Abstract
The title compound [systematic name: 7-bromo-5-(2-chlorophenyl)-1H-1,4-benzodiazepin-2(3H)-one] (β-polymorph), C15H10BrClN2O, has been obtained via cryomodification of the known α-polymorph of phenazepam [Karapetyan et al. (1979 ▶). Bioorg. Khim. 5, 1684–1690]. In both polymorphs, the molecules, which differ only in the dihedral angles between the aromatic rings [75.4 (2)° and 86.2 (3)° in the α- and β-polymorphs, respectively], are linked into centrosymmetric dimers via N—H⋯O hydrogen bonds. In the crystal structure of the β-polymorph, weak intermolecular C—H⋯O hydrogen bonds further link these dimers into layers parallel to bc plane.
Related literature
For details of the synthesis via cryomodification, see: Sergeev & Komarov (2006 ▶). For the crystal structure of the α-polymorph of phenazepam, see: Karapetyan et al. (1979 ▶). For details of the indexing algorithm, see: Werner et al. (1985 ▶). The methodology of the refinement (including applied restraints) has been described in detail by Ryabova et al. (2005 ▶). For the March–Dollase orientation correction, see: Dollase (1986 ▶) and for the split-type pseudo-Voigt profile, see: Toraya (1986 ▶). 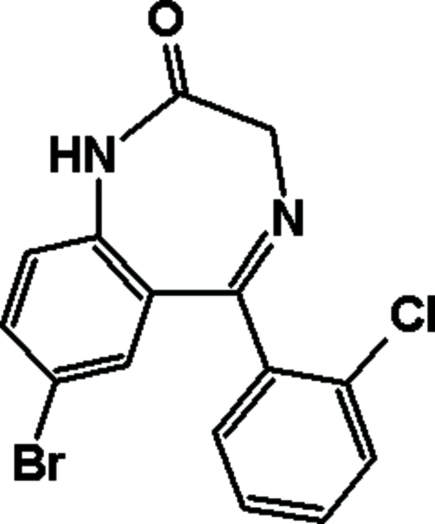
Experimental
Crystal data
C15H10BrClN2O
M r = 349.61
Monoclinic,
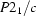
a = 14.8006 (19) Å
b = 11.6756 (14) Å
c = 8.4769 (9) Å
β = 93.679 (17)°
V = 1461.8 (3) Å3
Z = 4
Cu Kα1 radiation, λ = 1.54059 Å
μ = 5.49 mm−1
T = 295 K
Flat sheet, 15 × 1 mm
Data collection
Guinier camera G670 diffractometer
Specimen mounting: thin layer in the specimen holder of the camera
Data collection mode: transmission
Scan method: continuous
2θmin = 5.00°, 2θmax = 80.00°, 2θstep = 0.01°
Refinement
R p = 0.013
R wp = 0.017
R exp = 0.012
R Bragg = 0.059
χ2 = 2.250
7501 data points
128 parameters
64 restraints
H-atom parameters not refined
Data collection: G670 Imaging Plate Guinier Camera Software (Huber, 2002 ▶); cell refinement: MRIA (Zlokazov & Chernyshev, 1992 ▶); data reduction: G670 Imaging Plate Guinier Camera Software; method used to solve structure: simulated annealing (Zhukov et al., 2001 ▶); program(s) used to refine structure: MRIA; molecular graphics: PLATON (Spek, 2009 ▶); software used to prepare material for publication: MRIA and SHELXL97 (Sheldrick, 2008 ▶).
Supplementary Material
Crystal structure: contains datablocks I, global. DOI: 10.1107/S1600536810037402/lh5126sup1.cif
Rietveld powder data: contains datablocks I. DOI: 10.1107/S1600536810037402/lh5126Isup2.rtv
Additional supplementary materials: crystallographic information; 3D view; checkCIF report
Table 1. Hydrogen-bond geometry (Å, °).
| D—H⋯A | D—H | H⋯A | D⋯A | D—H⋯A |
|---|---|---|---|---|
| N8—H8⋯O10i | 0.86 | 2.15 | 2.865 (16) | 141 |
| C11—H11B⋯O10ii | 0.97 | 2.18 | 3.03 (2) | 145 |
Symmetry codes: (i) 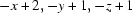 ; (ii)
; (ii) 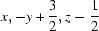 .
