetic relationship between ato, EGF signaling, and the Abd-A Hox factor in promoting the competency of the Drosophila ectoderm to generate ch organ SOP cells. First, we show that ectopic Ato promotes the formation of twice as many ch organ SOP cells in the abdomen than the thorax. Moreover, this enhancement of ch organ SOP cell development comes at the expense of oenocyte formation. Second, we show that the Abd-A Hox factor synergizes with Ato to promote the formation of 2° ch organ SOPs that require Spi-mediated signaling for their specification. Lastly, we show that the co-expression of Ato with the Senseless (Sens) zinc finger protein that is essential for nearly all peripheral nervous system development also promotes the formation of many ch organ SOP cells (Nolo et al., 2000). Importantly, Ato and Sens induce significantly more ch organ SOP cells in the abdomen than in the thorax, and surprisingly do so using both Spi-dependent and Spi-independent mechanisms. In total, these experiments reveal that genetic interactions between the EGF pathway, the Abd-A Hox factor, and the Sens zinc finger protein enhance the sensory organ promoting activity of Ato to result in region-specific neurogenesis in the Drosophila abdomen.
RESULTS
Atonal and the specification of abdominal cell fates
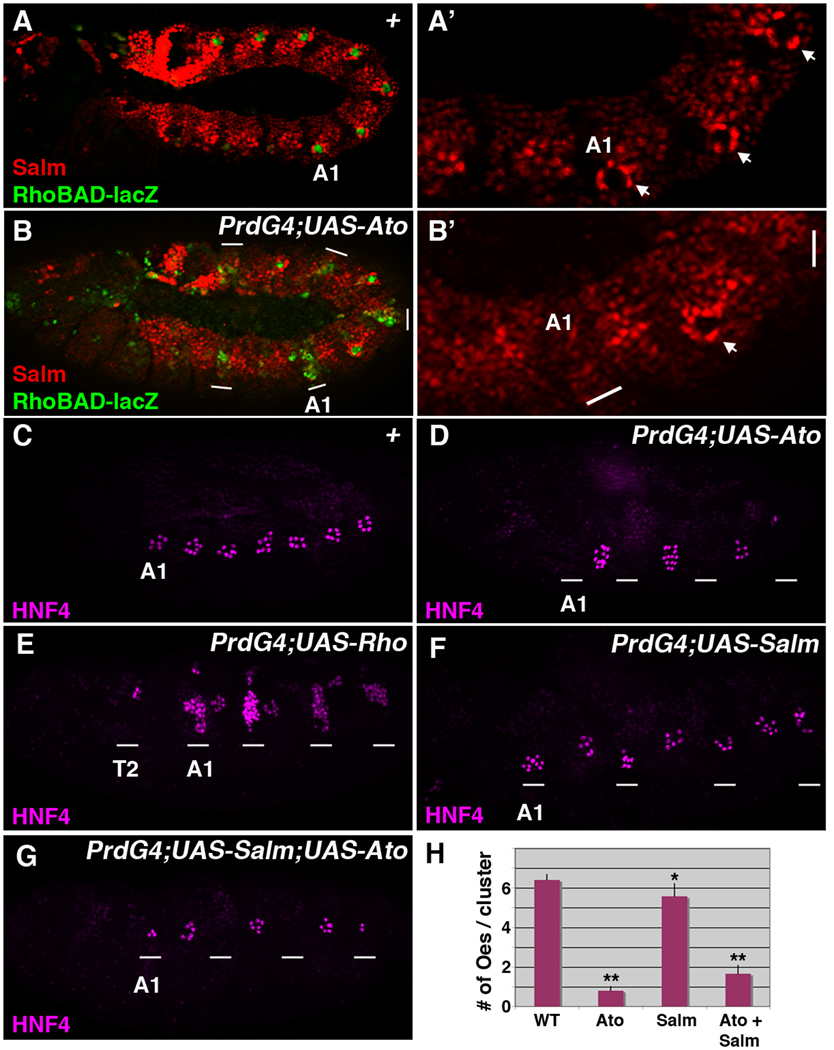
Expression of the Rho protease within a set of abdominal ch organ SOP cells triggers the secretion of the Spi EGF ligand to recruit abdomen-specific oenocytes and 2° ch organ SOP cells (Figure 1). We recently identified a rho enhancer (RhoBAD) that is expressed within the same abdominal C1 SOP cells that induce oenocyte formation (Figure 2A) and found that both RhoBAD activity and oenocyte recruitment are dependent upon the Ato proneural factor (Gebelein, 2008; Li-Kroeger et al., 2008; Witt et al., 2010). Moreover, the ectopic expression of Ato in every other segment of the developing embryo using the PrdG4 driver results in the stimulation of RhoBAD-lacZ activity in additional cells (Figure 2B). These findings indicate that ectopic Ato activates additional rho expression, which should subsequently enhance Spi secretion to induce more oenocytes. However, expression analysis of PrdG4;UAS-Ato embryos for an early oenocyte marker (high levels of Salm in cells surrounding C1 SOP cells) revealed a sharp decrease in oenocytes within the PrdG4-driven Ato-expressing segments (Figure 2B). Analysis of older embryos using an additional marker of oenocyte fate (HNF4, (Palanker et al., 2009)) confirmed this finding, with most Ato-expressing segments showing a complete loss of oenocytes (Figure 2C and 2D). Quantification of the number of oenocytes that develop within PrdG4;UAS-Ato embryos revealed that only 0.8 ± 0.2 oenocytes formed in the PrdG4-on Ato-expressing segments while 6.4 ± 0.3 oenocytes formed in the PrdG4-off non-Ato expressing segments (Figure 2H, p-value < 0.001). In contrast, the direct activation of rho (PrdG4;UAS-Rho) induced the formation of a large number of oenocytes (Figure 2E). Thus, Ato stimulates RhoBAD activity yet inhibits the formation of an EGF-dependent cell type (oenocytes).
Figure 2. Atonal inhibits oenocyte formation.
(A–B) Lateral views of a wild type stage 11 RhoBAD-lacZ Drosophila embryo (A-A’) and a stage 11 PrdG4;UAS-Ato;RhoBAD-lacZ embryo (B-B’) immunostained for β-gal (green) and Salm (red). The first abdominal segment is labeled (A1). In wild type embryos, the cells surrounding the β-gal-positive cell show an up-regulation of Salm (arrows in A’). In contrast, expression of Ato (B) in every other segment using PrdG4 (white line marks regions of PrdG4 activity) stimulates RhoBAD-lacZ activity yet decreases Salm expression compared to the PrdG4-off segments (A2 segment, arrow in B’). (C–G) Lateral views of stage 16 wild type stage (C), PrdG4;UAS-Ato (D), PrdG4;UAS-Rho (E), PrdG4;UAS-Salm (F), and PrdG4;UAS-Salm;UAS-Ato (G) embryos immunostained for HNF4 (purple). The first abdominal segment (A1) of each embryo is labeled and the white lines mark segments where PrdG4 is active. Note, the wild type embryo (C) has clusters of oenocytes in seven abdominal segments (A1–A7), whereas Ato mis-expression (D) inhibits oenocyte formation. In contrast, segments of PrdG4;UAS-Rho embryos expressing the Rho protease induce extra oenocytes (E). The expression of Salm results in a modest decrease in oenocytes, whereas the co-expression of both Ato and Salm results in a significant decrease in oenocytes. (H) Quantification of oenocyte numbers per cluster comparing wild type segments with segments expressing either Ato, Salm, or Ato and Salm from at least ten embryos. * denotes p-value < 0.01 and ** denotes p-value < 0.001 using ANOVA.
Since PrdG4 drives gene expression in oenocyte precursor cells as well as ch organ SOPs, the loss of oenocytes could be explained by: 1) Ato inducing oenocyte precursors to undergo apoptosis, 2) Ato interfering with the reception of Spi (EGF), 3) Ato repressing Salm expression and thereby suppresses oenocyte fate downstream of EGF signaling, and/or 4) Ato re-programming the precursors to a different fate. To address these possibilities, we first examined Ato-expressing segments for the induction of cell death using a marker of apoptosis (anti-cleaved caspase-3) and did not detect an appreciable difference in cell death (data not shown). Next, we examined the reception of Spi (EGF) using an antibody against activated ERK (phospho-ERK), a downstream effector of the EGF pathway (Gabay et al., 1997). In wild type embryos, anti-phospho-ERK labels the Salm-positive whorls of oenocytes surrounding the abdominal C1 SOP (marked by RhoBAD-lacZ activity, Figure 3A). In contrast, thoracic segments that lack oenocytes also lack significant β-gal expression and fail to accumulate phospho-ERK staining around the C1 SOP. Analysis of PrdG4;UAS-Ato embryos revealed that phospho-ERK is visible in whorls of cells in abdominal segments that ectopically express Ato (Figure 3B). Moreover, consistent with Ato inducing additional RhoBAD-lacZ expressing cells, some Ato-expressing segments have an extra phospho-ERK-positive whorl of cells (arrows in A1 segment, Figure 3B’ and 3B”). These data suggest that Ato does not interfere with either the production or reception of the EGF signal. Nevertheless, we wanted to further exclude this possibility by using PrdG4 to provide high levels of Ato and Rho in the same cells. Expressed by itself, the Rho protease is sufficient to trigger high levels of phospho-ERK throughout the PrdG4 expression domain resulting in the induction of many Spalt-positive oenocytes (Figure 3C). In contrast, co-expression of Ato and Rho (PrdG4;UAS-Ato;UAS-Rho) suppresses the induction of Salm-positive cells within the PrdG4 expression domain, even though these segments exhibit high levels of phospho-ERK (Figure 3D). As phospho-ERK lies downstream of the activated EGF-receptor, these results show that Ato does not inhibit oenocyte formation by interfering with the production or the reception of the Spi signal.
