Abstract
The title compound, C19H20O3, was obtained from 1,4-bis(4-methylphenyl)but-3-yn-2-one in the presence of carbon monoxide by Ni(CN)2 catalysis in a basic aqueous medium. Intermolecular O—H⋯O hydrogen bonds lead to the formation of hydrogen-bonded carboxylic acid dimers [graph-set motif R 2 2(8)]. Weak C—H⋯O hydrogen bonds between neighbouring dimers further extend the structure to give rise to a three-dimensional supramolecular network.
Related literature
For general background to transition metal-mediated carbonylation reactions, see: Collins (1999 ▶); Arzoumanian et al. (1995 ▶). For a similar structure, see: Garcia-Gutierrez et al. (2004 ▶). For bond length values, see: Allen et al. (1987 ▶). For hydrogen-bonding motifs, see: Bernstein et al. (1995 ▶).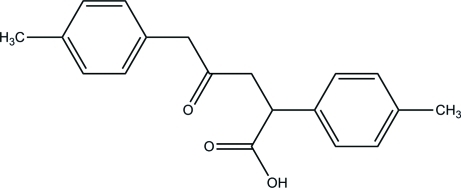
Experimental
Crystal data
C19H20O3
M r = 296.35
Monoclinic,
a = 11.846 (2) Å
b = 13.155 (3) Å
c = 11.755 (2) Å
β = 115.98 (3)°
V = 1646.7 (7) Å3
Z = 4
Mo Kα radiation
μ = 0.08 mm−1
T = 293 K
0.25 × 0.22 × 0.19 mm
Data collection
Bruker APEXII area-detector diffractometer
12947 measured reflections
2956 independent reflections
1474 reflections with I > 2σ(I)
R int = 0.062
Refinement
R[F 2 > 2σ(F 2)] = 0.050
wR(F 2) = 0.173
S = 1.01
2956 reflections
202 parameters
H-atom parameters constrained
Δρmax = 0.24 e Å−3
Δρmin = −0.18 e Å−3
Data collection: APEX2 (Bruker, 2004 ▶); cell refinement: APEX2 and SAINT (Bruker, 2004 ▶); data reduction: SAINT; program(s) used to solve structure: SHELXS97 (Sheldrick, 2008 ▶); program(s) used to refine structure: SHELXL97 (Sheldrick, 2008 ▶); molecular graphics: XP in SHELXTL (Sheldrick, 2008 ▶); software used to prepare material for publication: SHELXL97.
Supplementary Material
Crystal structure: contains datablocks I, global. DOI: 10.1107/S1600536810037323/zl2309sup1.cif
Structure factors: contains datablocks I. DOI: 10.1107/S1600536810037323/zl2309Isup2.hkl
Additional supplementary materials: crystallographic information; 3D view; checkCIF report
Table 1. Hydrogen-bond geometry (Å, °).
| D—H⋯A | D—H | H⋯A | D⋯A | D—H⋯A |
|---|---|---|---|---|
| C17—H17⋯O1i | 0.93 | 2.50 | 3.418 (4) | 169 |
| C15—H15⋯O2ii | 0.93 | 2.56 | 3.452 (4) | 160 |
| O2—H2A⋯O3iii | 0.82 | 1.83 | 2.638 (2) | 169 |
Symmetry codes: (i) 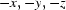 ; (ii)
; (ii) 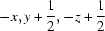 ; (iii)
; (iii) 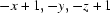 .
.
Acknowledgments
The work was supported by Zhongshan Polytechnic.
supplementary crystallographic information
Comment
Transition-metal-mediated carbonylation reactions are of great research interest in recent years (Collins, 1999). Amongst the many metal-mediated syntheses used, catalysis by nickel cyanide in aqueous media under phase transfer conditions has attracted particular attention (Arzoumanian et al., 1995) and numerous lactones and their hydrolysis products have been synthesized using this system. Herein, we chose 1,4-di(4-methylbenzyl)but-3-yn-2-one as a carbonylation substrate to be reacted in the presence of Ni(CN)2 and carbon monoxide in a biphasic toluene/basic aqueous medium to give the title compound.