.
Acknowledgments
This work was supported in part by the RFBR project 09–03-13557.
supplementary crystallographic information
Comment
Phenazepam is a benzodiazepine drug produced in Russia, which is used in the treatment of neurological disorders such as epilepsy, alcohol withdrawal syndrome and insomnia. The crystal structure of its α-polymorph has been reported by Karapetyan et al. (1979). Herewith we present the crystal structure of β-polymorph of phenazepam, which was obtained from the α-polymorph via cryomodification, i.e. through the preparation of metastable solid-phase from the vapor phase at low temperature (Sergeev & Komarov, 2006).
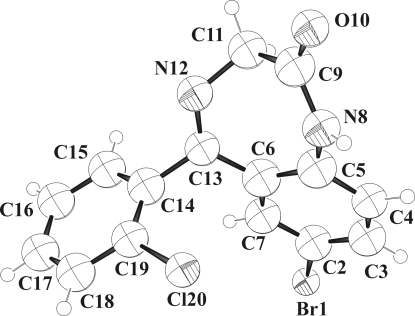
In β-polymorph (Fig. 1), two six-membered rings form a dihedral angle of 86.2 (3)°, while this dihedral angle is 75.4 (2)° in α-polymorph. In both polymorphs, intermolecular N—H···O hydrogen bonds (Table 1) link the molecules into centrosymmetric dimers. In the crystal structure of β-polymorph (in spite of α-polymorph), the non-classical intermolecular C—H···O hydrogen bonds (Table 1) link further these dimers into layers parallel to bc plane.
Experimental
The title β-polymorph of phenazepam has been obtained via cryomodification of α-polymorph of phenazepam. Cryomodification was realized by vapor deposition on a cold surface in vacuo at temperatures varying from 77 to 273 K following the known procedure (Sergeev & Komarov, 2006).
Refinement
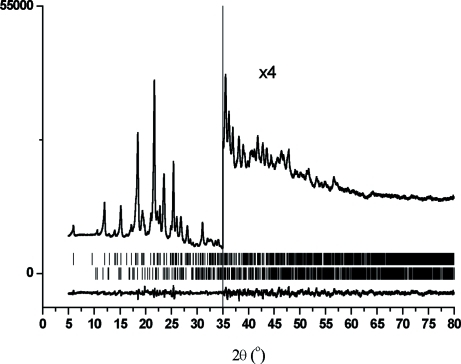
During the exposure, the specimen was spun in its plane to improve particle statistics. The triclinic unit-cell dimensions were determined with the indexing program TREOR (Werner et al., 1985), M20=37, using the first 35 peak positions. A number of weak unindexed lines (d-spacings of most significant ones were 8.54, 8.31, 6.90, 5.25 and 5.04 Å) demonstrated that the sample contained a small amount of α-polymorph. The crystal structure of β-polymorph was solved by simulated annealing procedure (Zhukov et al., 2001) and refined following the methodology described in (Ryabova et al., 2005). All non-H atoms were isotropically refined. H atoms were placed in geometrically calculated positions and not refined. The diffraction profiles and the differences between the measured and calculated profiles after the final two-phases Rietveld refinement are shown in Fig. 2. On the results of two-phases Rietveld refinement the ratio of β- and α-polymorphs in the sample was estimated as 1.000 (2) to 0.045 (2), respectively. For the α-polymorph, the atomic coordinates and displacement parameters were fixed to literature values (Karapetyan et al., 1979), so only scale factor and profile parameters were refined.
Figures
Fig. 1.
The molecular structure of β-polymorph with the atomic numbering and 50% displacement spheres.
Fig. 2.