Figure 3. Ato inhibition of oenocyte formation is not due to interference with EGF signaling.
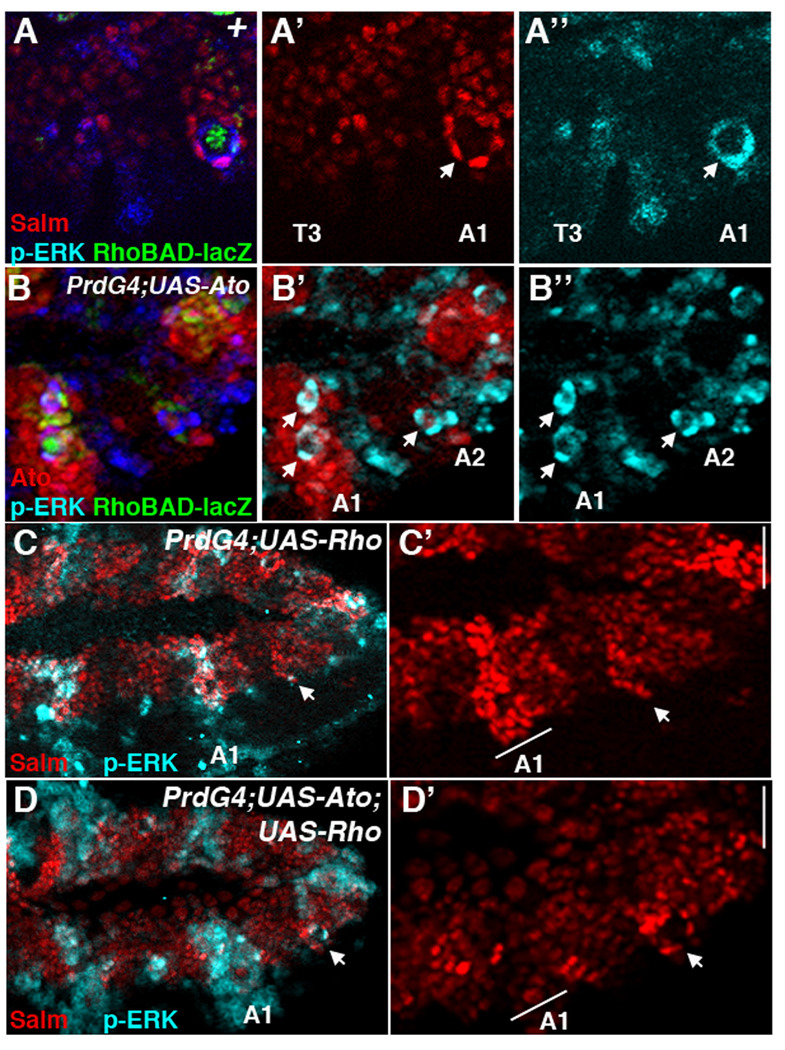
(A-A”) Lateral view of a wild type RhoBAD-lacZ stage 11 embryo showing thoracic (T3) and abdominal (A1) segments immunostained for β-gal (green), Salm (red), and phospho-ERK (blue). β-gal marks the C1 SOP cell that is surrounded by oenocytes (Salm-positive) that co-label with high levels of phospho-ERK. The thorax lacks these cells and significant phospho-ERK activity. (B-B”) Lateral view of a PrdG4; UAS-Ato;RhoBAD-lacZ stage 11 embryo immunostained for Ato (red), β-gal (green), and phospho-ERK (blue). Arrows denote phospho-Erk-positive cell whorls. Note that the first abdominal segment (A1) expresses high levels of Ato and has two visible phospho-ERK-positive cell whorls (arrows). (C–D) Lateral views of stage 11 PrdG4;UAS-Rho stage 11 (C-C’) and PrdG4;UAS-Ato;UAS-Rho stage 11 (D-D’) embryos immunostained for Salm (red) and phospho-ERK (blue). PrdG4 drive Rho expression to activate phospho-ERK in every other segment in both embryos. However, only the PrdG4-on segments expressing rho alone (C-C’) have many cells expressing high levels of Salm. In contrast, the PrdG4-on segments expressing both rho and ato have few cells expressing high levels of Salm. Note, that oenocyte whorls do form in the PrdG4-off segments of both embryos (arrow).
Next, we wanted to determine if Ato is suppressing oenocyte fate through the down-regulation of Salm expression (Figure 2B). Since Salm is required for the specification of oenocytes within abdominal segments (Elstob et al., 2001; Rusten et al., 2001), the decreased Salm expression observed in PrdG4;UAS-Ato segments could explain the subsequent loss of oenocyte formation (0.8 ± 0.2 per cluster, Figure 2D). To test this idea, we determined if the co-expression of Salm with Ato could rescue oenocyte formation. As shown in Figure 2, we compared oenocyte formation in PrdG4 embryos that express Ato alone, Salm alone, or both Ato and Salm using the HNF4 antibody (mature oenocyte marker). As previously described by Elstob et al (Elstob et al., 2001), the expression of Salm alone is insufficient to increase oenocyte numbers (Figure 2F) and actually has a small negative affect on oenocyte production (Salm-expressing segments generate 5.6 ± 0.6 oenocytes per cluster compared to 6.4 ± 0.2 oenocytes in control segments, Figure 2H, p-value < 0.01). In contrast, the co-expression of Ato and Salm resulted in a large loss of oenocytes with only 1.65 ± 0.4 forming per cluster (Figure 2G and 2H, p-value < 0.001). Taken together, these findings indicate that Ato does not block oenocyte formation through the down-regulation of Salm, suggesting Ato is reprogramming these cells to another cell fate downstream of the EGF signaling pathway.
Atonal promotes the formation of abdominal ch organs at the expense of oenocytes
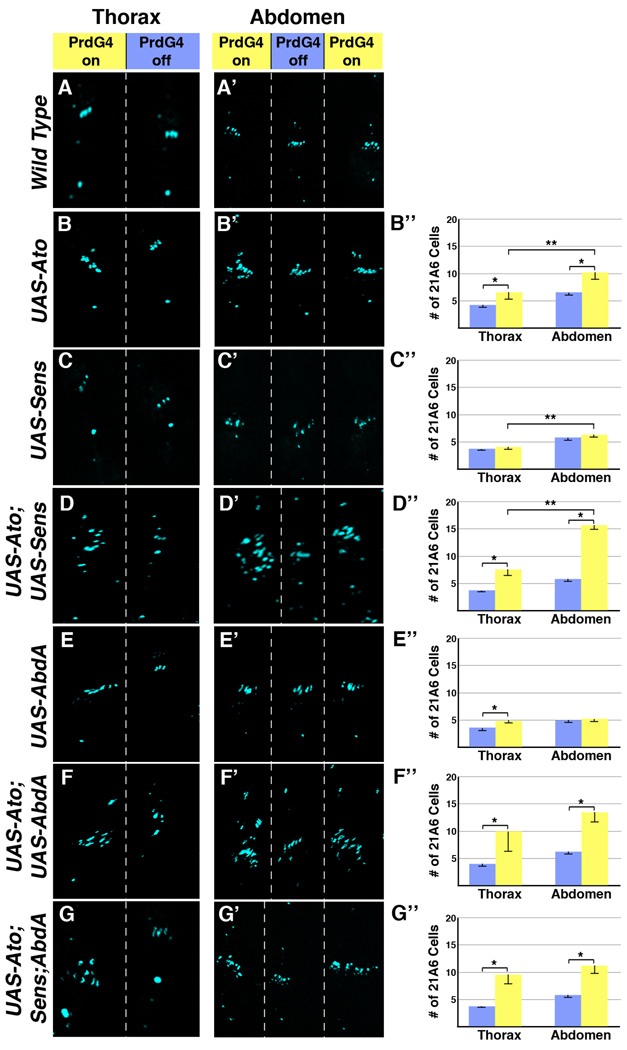
Previous studies demonstrated that expressing Ato using heat-shock or Gal4 drivers can induce the formation of ch organ scolopodia (Jarman and Ahmed, 1998; Jarman et al., 1993). To determine if the loss of abdominal oenocytes correlates with a gain in ch organ scolopodia, we quantified ch organ numbers in PrdG4;UAS-Ato embryos using the mAb21A6 antibody that marks the sensory cilium of ch organs (see Figure 1E). Since the curvature of the embryo makes it difficult to assess ch organ formation around the entire embryo, we limited our analysis to the dorsal/lateral PNS that contains the lch5 and lch1 organs within abdominal segments or the dch3 organ within thoracic segments (see Figure 1D–E). As shown in Figure 4B, an increase in 21A6-positive cells is observed in abdominal segments mis-expressing Ato (PrdG4-On) compared to segments not mis-expressing Ato (PrdG4-Off). However, the number of extra scolopodia (3.64 +/− 1.35) is less than the number of oenocytes that form in the PrdG4-off abdominal segments (6.4 +/− 0.2), indicating Ato does not convert all of the oenocyte precursors into sensory organ precursors. We also analyzed the ability of Ato to induce scolopodia in thoracic regions that lack oenocyte and 2° SOP recruitment. As shown in Figure 4B, we found that Ato induces additional scolopodia in the thorax, although to a significantly lesser extent than in abdominal segments (approximately half the number of mAb21A6-positive cilium, Figure 4B). These data show that expressing Ato in the ectoderm induces more scolopodia in the abdomen than in the thorax, but that Ato does not convert all potential oenocyte precursors to a ch organ fate.