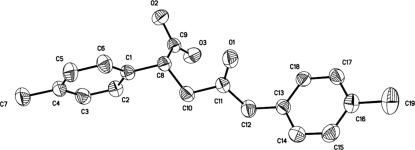
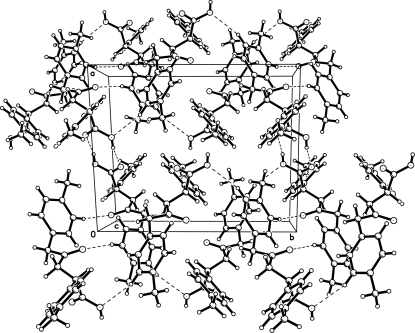
The structure of the title compound is depicted in Fig. 1. The C—C bond lengths show normal values (Allen et al., 1987), and the C—O and C═O bond lengths are comparable to those observed in simliar structures (Garcia-Gutierrez et al., 2004). The molecules form dimers with neighboring molecules through O—H···O hydrogen bonding with an R22(8) graph set motif (Bernstein et al., 1995). These dimers are further linked by C—H···O hydrogen bonds (Table 1) to form a three-dimensional supramolecular network (Fig. 2).
Experimental
A typical experiment was performed as follows: in a round-bottomed flask toluene (25 ml) and 1 M aqueous NaOH (10 ml) were degassed and saturated with CO under atmospheric pressure before Ni(CN)2.4H2O (1.0 mmol) and tetrabutylammonium bromide (0.3 mmol) were introduced, and the mixture was kept at room temperature overnight with stirring while CO was slowly (2–3 min) bubbled through the solution. To the yellow two-phase mixture was then added 10 mmol of 1,4-di(4-methylbenzyl)but-3-yn-2-one, and stirring and flow of CO at a flow rate of 3 ml min-1 were maintained for 5 h at 393 K. At the end of the reaction, ethyl ether (2 × 20 ml) was used to eliminate the impurities. The aqueous phase was acidified with diluted HCl at pH = 1. Ethyl ether (2 × 20 ml) was used to extract the product. The organic phase was dried over Na2SO4 and evaporated to obtain a yellow powder. During recrystallization, the yellow block crystals were obtained by slow evaporation of the solvent with a yield of 68%. m.p. 476–478 K; IR (KBr) cm-1: 3052, 2980, 2948, 1716, 1705, 1669, 1607, 1573, 1465, 1416, 1379, 1345, 1285, 1246, 1232, 1217, 1186, 1150, 1068, 1044, 995, 972, 850.
Refinement
All H atoms attached to C and O atoms were fixed geometrically and treated as riding with C—H = 0.93 or 0.96 Å and O—H = 0.82 Å, and Uiso(H) = 1.2Ueq(C) and Uiso(H) = 1.5Ueq(O).
Figures
Fig. 1.
ORTEP represention of atom numbering diagram for the title compound, showing 30% probability displacement ellipsoids.
Fig. 2.
View of the three-dimensional structure of the title compound. H-bonds are shown as dashed lines.