The Rietveld plot, showing the observed and difference profiles for the sample under study. The vertical bars above the difference profile show the reflection positions for α-polymorph (bottom) and β-polymorph (top).
Crystal data
| C15H10BrClN2O | F(000) = 696 |
| Mr = 349.61 | Dx = 1.589 Mg m−3 |
| Monoclinic, P21/c | Cu Kα1 radiation, λ = 1.54059 Å |
| Hall symbol: -P 2ybc | µ = 5.49 mm−1 |
| a = 14.8006 (19) Å | T = 295 K |
| b = 11.6756 (14) Å | Particle morphology: no specific habit |
| c = 8.4769 (9) Å | light grey |
| β = 93.679 (17)° | flat sheet, 15 × 1 mm |
| V = 1461.8 (3) Å3 | Specimen preparation: Prepared at 77 K and 6.6 10-6 kPa |
| Z = 4 |
Data collection
| Guinier camera G670 diffractometer | Data collection mode: transmission |
| Radiation source: line-focus sealed tube | Scan method: continuous |
| Curved Germanium (111) | 2θmin = 5.00°, 2θmax = 80.00°, 2θstep = 0.01° |
| Specimen mounting: thin layer in the specimen holder of the camera |
Refinement
| Refinement on Inet | Profile function: split-type pseudo-Voigt (Toraya, 1986) |
| Least-squares matrix: full with fixed elements per cycle | 128 parameters |
| Rp = 0.013 | 64 restraints |
| Rwp = 0.017 | 0 constraints |
| Rexp = 0.012 | H-atom parameters not refined |
| RBragg = 0.059 | Weighting scheme based on measured s.u.'s |
| χ2 = 2.250 | (Δ/σ)max = 0.004 |
| 7501 data points | Background function: Chebyshev polynomial up to the 5th order |
| Excluded region(s): none | Preferred orientation correction: March-Dollase (Dollase, 1986); direction of preferred orientation 001, texture parameter r = 0.93(1). |
Special details
| Geometry. All e.s.d.'s (except the e.s.d. in the dihedral angle between two l.s. planes) are estimated using the full covariance matrix. The cell e.s.d.'s are taken into account individually in the estimation of e.s.d.'s in distances, angles and torsion angles; correlations between e.s.d.'s in cell parameters are only used when they are defined by crystal symmetry. An approximate (isotropic) treatment of cell e.s.d.'s is used for estimating e.s.d.'s involving l.s. planes. |
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2)
| x | y | z | Uiso*/Ueq | ||
| Br1 | 0.77280 (15) | 0.40430 (17) | −0.3314 (2) | 0.0470 (11)* | |
| C2 | 0.8173 (12) | 0.4317 (13) | −0.1182 (18) | 0.074 (9)* | |
| C3 | 0.8911 (12) | 0.3743 (12) | −0.044 (2) | 0.075 (8)* | |
| H3 | 0.9200 | 0.3154 | −0.0943 | 0.090* | |
| C4 | 0.9202 (11) | 0.4080 (13) | 0.109 (2) | 0.063 (9)* | |
| H4 | 0.9684 | 0.3696 | 0.1616 | 0.076* | |