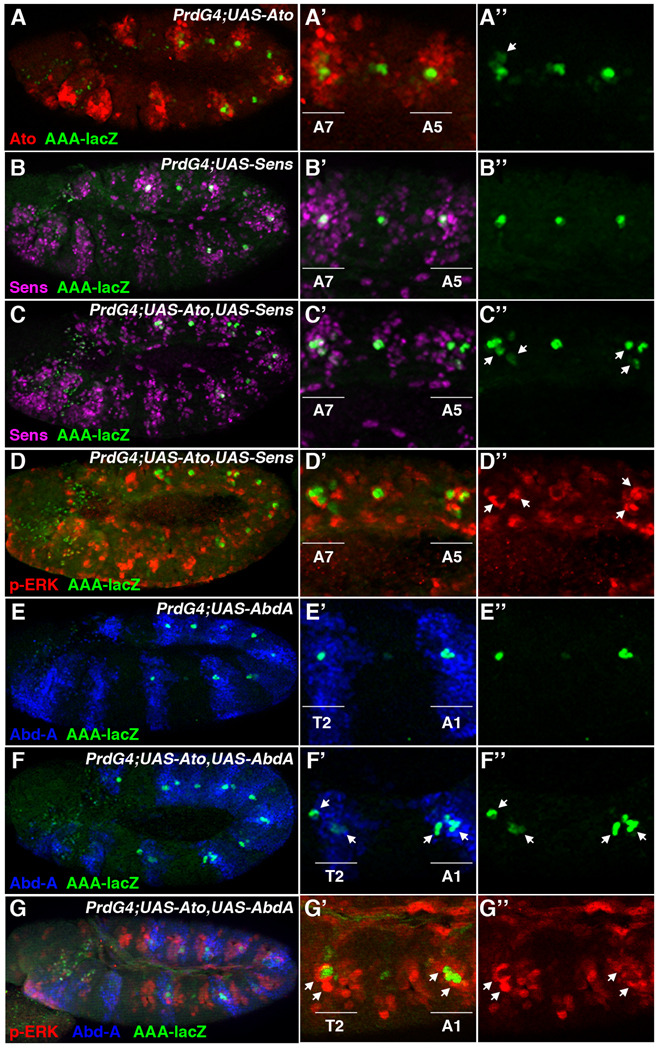
Figure 4. Ato synergizes with Abd-A to promote ch organ formation in the abdomen.
PrdG4 Drosophila embryos with UAS-transgenes were immunostained for a marker (mAb21A6, blue) of the scolopodial sensory cilia. Close up views of the dorsal and lateral PNS of the thorax and abdomen are shown and segments that express (PrdG4-on, yellow) and do not express (PrdG4-off, blue) PrdG4 are highlighted at top. Graphs at right quantify data from at least 10 embryos. * denotes significant difference (P-value of at least 0.01) in scolopodia numbers between PrdG4-on and PrdG4-off within a body region (thorax or abdomen). ** denotes significant difference (P-value of at least 0.01) in scolopodia numbers in PrdG4-on segments between body regions (thorax versus abdomen). (A-A’) Immunostaining of scolopodial sensory cilia within wild type thoracic and abdominal segments that lack UAS-transgene activity. (B-B”) PrdG4;UAS-Ato embryos have significantly more scolopodia in PrdG4-on abdominal segments compared to either abdominal PrdG4-off or thoracic PrdG4-on segments. Ato induces 3.7 +/− 1.4 extra scolopodia in abdominal segments and 2.1 +/− 1.5 scolopodia in thoracic segments (P-value between PrdG4-on in abdomen and thorax is less than 0.001). (C-C”) Ectopic Sens (PrdG4;UAS-Sens) has little affect on the number of scolopodia in either the thorax or abdomen (0.6 +/− 0.3 extra in abdomen versus 0.4 +/− 0.2 extra in thorax, no significant difference). (D-D”) Co-expression of Ato and Sens (PrdG4;UAS-Ato;UAS-Sens) induces significantly more scolopodia in the abdomen than in the thorax (9.8 +/− 0.4 extra in abdomen versus 4.4 +/− 0.9 in thorax, p-value < 0.001). (E-E”) PrdG4;UAS-Abd-A embryos have two additional scolopodia in the thorax but no additional scolopodia in the abdomen (0.1 +/− 0.1 extra in abdomen versus 1.9 +/− 0.1 in thorax). (F-F”) Co-expression of Ato and Abd-A (PrdG4;UAS-Ato;UAS-Abd-A) significantly enhances the number of scolopodia in both the thorax and abdomen (8.6 +/− 4.0 extra in abdomen versus 7.2 +/− 4.5 in thorax). (G-G”) Co-expression of Ato, Sens, and Abd-A (PrdG4;UAS-Ato,UAS-Sens;UAS-Abd-A) significantly enhances the number of scolopodia in both the thorax and abdomen (6.4 +/− 2.8 extra in abdomen versus 5.1 +/−1.0 in thorax).
Senseless and Abdominal-A synergize with Ato to promote ch organ formation
While the broad expression of Ato can enhance ch organ formation, surprisingly few extra ch organs are induced in either the thorax or abdomen. This finding suggests that many cells are not competent to respond to Ato. Previous studies demonstrated that the neural promoting activities of the bHLH proneural factors can be stimulated by the Sens zinc finger protein (Acar et al., 2006; Jafar-Nejad et al., 2003; Nolo et al., 2000). In particular, Sens has been shown to synergize with the Scute proneural factor to greatly enhance the formation of sensory bristles (Jafar-Nejad et al., 2003; Nolo et al., 2000). To determine if Sens can synergize with Ato to promote the formation of embryonic ch organs, we used PrdG4 to express Sens alone or in combination with Ato and analyzed both the thoracic and abdominal segments for scolopodia formation. As shown in Figure 4C, the expression of Sens alone (PrdG4;UAS-Sens) did not significantly affect scolopodia numbers in either the abdomen or the thorax. In contrast, the co-expression of both Ato and Sens (PrdG4;UAS-Ato;UAS-Sens) greatly increased the number of scolopodia in both regions of the embryo as compared to either factor alone (Figure 4D). Moreover, we found that Ato and Sens co-expression induced twice as many extra scolopodia in the abdomen (9.8 ± 0.39) as in the thorax (4.90 ± 0.58). These data indicate that, similar to Ato alone, Ato-Sens co-expression promotes neural fates more strongly in the abdomen than in the thorax.
An obvious candidate for providing enhanced abdominal neural competency in response to the Ato-Sens pathway is the Abd-A Hox factor. abd-A is expressed within all the abdominal segments that induce oenocytes and 2° ch organ SOP cells and has been shown to convert the dch3 thoracic ch organ to an lch5 fate when expressed within the thorax (Brodu et al., 2002; Gebelein and Mann, 2007; Heuer and Kaufman, 1992; Li-Kroeger et al., 2008; Wong and Merritt, 2002). To determine if Abd-A can synergize with Ato to enhance ch organ formation, we co-expressed both factors and assayed for scolopodia formation. As expected, ectopic expression of Abd-A alone (PrdG4;UAS-AbdA) induced two additional scolopodia within the thorax but did not alter ch organ formation in abdominal segments that already express this Hox factor (Figure 4E). However, when both Abd-A and Ato are co-expressed, scolopodia numbers are dramatically increased (Figure 4F). Whereas Abd-A alone fails to induce more abdominal scolopodia and Ato alone induces three to four extra abdominal scolopodia, Ato and Abd-A together induce an average of eight extra scolopodia per segment. Co-expression of Ato and Abd-A in the thorax induces a similar number of extra scolopodia. In contrast, the co-expression of Ato with a thoracic Hox factor (Antennapedia, Antp) failed to significantly affect the ability of Ato to stimulate ch organ SOP cells (data not shown). Thus, ato can synergize with a specific Hox factor (abd-A) to promote neural competency.
Since both Sens and Abd-A synergize with Ato to promote scolopodial formation, we next assayed if the expression of all three factors could further cooperate to induce additional sensory organ formation. To do so, we analyzed PrdG4;UAS-Ato,UAS-Sens;UAS-AbdA embryos and found that the number of scolopodia formed is similar to the co-expression of Ato and Abd-A (compare Figure 4G with 4F). Hence, whenever Ato is co-expressed with either Sens or Abd-A, the number of scolopodia formed is significantly increased compared to Ato expression alone. However, no further synergy in promoting sensory organ formation is observed when Ato, Sens, and Abd-A are all co-expressed.