Crystal data
| C19H20O3 | F(000) = 632 |
| Mr = 296.35 | Dx = 1.195 Mg m−3 |
| Monoclinic, P21/c | Mo Kα radiation, λ = 0.71073 Å |
| Hall symbol: -P 2ybc | Cell parameters from 2279 reflections |
| a = 11.846 (2) Å | θ = 2.3–28.0° |
| b = 13.155 (3) Å | µ = 0.08 mm−1 |
| c = 11.755 (2) Å | T = 293 K |
| β = 115.98 (3)° | Block, yellow |
| V = 1646.7 (7) Å3 | 0.25 × 0.22 × 0.19 mm |
| Z = 4 |
Data collection
| Bruker APEXII area-detector diffractometer | 1474 reflections with I > 2σ(I) |
| Radiation source: fine-focus sealed tube | Rint = 0.062 |
| graphite | θmax = 25.2°, θmin = 3.1° |
| φ and ω scan | h = −14→14 |
| 12947 measured reflections | k = −15→15 |
| 2956 independent reflections | l = −14→14 |
Refinement
| Refinement on F2 | Primary atom site location: structure-invariant direct methods |
| Least-squares matrix: full | Secondary atom site location: difference Fourier map |
| R[F2 > 2σ(F2)] = 0.050 | Hydrogen site location: inferred from neighbouring sites |
| wR(F2) = 0.173 | H-atom parameters constrained |
| S = 1.01 | w = 1/[σ2(Fo2) + (0.085P)2] where P = (Fo2 + 2Fc2)/3 |
| 2956 reflections | (Δ/σ)max < 0.001 |
| 202 parameters | Δρmax = 0.24 e Å−3 |
| 0 restraints | Δρmin = −0.18 e Å−3 |
Special details
| Geometry. All esds (except the esd in the dihedral angle between two l.s. planes) are estimated using the full covariance matrix. The cell esds are taken into account individually in the estimation of esds in distances, angles and torsion angles; correlations between esds in cell parameters are only used when they are defined by crystal symmetry. An approximate (isotropic) treatment of cell esds is used for estimating esds involving l.s. planes. |
| Refinement. Refinement of F2 against ALL reflections. The weighted R-factor wR and goodness of fit S are based on F2, conventional R-factors R are based on F, with F set to zero for negative F2. The threshold expression of F2 > 2sigma(F2) is used only for calculating R-factors(gt) etc. and is not relevant to the choice of reflections for refinement. R-factors based on F2 are statistically about twice as large as those based on F, and R- factors based on ALL data will be even larger. |
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2)
| x | y | z | Uiso*/Ueq | ||
| C1 | 0.2848 (2) | 0.06339 (18) | 0.6978 (3) | 0.0586 (7) | |
| C2 | 0.3764 (2) | 0.1318 (2) | 0.7725 (3) | 0.0695 (8) | |
| H2 | 0.4156 | 0.1729 | 0.7360 | 0.083* | |
| C3 | 0.4101 (3) | 0.1396 (2) | 0.9004 (3) | 0.0772 (9) | |
| H3 | 0.4722 | 0.1858 | 0.9485 | 0.093* | |
| C4 | 0.3548 (3) | 0.0817 (2) | 0.9587 (3) | 0.0744 (8) | |
| C5 | 0.2638 (3) | 0.0149 (3) | 0.8837 (3) | 0.0859 (9) | |