| C5 | 0.8784 (12) | 0.4986 (15) | 0.1866 (18) | 0.076 (8)* | |
| C6 | 0.8023 (12) | 0.5539 (13) | 0.110 (2) | 0.076 (9)* | |
| C7 | 0.7727 (11) | 0.5187 (16) | −0.0430 (18) | 0.071 (8)* | |
| H7 | 0.7228 | 0.5540 | −0.0945 | 0.085* | |
| N8 | 0.9112 (9) | 0.5266 (11) | 0.3406 (13) | 0.064 (6)* | |
| H8 | 0.9247 | 0.4696 | 0.4020 | 0.077* | |
| C9 | 0.9248 (12) | 0.6346 (12) | 0.406 (2) | 0.072 (8)* | |
| O10 | 0.9656 (7) | 0.6458 (8) | 0.5370 (13) | 0.057 (5)* | |
| C11 | 0.8837 (11) | 0.7351 (13) | 0.311 (2) | 0.070 (8)* | |
| H11A | 0.8966 | 0.8060 | 0.3674 | 0.084* | |
| H11B | 0.9113 | 0.7396 | 0.2101 | 0.084* | |
| N12 | 0.7856 (8) | 0.7217 (10) | 0.2827 (16) | 0.062 (7)* | |
| C13 | 0.7513 (11) | 0.6468 (13) | 0.1858 (19) | 0.061 (8)* | |
| C14 | 0.6501 (11) | 0.6452 (14) | 0.1602 (18) | 0.071 (9)* | |
| C15 | 0.6074 (12) | 0.7367 (12) | 0.077 (2) | 0.074 (8)* | |
| H15 | 0.6425 | 0.7953 | 0.0384 | 0.089* | |
| C16 | 0.5134 (11) | 0.7411 (11) | 0.051 (2) | 0.075 (8)* | |
| H16 | 0.4864 | 0.8028 | −0.0033 | 0.090* | |
| C17 | 0.4600 (11) | 0.6530 (13) | 0.106 (2) | 0.073 (9)* | |
| H17 | 0.3976 | 0.6540 | 0.0834 | 0.087* | |
| C18 | 0.5002 (12) | 0.5633 (14) | 0.1936 (17) | 0.074 (9)* | |
| H18 | 0.4646 | 0.5067 | 0.2356 | 0.089* | |
| C19 | 0.5943 (12) | 0.5595 (13) | 0.2180 (16) | 0.065 (8)* | |
| Cl20 | 0.6428 (3) | 0.4396 (4) | 0.3145 (5) | 0.054 (2)* |
Geometric parameters (Å, °)
| Br1—C2 | 1.910 (15) | C11—N12 | 1.46 (2) |
| C2—C7 | 1.39 (2) | C11—H11A | 0.9704 |
| C2—C3 | 1.40 (2) | C11—H11B | 0.9697 |
| C3—C4 | 1.40 (2) | N12—C13 | 1.28 (2) |
| C3—H3 | 0.9297 | C13—C14 | 1.50 (2) |
| C4—C5 | 1.41 (2) | C14—C19 | 1.41 (2) |
| C4—H4 | 0.9299 | C14—C15 | 1.41 (2) |
| C5—N8 | 1.402 (19) | C15—C16 | 1.40 (2) |
| C5—C6 | 1.42 (2) | C15—H15 | 0.9299 |
| C6—C7 | 1.41 (2) | C16—C17 | 1.39 (2) |
| C6—C13 | 1.49 (2) | C16—H16 | 0.9299 |
| C7—H7 | 0.9301 | C17—C18 | 1.40 (2) |
| N8—C9 | 1.389 (19) | C17—H17 | 0.9301 |
| N8—H8 | 0.8600 | C18—C19 | 1.40 (3) |
| C9—O10 | 1.23 (2) | C18—H18 | 0.9303 |
| C9—C11 | 1.53 (2) | C19—Cl20 | 1.751 (16) |
| C7—C2—C3 | 121.6 (14) | C9—C11—H11A | 109.4 |
| C7—C2—Br1 | 114.3 (11) | N12—C11—H11B | 109.4 |
| C3—C2—Br1 | 124.1 (12) | C9—C11—H11B | 109.4 |
| C4—C3—C2 | 118.1 (15) | H11A—C11—H11B | 108.0 |
| C4—C3—H3 | 120.9 | C13—N12—C11 | 121.6 (14) |
| C2—C3—H3 | 121.0 | N12—C13—C6 | 125.6 (14) |
| C3—C4—C5 | 121.7 (15) | N12—C13—C14 | 116.8 (14) |
| C3—C4—H4 | 119.2 | C6—C13—C14 | 117.3 (14) |
| C5—C4—H4 | 119.2 | C19—C14—C15 | 117.4 (15) |
| N8—C5—C4 | 118.1 (15) | C19—C14—C13 | 124.2 (14) |