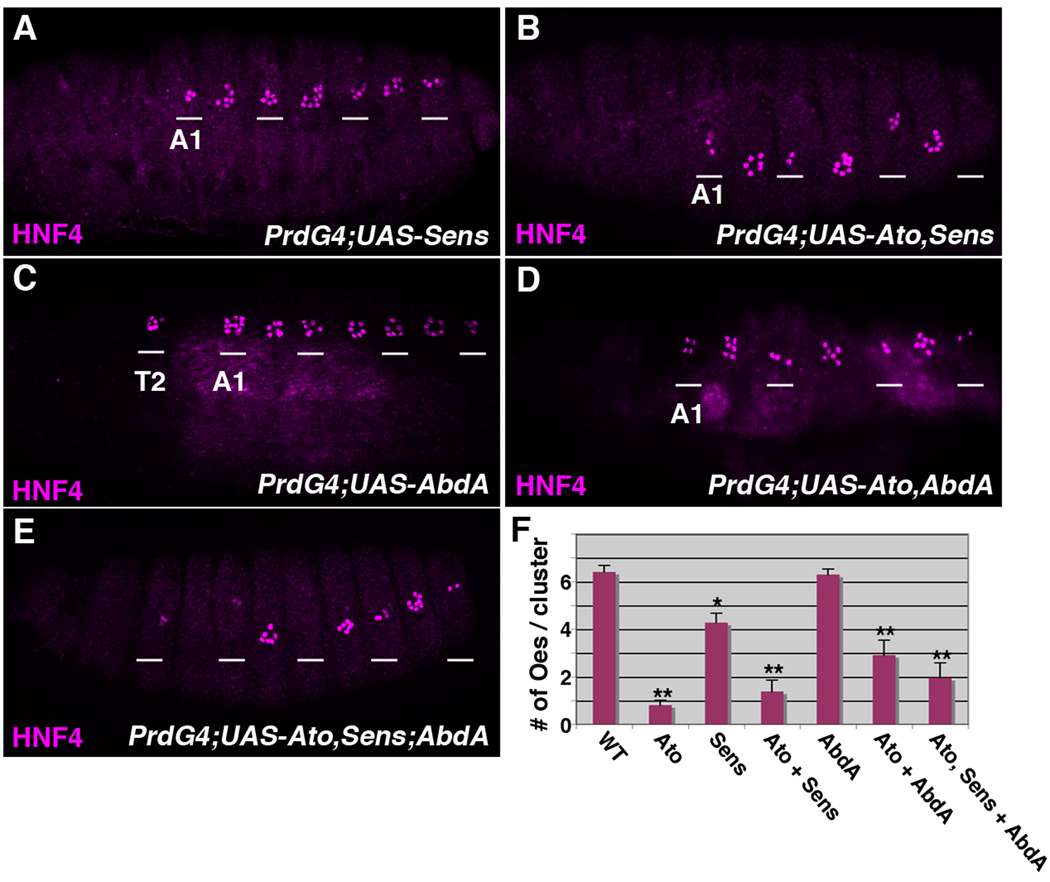
Lastly, we analyzed if the enhanced sensory organ formation observed when Ato is co-expressed with Sens and/or Abd-A also correlates with a loss in oenocyte production. As shown in Figure 5, the ectopic expression of Sens alone resulted in a modest decrease in oenocyte production (from 6.4 ± 0.3 per cluster in wild type segments to 4.3 ± 0.4 per cluster in Sens expressing segments, Figure 5A and 5F), whereas the co-expression of Ato and Sens resulted in significantly fewer oenocytes (1.4 ± 0.5 per cluster, Figure 5B and 5F). Similarly, while the expression of Abd-A had no affect on oenocyte numbers in the abdomen (6.3 ± 0.2 per cluster), the co-expression of Ato and Abd-A (2.9 ± 0.6 per cluster) as well as Ato, Sens and Abd-A (2.0 ± 0.6 per cluster) resulted in significantly fewer oenocytes (Figure 5C–F). Thus, the expression of Ato consistently promotes ch organ development at the expense of oenocyte formation.
Figure 5. The co-expression of Ato with Sens and/or AbdA inhibits oenocyte formation.
Lateral views of stage 16 PrdG4;UAS-Sens (A), PrdG4;UAS-Ato,UAS-Sens (B), PrdG4;UAS-AbdA (C), PrdG4;UAS-Ato,UAS-AbdA (D) and PrdG4;UAS-Ato,UAS-Sens;UAS-AbdA (E) embryos immunostained with the HNF4 (purple) oenocyte marker. The wild type control and PrdG4;UAS-Ato embryos are shown in Figure 2. The first abdominal segment (A1) of each embryo is labeled and the white lines mark segments where PrdG4 is active. The expression of Sens alone causes a modest decrease in oenocyte number (A), whereas the expression of Abd-A alone does not significantly alter oenocyte numbers within the abdomen but does induce their formation within the thoracic T2 segment (C). Note, that whenever Ato is co-expressed with Sens (B), AbdA (D), or both factors (E) significantly fewer oenoctyes are observed. (F) Quantification of oenocyte numbers per cluster from at least ten embryos comparing wild type segments (taken from Figure 2) with segments expressing either Ato (taken from Figure 2), Sens, Ato and Sens, Abd-A, Ato and Abd-A, and all three factors. * denotes p-value < 0.01 and ** denotes p-value < 0.001 using ANOVA.
The Ato proneural pathway and Abd-A promote EGF signaling
Two types of ch organ SOP cells are made within the embryonic ectoderm: 1° ch organ SOP cells that develop independent of EGF signaling within both the thorax and abdomen, and 2° ch organ SOP cells that are dependent upon rho-mediated EGF signaling and develop only within the abdomen (Figure 1, and (Lage et al., 1997; Okabe and Okano, 1997). Since Ato-Sens co-expression stimulated the formation of more ch organ scolopodia in the abdomen relative to the thorax, we postulated that this proneural pathway may enhance sensory organ formation by increasing the number of rho expressing cells within the abdomen. We addressed this possibility by analyzing the expression of the RhoAAA-lacZ reporter that marks the abdominal Spi-secreting C1 SOP cells in the early embryo (Witt et al., 2010). As shown in Figure 6, we found that Sens expression alone had no effect on the number of RhoAAA-positive cells, whereas Ato alone had a modest effect with a minority of segments containing additional weakly positive RhoAAA cells (Figure 6A and 6B). However, their co-expression resulted in the induction of additional RhoAAA-positive cells within the abdomen compared to control segments (Figure 6C). Moreover, the abdominal segments showing increased Rho enhancer activity also displayed increased phospho-ERK activity in nearby cells, congruent with the prediction that these cells secrete the Spi ligand (Figure 6D). In contrast, thoracic segments co-expressing Ato and Sens did not have significantly enhanced phospho-ERK activity (Supplemental Figure 1). Thus, the Ato-Sens pathway can increase EGF signaling within the abdomen.
Figure 6. Ato and Abd-A increase Rho enhancer activity and phospho-ERK signaling.
(A-A”) Lateral view of a stage 11 PrdG4;UAS-Ato;RhoAAA-lacZ embryo immunostained for β-gal (green) and Ato (red). Ectopic Ato has a small affect on RhoAAA activity in the thoracic segment (T2), and occasionally enhances RhoAAA activity in additional cells of the abdomen (arrow). (B-B”) Lateral view of a stage 11 PrdG4;UAS-Sens;RhoAAA-lacZ Drosophila embryo immunostained for β-gal (green) and Sens (purple). Ectopic Sens does not alter the number of RhoAAA-positive cells within either the thorax or the abdomen. (C-C”) Lateral view of a stage 11 PrdG4;UAS-Ato UAS-Sens;RhoAAA-lacZ Drosophila embryo immunostained for β-gal (green) and Sens (purple). Co-expression of Ato and Sens significantly stimulates the number of RhoAAA-positive cells within the abdomen but not the thorax. (D-D”) Lateral view of the same embryo in C immunostained for phospho-ERK (red) and β-gal (green). Close-up view of PrdG4-On and PrdG4-Off abdominal segments reveals that the co-expression of Ato and Sens induces additional phospho-ERK (arrows) compared to a control abdominal segment. (E-E”) Lateral view of a stage 11 PrdG4;UAS-Abd-A;RhoAAA-lacZ Drosophila embryo immunostained for β-gal (green) and Abd-A (blue). Ectopic Abd-A expression enhances RhoAAA activity in the thoracic segment (T2), but has no additional affect on β-gal expression in the abdomen. (F-F”) Lateral view of a stage 11 PrdG4;UAS-Ato,UAS-AbdA;RhoAAA-lacZ Drosophila embryo immunostained for β-gal (green) and Abd-A (blue). Co-expression of Ato and AbdA activates RhoAAA activity in multiple cells of both the thoracic (T2) and abdominal segments (arrows). (G-G”) Lateral view of a stage 11 PrdG4;UAS-Ato,UAS-AbdA;RhoAAA-lacZ Drosophila embryo immunostained for phospho-ERK (red), Abd-A (blue), and β-gal (green, in G’). Close-up view of PrdG4-On and PrdG4-Off segments reveals that the co-expression of Ato and Abd-A induces phospho-ERK (arrows) surrounding the β-gal-positive cells within thoracic and abdominal segments.
Since Abd-A also significantly stimulated the neural promoting activities of Ato, we next determined whether their co-expression could similarly enhance EGF signaling. In our previous studies, we demonstrated that Abd-A directly regulates the activity of Rho enhancers within the abdominal C1 SOP cells (Li-Kroeger et al., 2008; Witt et al., 2010). Consistent with these findings, PrdG4-mediated expression of Abd-A alone activated RhoAAA-lacZ expression in a single cell of the T2 thoracic segment but did not significantly alter rho enhancer activity in abdominal segments that already express this Hox factor (Figure 6E). However, the co-expression of both Ato and Abd-A frequently induced extra β-gal positive SOP cells in both the thorax and abdomen (Figure 6F). Congruent with this result, we found that the Ato/Abd-A expressing segments frequently induced more phospho-ERK activity than control segments (see arrows, Figure 6G). Altogether, these data show that the Ato proneural factor (or Ato/Sens) can synergize with Abd-A to promote rho expression and EGF signaling within the Drosophila abdomen.