| H5 | 0.2239 | −0.0254 | 0.9201 | 0.103* | |
| C6 | 0.2299 (3) | 0.0057 (2) | 0.7571 (3) | 0.0758 (8) | |
| H6 | 0.1680 | −0.0409 | 0.7099 | 0.091* | |
| C7 | 0.3949 (4) | 0.0898 (3) | 1.0989 (3) | 0.1073 (12) | |
| H7A | 0.4565 | 0.1427 | 1.1337 | 0.161* | |
| H7B | 0.4304 | 0.0263 | 1.1388 | 0.161* | |
| H7C | 0.3232 | 0.1057 | 1.1136 | 0.161* | |
| C8 | 0.2475 (2) | 0.05361 (18) | 0.5580 (2) | 0.0568 (7) | |
| H8 | 0.1909 | −0.0049 | 0.5272 | 0.068* | |
| C9 | 0.3590 (2) | 0.0320 (2) | 0.5332 (3) | 0.0581 (7) | |
| C10 | 0.1766 (2) | 0.1458 (2) | 0.4817 (3) | 0.0654 (7) | |
| H10A | 0.1111 | 0.1638 | 0.5065 | 0.078* | |
| H10B | 0.2341 | 0.2028 | 0.5021 | 0.078* | |
| C11 | 0.1187 (2) | 0.1287 (2) | 0.3424 (3) | 0.0617 (7) | |
| C12 | 0.0992 (3) | 0.2191 (2) | 0.2583 (3) | 0.0764 (8) | |
| H12A | 0.0774 | 0.2768 | 0.2961 | 0.092* | |
| H12B | 0.1783 | 0.2348 | 0.2563 | 0.092* | |
| C13 | 0.0008 (2) | 0.20819 (18) | 0.1251 (3) | 0.0604 (7) | |
| C14 | −0.1074 (3) | 0.2653 (2) | 0.0815 (3) | 0.0827 (9) | |
| H14 | −0.1173 | 0.3129 | 0.1349 | 0.099* | |
| C15 | −0.2006 (3) | 0.2536 (2) | −0.0386 (4) | 0.0892 (10) | |
| H15 | −0.2723 | 0.2937 | −0.0646 | 0.107* | |
| C16 | −0.1916 (3) | 0.1845 (2) | −0.1219 (3) | 0.0729 (8) | |
| C17 | −0.0825 (3) | 0.1291 (2) | −0.0809 (3) | 0.0678 (8) | |
| H17 | −0.0718 | 0.0832 | −0.1355 | 0.081* | |
| C18 | 0.0115 (2) | 0.14094 (19) | 0.0405 (3) | 0.0638 (7) | |
| H18 | 0.0843 | 0.1023 | 0.0659 | 0.077* | |
| C19 | −0.2969 (3) | 0.1693 (3) | −0.2526 (4) | 0.1142 (13) | |
| H19A | −0.2762 | 0.1145 | −0.2939 | 0.171* | |
| H19B | −0.3088 | 0.2305 | −0.3010 | 0.171* | |
| H19C | −0.3729 | 0.1533 | −0.2460 | 0.171* | |
| O1 | 0.0871 (2) | 0.04379 (15) | 0.3000 (2) | 0.0947 (7) | |
| O2 | 0.41394 (17) | −0.05500 (14) | 0.5781 (2) | 0.0763 (6) | |
| H2A | 0.4777 | −0.0597 | 0.5674 | 0.114* | |
| O3 | 0.39643 (17) | 0.08983 (15) | 0.4773 (2) | 0.0817 (7) |
Atomic displacement parameters (Å2)
| U11 | U22 | U33 | U12 | U13 | U23 | |
| C1 | 0.0534 (14) | 0.0674 (16) | 0.0574 (18) | 0.0026 (11) | 0.0266 (13) | −0.0028 (13) |
| C2 | 0.0662 (17) | 0.0777 (18) | 0.065 (2) | −0.0016 (13) | 0.0293 (15) | −0.0019 (15) |
| C3 | 0.0674 (17) | 0.088 (2) | 0.064 (2) | 0.0083 (14) | 0.0171 (16) | −0.0111 (16) |
| C4 | 0.085 (2) | 0.085 (2) | 0.0540 (19) | 0.0263 (16) | 0.0306 (16) | 0.0051 (16) |
| C5 | 0.105 (2) | 0.097 (2) | 0.073 (3) | 0.0023 (18) | 0.055 (2) | 0.0100 (18) |
| C6 | 0.0804 (19) | 0.087 (2) | 0.069 (2) | −0.0133 (14) | 0.0415 (17) | −0.0067 (16) |
| C7 | 0.132 (3) | 0.126 (3) | 0.060 (2) | 0.048 (2) | 0.039 (2) | 0.010 (2) |
| C8 | 0.0558 (14) | 0.0617 (14) | 0.0549 (17) | −0.0005 (11) | 0.0262 (12) | −0.0015 (12) |