| N8—C5—C6 | 122.4 (15) | C15—C14—C13 | 118.4 (14) |
| C4—C5—C6 | 119.3 (14) | C16—C15—C14 | 121.2 (15) |
| C7—C6—C5 | 118.7 (15) | C16—C15—H15 | 119.4 |
| C7—C6—C13 | 118.3 (15) | C14—C15—H15 | 119.4 |
| C5—C6—C13 | 123.0 (14) | C17—C16—C15 | 120.0 (14) |
| C2—C7—C6 | 120.6 (15) | C17—C16—H16 | 120.0 |
| C2—C7—H7 | 119.7 | C15—C16—H16 | 120.0 |
| C6—C7—H7 | 119.7 | C16—C17—C18 | 120.0 (15) |
| C9—N8—C5 | 128.2 (13) | C16—C17—H17 | 120.0 |
| C9—N8—H8 | 115.9 | C18—C17—H17 | 120.0 |
| C5—N8—H8 | 115.9 | C19—C18—C17 | 119.4 (15) |
| O10—C9—N8 | 120.5 (13) | C19—C18—H18 | 120.3 |
| O10—C9—C11 | 123.4 (13) | C17—C18—H18 | 120.3 |
| N8—C9—C11 | 116.1 (14) | C18—C19—C14 | 121.9 (14) |
| N12—C11—C9 | 111.1 (13) | C18—C19—Cl20 | 118.0 (12) |
| N12—C11—H11A | 109.4 | C14—C19—Cl20 | 120.0 (13) |
Hydrogen-bond geometry (Å, °)
| D—H···A | D—H | H···A | D···A | D—H···A |
| N8—H8···O10i | 0.86 | 2.15 | 2.865 (16) | 141 |
| C11—H11B···O10ii | 0.97 | 2.18 | 3.03 (2) | 145 |
Symmetry codes: (i) −x+2, −y+1, −z+1; (ii) x, −y+3/2, z−1/2.
Footnotes
Supplementary data and figures for this paper are available from the IUCr electronic archives (Reference: LH5126).
References
- Dollase, W. A. (1986). J. Appl. Cryst.19, 267–272.
- Huber (2002). G670 Imaging Plate Guinier Camera Software Huber Diffraktionstechnik GmbH. Rimsting, Germany.
- Karapetyan, A. A., Andrianov, V. G., Struchkov, Yu. T., Bogatskii, A. V., Andronati, S. A. & Korotenko, T. I. (1979). Bioorg. Khim.5, 1684–1690.
- Ryabova, S. Yu., Rastorgueva, N. A., Sonneveld, E. J., Peschar, R., Schenk, H., Tafeenko, V. A., Aslanov, L. A. & Chernyshev, V. V. (2005). Acta Cryst. B61, 192–199. [DOI] [PubMed]
- Sergeev, G. B. & Komarov, V. S. (2006). Mol. Cryst. Liquid Cryst.456, 107–115.
- Sheldrick, G. M. (2008). Acta Cryst. A64, 112–122. [DOI] [PubMed]
- Spek, A. L. (2009). Acta Cryst. D65, 148–155. [DOI] [PMC free article] [PubMed]
- Toraya, H. (1986). J. Appl. Cryst.19, 440–447.
- Werner, P.-E., Eriksson, L. & Westdahl, M. (1985). J. Appl. Cryst.18, 367–370.
- Zhukov, S. G., Chernyshev, V. V., Babaev, E. V., Sonneveld, E. J. & Schenk, H. (2001). Z. Kristallogr.216, 5–9.
- Zlokazov, V. B. & Chernyshev, V. V. (1992). J. Appl. Cryst.25, 447–451.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Crystal structure: contains datablocks I, global. DOI: 10.1107/S1600536810037402/lh5126sup1.cif
Rietveld powder data: contains datablocks I. DOI: 10.1107/S1600536810037402/lh5126Isup2.rtv
Additional supplementary materials: crystallographic information; 3D view; checkCIF report