Segmental differences in Ato activity and the EGF pathway
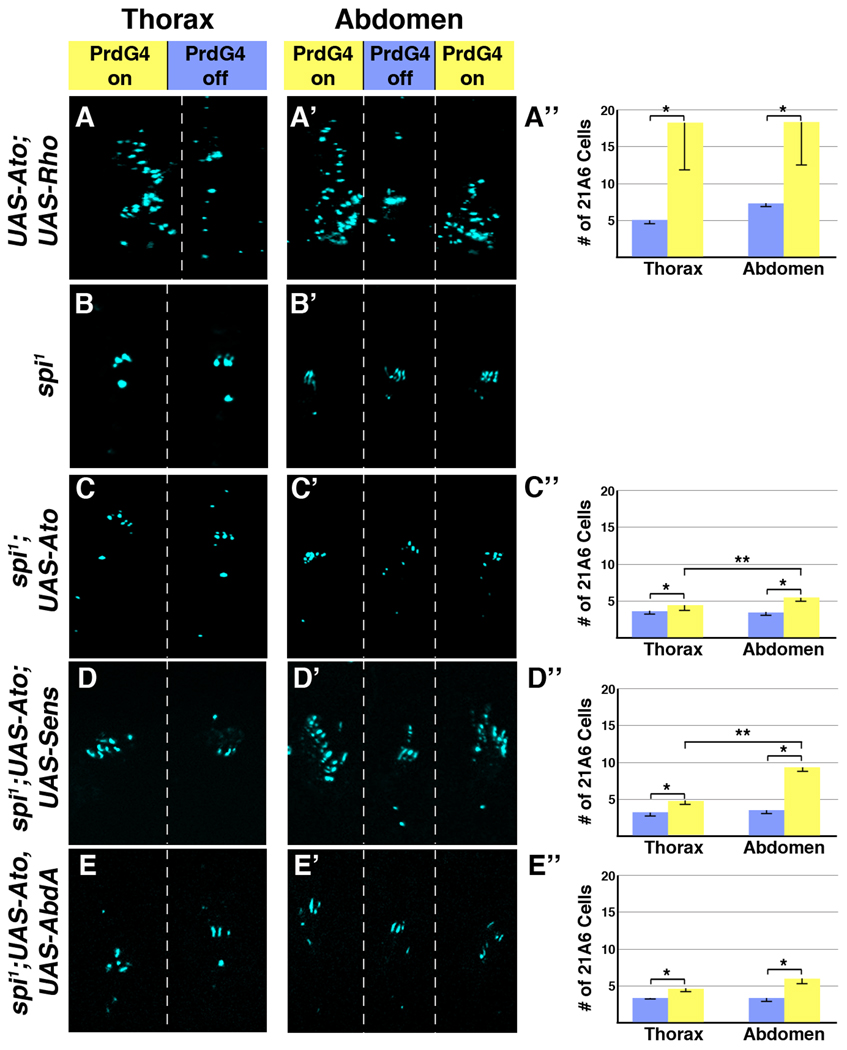
In total, our data suggest that the Ato-Sens proneural factors promote extra ch organ formation in the abdomen by increasing the number of rho-expressing 1° SOP cells that subsequently recruit additional 2° SOP cells. Hence, differences in EGF signaling between the thoracic and abdominal body regions would affect Ato’s ability to induce scolopodia. To further test this idea, we first “normalized” EGF signaling between the thorax and abdomen in two ways. First, we co-expressed both Ato and Rho using PrdG4 (PrdG4;UAS-Ato;UAS-Rho) and detected similar EGF signaling (phospho-ERK levels) between thoracic and abdominal segments expressing PrdG4 (PrdG4-On segments, see Figure 3D). Under these conditions, Ato and Rho co-expression results in the induction of a similar large number of 21A6-positive cells in the abdomen and thorax (Figure 7A). In contrast, ectopic Rho alone induced few extra 21A6-positive cells (one or two per segment) and a large number of oenocytes in both thoracic and abdominal segments (Figure 2E and data not shown, scolopodia numbers are difficult to quantify in Rho-expressing embryos due to a severe folding malformation caused by the conversion of dorsal ectoderm to an oenocyte cell fate). These findings further indicate that Ato promotes the formation of SOP cells at the expense of oenocyte production. Second, we assessed Ato’s ability to induce ch organ SOP cells in the absence of Spi signaling by mis-expressing Ato in spi1 mutant embryos, which lack 2° SOP recruitment and hence form equivalent numbers of scolopodia in the thorax and the abdomen (Figure 7B). Ectopic Ato expression in this background (PrdG4;spi1;UAS-Ato) results in approximately half the number of 21A6-positive scolopodial cells compared to Ato expression in wild type embryos (Figure 7C). These data show that Ato promotes a higher level of neural competency in the presence of EGF signaling. However, while small, we still detect an overall difference in the number of scolopodia induced in the abdomen compared to the thorax (an approximate two-fold enhancement of 21A6-positive cells in the abdomen, Figure 7C). Thus, while EGF signaling enhances Ato’s ability to induce ch organ fate, Ato retains the ability to induce more ch organ SOP cells in the abdomen than the thorax in the absence of Spi function.
Figure 7. The role of Spi-mediated signaling in ch organ SOP induction by Ato, Sens and Abd-A.
(A-A”) Ectopic expression of Rho and Ato (PrdG4;UAS-Ato;UAS-Rho) induces many more scolopodia in both the thorax and abdomen in PrdG4-on segments than does Ato alone (see Figure 4A). Ato and Rho co-expression induces 10.7 +/− 6.0 extra scolopodia in abdominal segments and 13.0 +/− 6.8 scolopodia in thoracic segments (no significant difference between abdomen and thorax). (B-B’) In spi1 null embryos, both the thoracic and abdominal segments have clusters of three scolopodia. (C-C”) In spi1 null embryos, ectopic Ato (PrdG4;UAS-Ato;spi1) induces a smaller number of scolopodia than it does in wild type embryos (see Figure 4A). However, Ato still induces approximately twice as many scolopodia in the abdomen as in the thorax (2.0 +/− 0.2 extra in abdomen versus 1.3 +/− 0.5 extra in thorax, p-value < 0.01). (D-D”) Co-expression of Ato and Sens in a spi1 mutant background (PrdG4;UAS-Ato;UAS-Sens) induces significantly more scolopodia in the abdomen than in the thorax (5.61 +/− 0.34 extra in abdomen versus 1.73 +/− 0.54 in thorax, p-value < 0.001). (E-E”) In spi1 null embryos, ectopic Ato and Abd-A (PrdG4;UAS-Ato,UAS-AbdA;spi1) induce significantly fewer scolopodia than in a wild type background revealing that the synergistic specification of scolopodia is dependent upon EGF signaling. Moreover, the number of scolopodia induced by Ato and Abd-A together was not significantly different than Ato alone in a spi1 mutant background.
We next wanted to determine if the ability of Sens and Abd-A to stimulate the production of abdominal ch organs with Ato is dependent upon Spi-mediated EGF signaling. To do so, we used PrdG4 to drive the co-expression of Ato with either Abd-A or Sens in a spi1 mutant background. In both cases, we found a significant reduction in the production of scolopodia compared to when these factors are expressed in a wild type background (Figure 7D and 7E). These results are consistent with Ato-Sens and Ato-AbdA co-expression stimulating the EGF pathway within 1° SOP cells to enhance the recruitment of 2° ch organ SOP cells (see Figure 6). We also found that co-expression of Ato and Abd-A in the absence of spitz results in the same modest increase in 21A6-positive cells as Ato expression alone (compare Figure 7C and 7E). This finding indicates that Ato and Abd-A depend upon Spi-mediated signaling to enhance ch organ formation. Surprisingly, however, Ato-Sens co-expression still induced significantly more scolopodia in the abdomen (5.61 ± 0.34) than the thorax (1.73 ± 0.54) in the spitz mutant background. Thus, unlike Ato and Abd-A, Ato and Sens enhance neurogenesis in both Spi-dependent and Spi-independent manners within the Drosophila abdomen.
DISCUSSION
How proneural pathways that specify sensory precursor cells throughout the body are integrated with region-specific patterning genes to yield the correct type and number of sensory organs is not well understood. In this study, we show that three factors enhance the ability of Ato to promote ch organ SOP cell fate in the Drosophila abdomen; the EGF pathway mediated by the Spi ligand, the Abd-A Hox factor, and the Sens zinc finger transcription factor (see Table 1 for numbers of extra scolopodia and Figure 8 for summary schematics for each of the different genetic experiments). In addition, our data demonstrate that Ato can play a role in the Spi-secreting cell by activating rho enhancer activity in the 1° ch organ SOP cells as well as in the Spi-receiving cell to promote ch organ SOP fate at the expense of oenocytes (Figure 8I). Here, we discuss the implications of these findings in conjunction with prior publications on the regulation of rhomboid gene expression in abdominal ch organ SOP cells.
TABLE 1. Quantification of the number of induced (extra) ch organs and number of oenocytes per abdominal cluster.
The number of scolopodia was determined using mAb21A6 counts as described in the Materials and methods.
| Genotype | # of Scolopodia Greater than Wild Type | # of Oes | |
|---|---|---|---|
| Thorax | Abdomen | ||
| UAS-Ato | 2.11 ±1.51 | 3.64 ±1.35* | 0.8 ± 0.2** |
| UAS-Ato, Rho | 13.00 ±6.77 | 10.67 ±6.00 | ND |
| UAS-Ato, AbdA | 6.50 ± 3.80 | 7.50 ±1.76 | 2.9 ± 0.6** |
| UAS-Ato, Sens | 4.90 ± 0.58 | 9.80 ± 0.39* | 1.4 ± 0.5** |
| UAS-AbdA | 1.88 ± 0.04 | 0.10 ± 0.04 | 6.3 ± 0.2 |
| UAS-Sens | 0.40 ± 0.20 | 0.64 ± 0.32 | 4.3 ± 0.4** |
| UAS-Ato, Sens, AbdA | 6.36 ± 2.83 | 5.10 ± 1.01 | 2.0 ± 0.6** |
| spi1;UAS-Ato | 1.30 ± 0.45 | 2.00 ± 0.17* | ND |
| spi1 ;UAS-Ato, AbdA | 1.67 ± 0.11 | 2.16 ± 0.26 | ND |
| spi1;UAS-Ato, Sens | 1.73 ± 0.54 | 5.61 ± 0.34* | ND |
Standard error is noted and denotes significance between the thorax and abdomen for each experimental condition (P-value < 0.01, ANOVA). The number of oenocytes per cluster was determined using antibodies to HNF4 (see Materials and methods).
Standard error is noted and denotes significance from wild type segments (6.4 ± 0.3 per cluster, P-value < 0.01).