| C9 | 0.0578 (15) | 0.0647 (15) | 0.0563 (17) | 0.0003 (12) | 0.0294 (13) | −0.0026 (13) |
| C10 | 0.0601 (15) | 0.0771 (17) | 0.0588 (19) | 0.0056 (12) | 0.0258 (13) | −0.0081 (13) |
| C11 | 0.0621 (15) | 0.0671 (17) | 0.0547 (18) | −0.0022 (12) | 0.0245 (13) | −0.0054 (13) |
| C12 | 0.086 (2) | 0.0749 (18) | 0.064 (2) | −0.0135 (14) | 0.0293 (16) | −0.0020 (15) |
| C13 | 0.0696 (16) | 0.0574 (14) | 0.0578 (18) | −0.0044 (12) | 0.0312 (14) | 0.0008 (13) |
| C14 | 0.095 (2) | 0.084 (2) | 0.074 (2) | 0.0215 (16) | 0.0407 (19) | −0.0013 (16) |
| C15 | 0.080 (2) | 0.103 (2) | 0.083 (3) | 0.0331 (17) | 0.0347 (19) | 0.016 (2) |
| C16 | 0.0686 (17) | 0.0887 (19) | 0.060 (2) | −0.0010 (15) | 0.0266 (15) | 0.0068 (16) |
| C17 | 0.0831 (19) | 0.0671 (17) | 0.058 (2) | −0.0030 (14) | 0.0355 (16) | −0.0034 (13) |
| C18 | 0.0693 (16) | 0.0627 (16) | 0.064 (2) | 0.0077 (12) | 0.0336 (15) | 0.0056 (13) |
| C19 | 0.083 (2) | 0.169 (4) | 0.076 (3) | −0.010 (2) | 0.021 (2) | 0.009 (2) |
| O1 | 0.1269 (17) | 0.0755 (14) | 0.0615 (14) | −0.0101 (12) | 0.0226 (12) | −0.0026 (11) |
| O2 | 0.0787 (13) | 0.0734 (12) | 0.0948 (17) | 0.0158 (9) | 0.0547 (12) | 0.0179 (11) |
| O3 | 0.0835 (14) | 0.0767 (12) | 0.1091 (19) | 0.0139 (9) | 0.0646 (13) | 0.0223 (12) |
Geometric parameters (Å, °)
| C1—C6 | 1.372 (4) | C10—H10B | 0.9700 |
| C1—C2 | 1.387 (3) | C11—O1 | 1.214 (3) |
| C1—C8 | 1.508 (4) | C11—C12 | 1.498 (4) |
| C2—C3 | 1.380 (4) | C12—C13 | 1.494 (4) |
| C2—H2 | 0.9300 | C12—H12A | 0.9700 |
| C3—C4 | 1.368 (4) | C12—H12B | 0.9700 |
| C3—H3 | 0.9300 | C13—C14 | 1.376 (4) |
| C4—C5 | 1.371 (4) | C13—C18 | 1.378 (4) |
| C4—C7 | 1.506 (4) | C14—C15 | 1.367 (4) |
| C5—C6 | 1.366 (4) | C14—H14 | 0.9300 |
| C5—H5 | 0.9300 | C15—C16 | 1.375 (4) |
| C6—H6 | 0.9300 | C15—H15 | 0.9300 |
| C7—H7A | 0.9600 | C16—C17 | 1.374 (4) |
| C7—H7B | 0.9600 | C16—C19 | 1.509 (4) |
| C7—H7C | 0.9600 | C17—C18 | 1.381 (4) |
| C8—C9 | 1.499 (3) | C17—H17 | 0.9300 |
| C8—C10 | 1.523 (3) | C18—H18 | 0.9300 |
| C8—H8 | 0.9800 | C19—H19A | 0.9600 |
| C9—O3 | 1.209 (3) | C19—H19B | 0.9600 |
| C9—O2 | 1.308 (3) | C19—H19C | 0.9600 |
| C10—C11 | 1.489 (4) | O2—H2A | 0.8200 |
| C10—H10A | 0.9700 | ||
| C6—C1—C2 | 116.8 (3) | C8—C10—H10B | 108.9 |
| C6—C1—C8 | 121.9 (2) | H10A—C10—H10B | 107.7 |
| C2—C1—C8 | 121.3 (3) | O1—C11—C10 | 120.1 (3) |
| C3—C2—C1 | 120.7 (3) | O1—C11—C12 | 121.9 (3) |
| C3—C2—H2 | 119.6 | C10—C11—C12 | 118.0 (2) |
| C1—C2—H2 | 119.6 | C13—C12—C11 | 116.1 (2) |
| C4—C3—C2 | 122.0 (3) | C13—C12—H12A | 108.3 |
| C4—C3—H3 | 119.0 | C11—C12—H12A | 108.3 |
| C2—C3—H3 | 119.0 | C13—C12—H12B | 108.3 |
| C3—C4—C5 | 116.7 (3) | C11—C12—H12B | 108.3 |