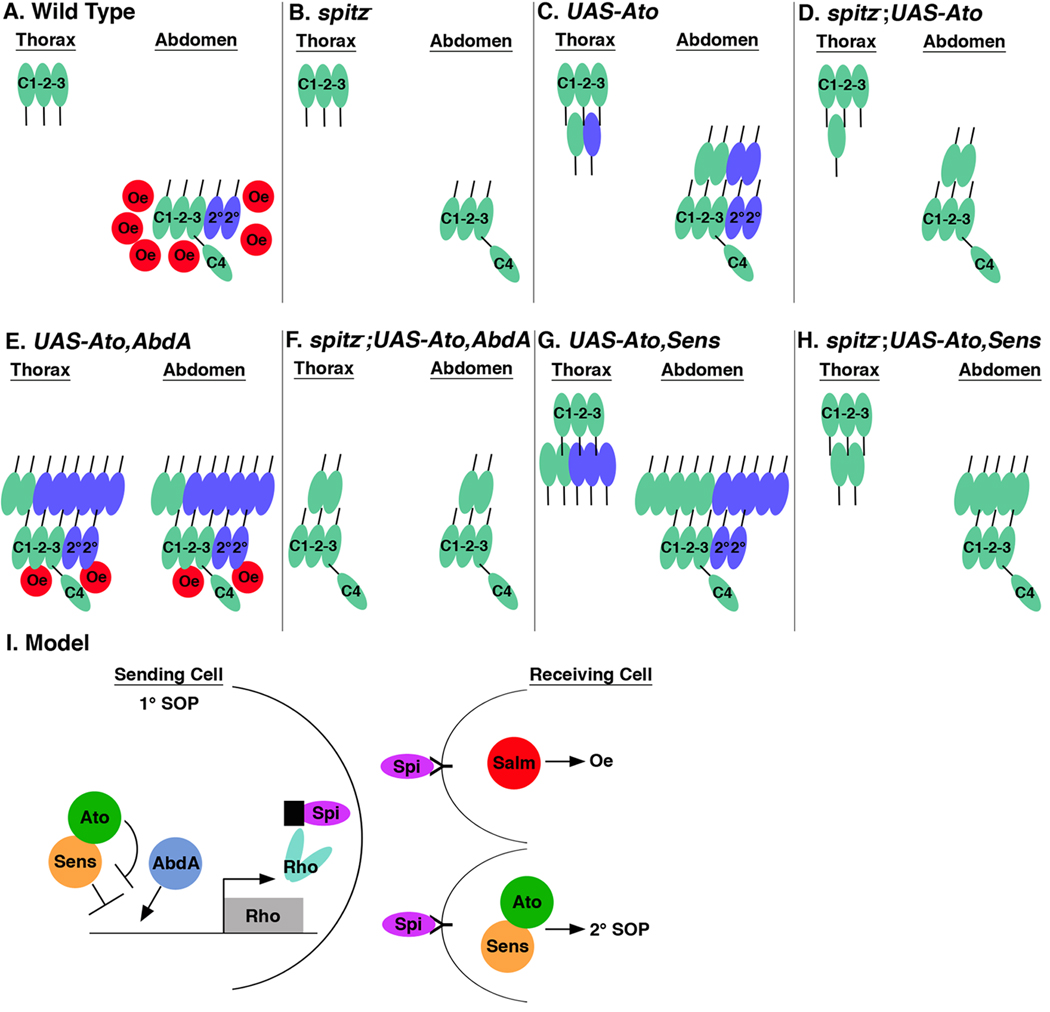
Figure 8. Model for the regulation of ch organ and oenocyte formation by Ato, Abd-A, and Sens.
Schematics summarize the regulation of primary ch organ (EGF-independent, green), secondary ch organ (EGF dependent, blue), and oenocyte (red) development under wild type and spitz mutant conditions. (A) Wild type thoracic segments (T2/T3) contain three 1° scolopodia (C1-C3), whereas the abdominal segments also have two 2° scolopodia, an lch1 organ (C4), and oenocytes (Oe). (B) In spitz1 mutant segments, neither 2° scolopodia nor Oes form within the abdominal segments. (C) A typical Ato-expressing thoracic segment (PrdG4;UAS-Ato) has an extra 1° scolopodia (green) and an extra 2° scolopodia (blue), whereas abdominal segments have two extra 1° scolopodia (green), two extra 2° scolopodia (blue), and no Oes. (D) In the absence of spi, the number of induced scolopodia in Ato-expressing segments (spi1; PrdG4;UAS-Ato) is decreased by half in both the thoracic and abdominal segments as no 2° SOPs are recruited. (E) Ato and Abd-A expressing thoracic and abdominal segments (PrdG4;UAS-Ato,UAS-AbdA) develop similarly with approximately two extra 1° scolopodia (green) and six extra 2° scolopodia (blue) as well as two-three oenocytes. (F) In the absence of spi, Ato and Abd-A expressing segments (spi1; PrdG4;UAS-Ato,UAS-AbdA) only induce an average of two extra 1° scolopodia. (G) A typical Ato and Sens expressing thoracic segment (PrdG4;UAS-Ato,UAS-Sens) develops an average of two extra 1° scolopodia (green) and three extra 2° scolopodia (blue), whereas abdominal segments have five extra 1° and five extra 2° scolopodia. (H) In the absence of spi, Ato and Sens expressing segments (spi1; PrdG4;UAS-Ato,UAS-Sens) induce an average of two 1° scolopodia in the thorax and five 1° scolopodia in the abdomen. (I) Model for the regulation of 2° scolopodia and oenocyte formation by the Ato, Abd-A, and Sens transcription factors. Both oenocyte and 2° SOP cell formation are dependent upon Spi-mediated EGF secretion from a sending cell. If a 1° ch organ SOP cell expresses Ato and Abd-A, then Sens-mediated repression of rhomboid (rho) expression is abolished, the Rho protein is expressed, and Spi is secreted. Spi binds the EGF-receptor on the receiving cell and if the receiving cell expresses the Salm transcription factor then oenocytes are induced. In contrast, if the Spi-receiving cell expresses the Ato/Sens transcription factors then oenocyte formation is inhibited and 2° SOP cells develop.
Choice of cell fate: the role of Ato and Salm in specifying ch organ SOP cells versus oenocytes
EGF signaling is used reiteratively throughout development to specify the formation of distinct cell types along the body plan (Shilo, 2005). In the embryonic Drosophila abdomen, EGF signaling initiated by the activation of rho in a set of ch organ SOP cells induces the formation of both a cluster of abdomen-specific oenocytes as well as a set of 2° ch organ SOP cells (Figure 1). But how does the EGF-receiving cell know whether to become a larval oenocyte that is specialized to process lipids or a ch organ SOP cell that forms part of the peripheral nervous system? Previous studies have shown that oenocyte specification requires at least two inputs: 1) the reception of relatively high levels of EGF signaling and 2) the expression of the Spalt transcription factors (Elstob et al., 2001; Rusten et al., 2001). Hence, oenocytes develop in close proximity to the abdominal C1 SOP cells that lie within a Spalt expression domain and express high levels of rho (Figure 1). In contrast, 2° SOP cells require less EGF signaling and form if the receiving cells lack Spalt. Consistent with this model, genetic studies have shown that oenocytes fail to develop and one to two additional ch organ SOP cells are specified in Spalt mutant embryos, whereas ectopic Spalt expression in the ventral ectoderm inhibits the recruitment of 2° SOP cells (Elstob et al., 2001; Rusten et al., 2001). Thus, Spalt promotes oenocyte development and antagonizes 2° ch organ specification in the Drosophila embryo.
We provide evidence that ato has the opposite effect as Spalt: it promotes ch organ SOP cells at the expense of oenocyte specification (Figure 8C). In Witt et al, we showed that ato loss-of-function results in decreased RhoBAD enhancer activity in C1 SOP cells and induces fewer oenocytes (Witt et al., 2010). This data is consistent with EGF signaling being compromised in ato mutant embryos and oenocyte specification being dependent upon the reception of high levels of Spi. Here, we show that Ato gain-of-function stimulates RhoBAD expression yet results in the inhibition of oenocyte formation (Figure 2). Importantly, the loss of oenocytes is not due to decreased EGF signaling as similar whorls of phospho-ERK-positive cells and even extra phoshpo-ERK staining are observed in Ato-expressing segments compared with non-expressing segments (Figure 3). In addition, we did not detect a difference in cell death between Ato-expressing and non-Ato-expressing segments (using an anti-cleaved Caspase3 marker, data not shown), indicating the oenocyte loss is not due to apoptosis. Instead, Ato promotes the formation of additional ch organ SOP cells in abdominal segments that normally form oenocytes. Moreover, while the broad activation of EGF signaling (PrdG4;UAS-Rho) induces many extra oenocytes and a few scolopodia, the co-expression of Ato and Rho induces many scolopodia and few oenocytes. These data suggest that if the Spi-receiving cell expresses high Ato relative to Salm then ch organ development occurs whereas if the Spi-receiving cell expresses high Salm relative to Ato then oenocytes are formed (Figure 8I). Thus, Ato plays a role in both the Spi-secreting (induction of rho expression) and Spi-receiving cell to dictate the choice of cell fate.
Ato and Abd-A synergize to induce ch organ SOP cells in an EGF-dependent manner
The broad expression of Ato within the ectoderm revealed differences in sensory organ competency between the thorax and abdomen. In particular, we found that Ato induced approximately twice as many ch organ SOP cells in the abdomen as in the thorax (Figure 8C). Moreover, the co-expression of Ato with the Abd-A Hox factor induced significantly more ch organ cell formation than expression of either factor alone (none by Abd-A, four by Ato, and eight by Ato/Abd-A, see Table 1 and Figure 8E). These data suggest that Ato and Abd-A synergize to enhance ch organ SOP formation in the abdomen and prompted us to examine if these SOP cells are predominantly 1° or 2° cells. We addressed this problem by first showing that the co-expression of Ato and Abd-A stimulates Rho enhancer activity (RhoAAA) within additional cells and results in enhanced phospho-ERK staining. Second, we showed that Ato and Abd-A require the EGF pathway to enhance ch organ development as co-expression of both factors in a spi mutant embryo failed to promote more ch organs than expression of Ato alone (Figure 8D and 8F). These data indicate that the co-expression of Ato and Abd-A enhances the ability of 1° ch organ SOP cells to activate rho, stimulates Spi secretion and, since the receiving cell expresses Ato, 2° SOPs form instead of oenocytes. The net result is that Ato and Abd-A synergize to activate the EGF pathway to promote region-specific neurogenesis within the Drosophila abdomen.
Ato and Sens enhance ch organ SOP cell formation in the abdomen in an EGF-dependent and an EGF-independent manner
The Sens transcription factor is essential for the formation of much of the peripheral nervous system in Drosophila and previous studies revealed that Sens can stimulate the sensory bristle-forming activity of the Scute and Achaete proneural factors in the wing disc (Acar et al., 2006; Jafar-Nejad et al., 2003; Nolo et al., 2000). Similarly, we found that Sens stimulates the ability of Ato to generate internal stretch receptors in the embryo and that Ato and Sens promote more sensory organ development in the abdomen than in the thorax (Figure 8G). In addition, while the overall number of ch organs formed by Ato and Sens co-expression is decreased in spi mutant embryos, we still observe significantly more ch organ SOP cells in the abdomen than in the thorax in this EGF-compromised genetic background (Figure 8H). Thus, Ato and Sens can stimulate abdominal ch organ SOP cell development in the presence or absence of Spi-mediated cell signaling.
So, what is the relationship between Ato, Sens, and Abd-A in regulating both EGF signaling and region-specific sensory organ formation? We previously found that Ato, Sens, and Abd-A control EGF signaling through the regulation of a cis-regulatory element within the rho locus (RhoBAD) (Li-Kroeger et al., 2008; Witt et al., 2010). RhoBAD acts in abdominal C1 SOP cells to induce oenocyte formation, and Ato and Abd-A both stimulate RhoBAD expression, at least in part, by limiting the ability of Sens to repress RhoBAD activity (Figure 8I). Moreover, they do so using different mechanisms. An Abd-A Hox complex containing Extradenticle and Homothorax directly competes with the Sens repressor for overlapping binding sites in RhoBAD (Li-Kroeger et al., 2008). In contrast, Ato does not directly bind RhoBAD but does directly interact with Sens to limit its ability to bind and repress Rho enhancer activity (Witt et al., 2010). Consequently, SOPs that co-express Ato and Abd-A are likely to limit the ability of Sens to repress Rho and thereby increase the number of ch organ SOP cells that secrete Spi. Consistent with this prediction, the co-expression of Ato and Sens preferentially stimulates Rho enhancer activity within abdominal segments compared to thoracic segments. Each SOP cell that expresses rho would further enhance sensory organ development through the recruitment of 2° SOP cells via Spi-mediated signaling. Hence, the genetic removal of spi results in a significant decrease in the number of ch organ SOP cells that develop in response to Ato and Sens. Thus, the ato-sens genetic pathway, which is used throughout the body to promote SOP formation, interacts with an abdominal Hox factor to stimulate EGF signaling and promote additional cell fate specification in the abdomen.
While the above model fits well with most of our data, we did observe two unexpected findings when comparing the ability of Ato-Sens co-expression to induce ch organ development in the presence and absence of spi function: First, we predicted that Ato-Sens co-expression in the thoracic regions, which lack Abd-A, should predominantly induce the formation of 1° ch organ SOP cells that do not require EGF signaling for their development. However, we found that significantly fewer ch organs form in the thorax of spi mutants, indicating that EGF signaling can enhance 2° sensory organ formation within thoracic segments that co-express Ato and Sens (compare Figure 8G with 8H). Interestingly, previous studies have shown that both rho and the Rho enhancers are weakly active within thoracic C1 SOP cells, but their levels do not reach a high enough threshold to induce oenocyte formation (Brodu et al., 2002; Witt et al., 2010). However, it is possible that the co-expression of Ato and Sens sufficiently sensitizes the receiving cells to respond to low levels of EGF signaling and become ch organ SOP cells (Figure 8I). The second unanticipated finding is that Ato and Sens co-expression still induced significantly more ch organ development within the abdomen (5–6 extra SOP cells) relative to the thorax (1–2 extra SOP cells) in the absence of Spi-mediated signaling. This finding suggests that Ato and Sens can genetically interact with the Abd-A Hox factor to promote sensory organ development in a Spi-independent manner. Currently, we do not understand how Abd-A enhances the proneural activity of the Ato-Sens factors in the absence of Spi signaling. One possibility is that Abd-A and Ato use similar mechanisms to limit Sens-mediated repression of additional target genes besides rho to stimulate ch organ development. Alternatively, Abd-A could independently regulate other factors such as those involved in the Notch-Delta pathway to enhance the competency of the ectoderm to respond to the Ato-Sens pathway. Intriguingly, a Hox factor (lin-39) in C elegans has been shown to directly regulate Notch signaling during vulval development (Takacs-Vellai et al., 2007), and the vertebrate Hoxb1 factor regulates neural stem cell progenitor proliferation and maintenance by modulating Notch signaling (Gouti and Gavalas, 2008). Since differential Notch-Delta signaling is a key pathway in deciding neural versus non-neural cell fates, the ability of Hox factors to modify this pathway could result in segmental differences in neurogenesis.
MATERIALS AND METHODS
Fly stocks and embryo staining
Fly stocks used were as follows: yw1118 and PrdG4 (Bloomington Stock Center); UAS-Ato (gift of Andrew Jarman); UAS-Sens (gift of Hugo Bellen); UAS-AbdA, and UAS-Antp (gift of Richard Mann); UAS-Rho and spi1 (gift of Gary Struhl); RhoBAD-lacZ (Li-Kroeger et al., 2008); and RhoAAA-lacZ, (Witt et al., 2010). Expression of lacZ (anti-β-gal, Abcam, 1:1000), Abd-A (GP4, 1:500) (Jarman et al., 1993; Li-Kroeger et al., 2008), mAb22C10 (DSHB, 1:50), mAb21A6 (DSHB, 1:50), Elav (DSHB, 1:50), Ato (1:5000) (Jarman et al., 1993), Sens (1:200) (Xie et al., 2007), Salm (1:1000) (Xie et al., 2007), HNF4 (Rat, 1:200) (Palanker et al., 2009), HNF4 (Rat and Guinea pig, 1:1000, see below), cleaved Caspase-3 (1:100, Cell Signaling (Ab# 9661)) and phospho-Erk (p-Erk, M8159 - Sigma, 1:50) was detected by indirect immunofluorescent antibody staining using an apotome-configured Zeiss fluorescent microscope. All flies were raised at 25°C. Embryos were harvested, fixed and immunostained using standard protocols except in the case of phospho-Erk embryos, which were fixed in 6% formaldehyde instead of 4%. Oenocyte identity was determined using anti-HNF4. All quantifications were done using a minimum of 10 Drosophila embryos (7 abdominal segments per embryo).
Antibody production
An HNF4 bacterial expression vector was generated using PCR to amplify and clone a HNF4 cDNA encoding amino acids 231–548 in-frame with an N-terminal 6-His tag (pET14b). The expression plasmid was transformed into BL21-CodonPlus (DE3)-RP bacteria (Stratagene). Protein expression was induced using 0.25 mM IPTG for 2 hours. Cells were lysed in 8M urea lysis buffer (ULB: 100 mM NaH2PO4, 10 mM Tris pH 8.0, 10 mM imidazole, 8M urea, 0.5% Igepal), centrifuged for 30 minutes at 16,000 g, and the supernatant mixed with Ni-NTA beads (Qiagen) for 2 hours at room temperature. Beads were washed three times with 10 mls ULB and protein was eluted in ULB plus 250 mM imidazole. Protein purity was confirmed using SDS-PAGE and coomassie blue gel staining and this protein sample was used to generate HNF4 antibodies in both rats and guinea pigs (Cocalico Biologicals).