| C3—C4—C7 | 121.2 (3) | H12A—C12—H12B | 107.4 |
| C5—C4—C7 | 122.1 (3) | C14—C13—C18 | 116.5 (3) |
| C6—C5—C4 | 122.0 (3) | C14—C13—C12 | 120.6 (3) |
| C6—C5—H5 | 119.0 | C18—C13—C12 | 122.8 (2) |
| C4—C5—H5 | 119.0 | C15—C14—C13 | 121.5 (3) |
| C5—C6—C1 | 121.7 (3) | C15—C14—H14 | 119.3 |
| C5—C6—H6 | 119.1 | C13—C14—H14 | 119.3 |
| C1—C6—H6 | 119.1 | C14—C15—C16 | 122.0 (3) |
| C4—C7—H7A | 109.5 | C14—C15—H15 | 119.0 |
| C4—C7—H7B | 109.5 | C16—C15—H15 | 119.0 |
| H7A—C7—H7B | 109.5 | C17—C16—C15 | 117.1 (3) |
| C4—C7—H7C | 109.5 | C17—C16—C19 | 121.2 (3) |
| H7A—C7—H7C | 109.5 | C15—C16—C19 | 121.7 (3) |
| H7B—C7—H7C | 109.5 | C16—C17—C18 | 120.8 (3) |
| C9—C8—C1 | 111.4 (2) | C16—C17—H17 | 119.6 |
| C9—C8—C10 | 110.0 (2) | C18—C17—H17 | 119.6 |
| C1—C8—C10 | 113.4 (2) | C13—C18—C17 | 122.0 (2) |
| C9—C8—H8 | 107.3 | C13—C18—H18 | 119.0 |
| C1—C8—H8 | 107.3 | C17—C18—H18 | 119.0 |
| C10—C8—H8 | 107.3 | C16—C19—H19A | 109.5 |
| O3—C9—O2 | 122.2 (2) | C16—C19—H19B | 109.5 |
| O3—C9—C8 | 123.4 (2) | H19A—C19—H19B | 109.5 |
| O2—C9—C8 | 114.4 (2) | C16—C19—H19C | 109.5 |
| C11—C10—C8 | 113.4 (2) | H19A—C19—H19C | 109.5 |
| C11—C10—H10A | 108.9 | H19B—C19—H19C | 109.5 |
| C8—C10—H10A | 108.9 | C9—O2—H2A | 109.5 |
| C11—C10—H10B | 108.9 |
Hydrogen-bond geometry (Å, °)
| D—H···A | D—H | H···A | D···A | D—H···A |
| C17—H17···O1i | 0.93 | 2.50 | 3.418 (4) | 169 |
| C15—H15···O2ii | 0.93 | 2.56 | 3.452 (4) | 160 |
| O2—H2A···O3iii | 0.82 | 1.83 | 2.638 (2) | 169 |
Symmetry codes: (i) −x, −y, −z; (ii) −x, y+1/2, −z+1/2; (iii) −x+1, −y, −z+1.
Footnotes
Supplementary data and figures for this paper are available from the IUCr electronic archives (Reference: ZL2309).
References
- Allen, F. H., Kennard, O., Watson, D. G., Brammer, L., Orpen, A. G. & Taylor, R. (1987). J. Chem. Soc. Perkin Trans. 2, pp. S1–19.
- Arzoumanian, H., Nuel, D., Jean, M., Cabrera, A., Garcia, J. L. & Rosas, N. (1995). Organometallics, 14, 5438–5441.
- Bernstein, J., Davis, R. E., Shimoni, L. & $ Chang, N.-L. (1995). Angew. Chem. Int. Ed.34, 1555–1573.
- Bruker (2004). APEX2 and SAINT Bruker AXS Inc., Madison, Wisconsin, USA.
- Collins, I. (1999). J. Chem. Soc. Perkin Trans. 1, pp. 1377–1395.
- Garcia-Gutierrez, J. L., Jimenez-Cruz, F. & Rosas Espinosa, N. (2004). Tetrahedron Lett.46, 803–805.
- Sheldrick, G. M. (2008). Acta Cryst. A64, 112–122. [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Crystal structure: contains datablocks I, global. DOI: 10.1107/S1600536810037323/zl2309sup1.cif
Structure factors: contains datablocks I. DOI: 10.1107/S1600536810037323/zl2309Isup2.hkl
Additional supplementary materials: crystallographic information; 3D view; checkCIF report