Supplementary Material
ACKNOWLEDGEMENTS
We thank Andrew Jarman, Hugo Bellen, Yuh Nung Jan, Gary Struhl, Richard Mann, Carl Thummel, the Bloomington Drosophila Stock Center (Indiana University), and the Developmental Studies Hybridoma Bank (University of Iowa) for reagents. This work was supported by a neurofibroma pilot award and an NIH grant (R01GM079428) to B.G. T.A.C. was supported by an NIH grant (R01EY017907).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Acar M, Jafar-Nejad H, Giagtzoglou N, Yallampalli S, David G, He Y, Delidakis C, Bellen HJ. Senseless physically interacts with proneural proteins and functions as a transcriptional co-activator. Development. 2006;133:1979–1989. doi: 10.1242/dev.02372. [DOI] [PubMed] [Google Scholar]
- Bertrand N, Castro DS, Guillemot F. Proneural genes and the specification of neural cell types. Nat Rev Neurosci. 2002;3:517–530. doi: 10.1038/nrn874. [DOI] [PubMed] [Google Scholar]
- Brodu V, Elstob PR, Gould AP. abdominal A specifies one cell type in Drosophila by regulating one principal target gene. Development. 2002;129:2957–2963. doi: 10.1242/dev.129.12.2957. [DOI] [PubMed] [Google Scholar]
- Brodu V, Elstob PR, Gould AP. EGF receptor signaling regulates pulses of cell delamination from the Drosophila ectoderm. Dev Cell. 2004;7:885–895. doi: 10.1016/j.devcel.2004.10.016. [DOI] [PubMed] [Google Scholar]
- Dominguez M, Campuzano S. asense, a member of the Drosophila achaete-scute complex, is a proneural and neural differentiation gene. Embo J. 1993;12:2049–2060. doi: 10.1002/j.1460-2075.1993.tb05854.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elstob PR, Brodu V, Gould AP. spalt-dependent switching between two cell fates that are induced by the Drosophila EGF receptor. Development. 2001;128:723–732. doi: 10.1242/dev.128.5.723. [DOI] [PubMed] [Google Scholar]
- Gabay L, Seger R, Shilo BZ. In situ activation pattern of Drosophila EGF receptor pathway during development. Science. 1997;277:1103–1106. doi: 10.1126/science.277.5329.1103. [DOI] [PubMed] [Google Scholar]
- Gebelein B. Fly. Austin: 2008. The control of EGF signaling and cell fate in the Drosophila abdomen; p. 2. [DOI] [PubMed] [Google Scholar]
- Gebelein B, Mann RS. Compartmental modulation of abdominal Hox expression by engrailed and sloppy-paired patterns the fly ectoderm. Dev Biol. 2007 doi: 10.1016/j.ydbio.2007.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goulding SE, White NM, Jarman AP. cato encodes a basic helix-loop-helix transcription factor implicated in the correct differentiation of Drosophila sense organs. Dev Biol. 2000;221:120–131. doi: 10.1006/dbio.2000.9677. [DOI] [PubMed] [Google Scholar]
- Gouti M, Gavalas A. Hoxb1 controls cell fate specification and proliferative capacity of neural stem and progenitor cells. Stem Cells. 2008;26:1985–1997. doi: 10.1634/stemcells.2008-0182. [DOI] [PubMed] [Google Scholar]
- Gupta BP, Rodrigues V. Atonal is a proneural gene for a subset of olfactory sense organs in Drosophila. Genes Cells. 1997;2:225–233. doi: 10.1046/j.1365-2443.1997.d01-312.x. [DOI] [PubMed] [Google Scholar]
- Gutierrez E, Wiggins D, Fielding B, Gould AP. Specialized hepatocyte-like cells regulate Drosophila lipid metabolism. Nature. 2007;445:275–280. doi: 10.1038/nature05382. [DOI] [PubMed] [Google Scholar]
- Heuer JG, Kaufman TC. Homeotic genes have specific functional roles in the establishment of the Drosophila embryonic peripheral nervous system. Development. 1992;115:35–47. doi: 10.1242/dev.115.1.35. [DOI] [PubMed] [Google Scholar]
- Jafar-Nejad H, Acar M, Nolo R, Lacin H, Pan H, Parkhurst SM, Bellen HJ. Senseless acts as a binary switch during sensory organ precursor selection. Genes Dev. 2003;17:2966–2978. doi: 10.1101/gad.1122403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jan YN, Jan LY. Neuronal cell fate specification in Drosophila. Curr Opin Neurobiol. 1994;4:8–13. doi: 10.1016/0959-4388(94)90025-6. [DOI] [PubMed] [Google Scholar]
- Jan YN, Jan YJ. In The Development of Drosophila melanogaster. Plainview, NY: Cold Spring Harbor Laboratory Press; 1993. The Peripheral Nervous System. [Google Scholar]
- Jarman AP, Ahmed I. The specificity of proneural genes in determining Drosophila sense organ identity. Mech Dev. 1998;76:117–125. doi: 10.1016/s0925-4773(98)00116-6. [DOI] [PubMed] [Google Scholar]
- Jarman AP, Grau Y, Jan LY, Jan YN. atonal is a proneural gene that directs chordotonal organ formation in the Drosophila peripheral nervous system. Cell. 1993;73:1307–1321. doi: 10.1016/0092-8674(93)90358-w. [DOI] [PubMed] [Google Scholar]
- Jarman AP, Grell EH, Ackerman L, Jan LY, Jan YN. Atonal is the proneural gene for Drosophila photoreceptors. Nature. 1994;369:398–400. doi: 10.1038/369398a0. [DOI] [PubMed] [Google Scholar]
- Jarman AP, Sun Y, Jan LY, Jan YN. Role of the proneural gene, atonal, in formation of Drosophila chordotonal organs and photoreceptors. Development. 1995;121:2019–2030. doi: 10.1242/dev.121.7.2019. [DOI] [PubMed] [Google Scholar]
- Lage P, Jan YN, Jarman AP. Requirement for EGF receptor signalling in neural recruitment during formation of Drosophila chordotonal sense organ clusters. Curr Biol. 1997;7:166–175. doi: 10.1016/s0960-9822(97)70087-3. [DOI] [PubMed] [Google Scholar]
- Lai EC. Notch signaling: control of cell communication and cell fate. Development. 2004;131:965–973. doi: 10.1242/dev.01074. [DOI] [PubMed] [Google Scholar]
- Lai EC, Orgogozo V. A hidden program in Drosophila peripheral neurogenesis revealed: fundamental principles underlying sensory organ diversity. Dev Biol. 2004;269:1–17. doi: 10.1016/j.ydbio.2004.01.032. [DOI] [PubMed] [Google Scholar]
- Li-Kroeger D, Witt LM, Grimes HL, Cook TA, Gebelein B. Hox and senseless antagonism functions as a molecular switch to regulate EGF secretion in the Drosophila PNS. Dev Cell. 2008;15:298–308. doi: 10.1016/j.devcel.2008.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nolo R, Abbott LA, Bellen HJ. Senseless, a Zn finger transcription factor, is necessary and sufficient for sensory organ development in Drosophila. Cell. 2000;102:349–362. doi: 10.1016/s0092-8674(00)00040-4. [DOI] [PubMed] [Google Scholar]
- Okabe M, Okano H. Two-step induction of chordotonal organ precursors in Drosophila embryogenesis. Development. 1997;124:1045–1053. doi: 10.1242/dev.124.5.1045. [DOI] [PubMed] [Google Scholar]
- Palanker L, Tennessen JM, Lam G, Thummel CS. Drosophila HNF4 regulates lipid mobilization and beta-oxidation. Cell Metab. 2009;9:228–239. doi: 10.1016/j.cmet.2009.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parks AL, Huppert SS, Muskavitch MA. The dynamics of neurogenic signalling underlying bristle development in Drosophila melanogaster. Mech Dev. 1997;63:61–74. doi: 10.1016/s0925-4773(97)00675-8. [DOI] [PubMed] [Google Scholar]
- Powell LM, Jarman AP. Context dependence of proneural bHLH proteins. Curr Opin Genet Dev. 2008;18:411–417. doi: 10.1016/j.gde.2008.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez I, Hernandez R, Modolell J, Ruiz-Gomez M. Competence to develop sensory organs is temporally and spatially regulated in Drosophila epidermal primordia. Embo J. 1990;9:3583–3592. doi: 10.1002/j.1460-2075.1990.tb07569.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rusten TE, Cantera R, Urban J, Technau G, Kafatos FC, Barrio R. Spalt modifies EGFR-mediated induction of chordotonal precursors in the embryonic PNS of Drosophila promoting the development of oenocytes. Development. 2001;128:711–722. doi: 10.1242/dev.128.5.711. [DOI] [PubMed] [Google Scholar]
- Shilo BZ. Regulating the dynamics of EGF receptor signaling in space and time. Development. 2005;132:4017–4027. doi: 10.1242/dev.02006. [DOI] [PubMed] [Google Scholar]
- Simpson P. Notch signalling in development: on equivalence groups and asymmetric developmental potential. Curr Opin Genet Dev. 1997;7:537–542. doi: 10.1016/s0959-437x(97)80083-4. [DOI] [PubMed] [Google Scholar]
- Simpson P, Carteret C. Proneural clusters: equivalence groups in the epithelium of Drosophila. Development. 1990;110:927–932. doi: 10.1242/dev.110.3.927. [DOI] [PubMed] [Google Scholar]
- Takacs-Vellai K, Vellai T, Chen EB, Zhang Y, Guerry F, Stern MJ, Muller F. Transcriptional control of Notch signaling by a HOX and a PBX/EXD protein during vulval development in C. elegans. Dev Biol. 2007;302:661–669. doi: 10.1016/j.ydbio.2006.09.049. [DOI] [PubMed] [Google Scholar]
- Villares R, Cabrera CV. The achaete-scute gene complex of D. melanogaster: conserved domains in a subset of genes required for neurogenesis and their homology to myc. Cell. 1987;50:415–424. doi: 10.1016/0092-8674(87)90495-8. [DOI] [PubMed] [Google Scholar]
- Witt LM, Gutzwiller LM, Gresser AL, Li-Kroeger D, Cook TA, Gebelein B. Atonal, senseless, and abdominal-A regulate rhomboid enhancer activity in abdominal sensory organ precursors. Dev Biol. 2010 doi: 10.1016/j.ydbio.2010.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong DC, Merritt DJ. The role of homeotic genes in determining the segmental pattern of chordotonal organs in Drosophila. Int J Dev Biol. 2002;46:475–481. [PubMed] [Google Scholar]
- Xie B, Charlton-Perkins M, McDonald E, Gebelein B, Cook T. Senseless functions as a molecular switch for color photoreceptor differentiation in Drosophila. Development. 2007;134:4243–4253. doi: 10.1242/dev.012781. [DOI] [PubMed] [Google Scholar]
- zur Lage PI, Powell LM, Prentice DR, McLaughlin P, Jarman AP. EGF receptor signaling triggers recruitment of Drosophila sense organ precursors by stimulating proneural gene autoregulation. Dev Cell. 2004;7:687–696. doi: 10.1016/j.devcel.2004.09.015. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.