Abstract
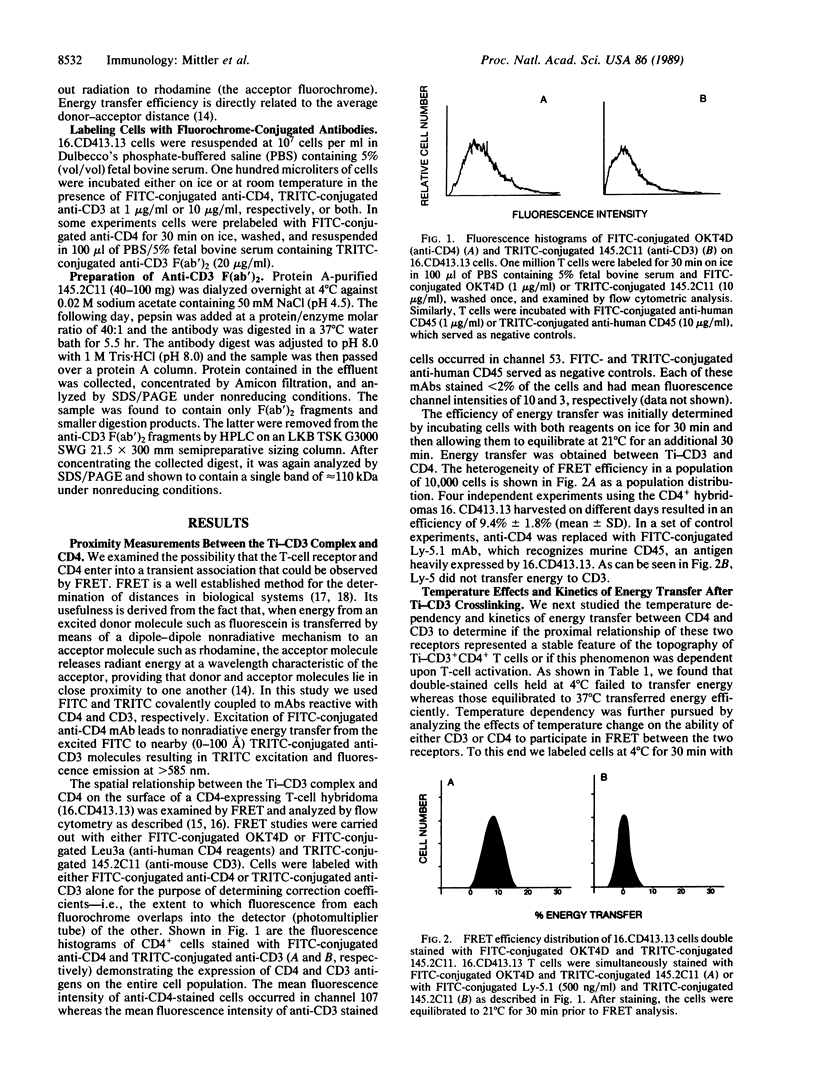
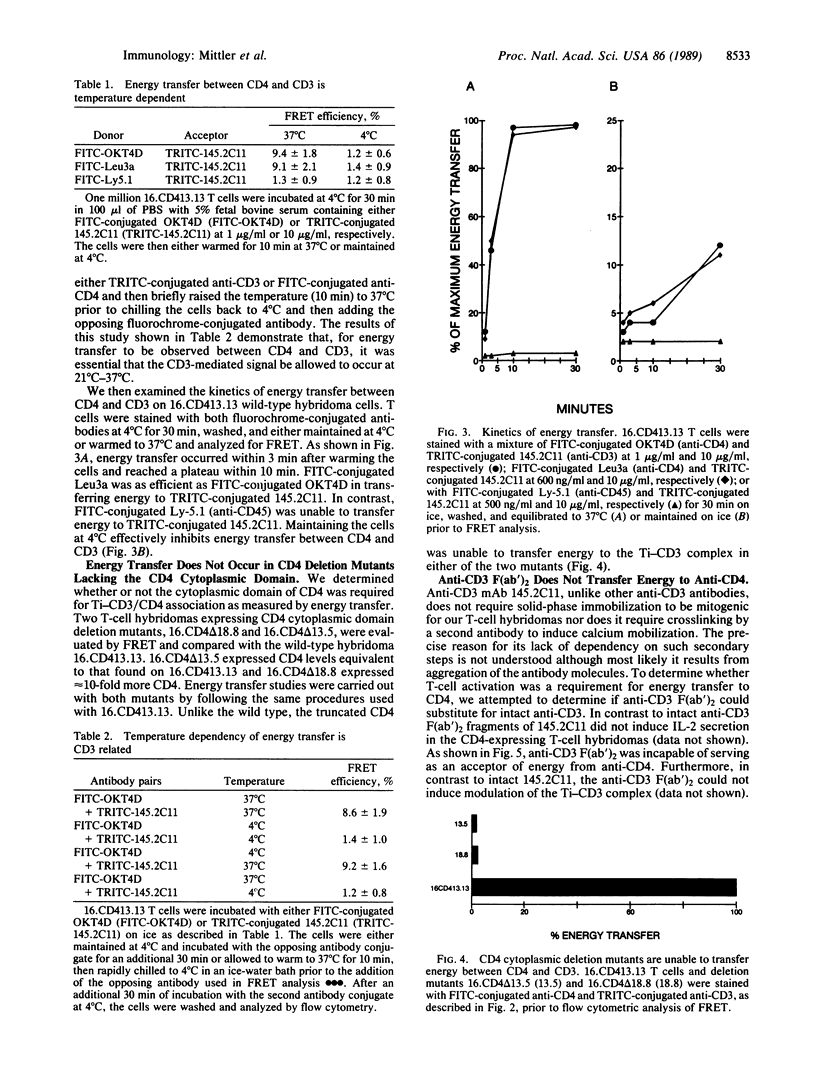
By employing flow cytometric analysis and fluorescence resonance energy transfer (FRET), we examined the physical relationship between the T-cell receptor-CD3 complex (Ti-CD3) and the CD4 molecule on helper T cells. Through the use of an L3T4-negative murine T-cell hybridoma infectant expressing the human CD4 gene and having antigen specificity for HLA-DR, we show that binding of the Ti-CD3 complex with an anti-CD3 monoclonal antibody induces its redistribution proximal to cell-surface CD4. FRET efficiency was 9.4% on cells labeled with rhodaminated anti-CD3 and fluoresceinated anti-CD4. FRET was found to be temperature dependent, since similarly treated cells held at 4 degrees C displayed a FRET efficiency of less than 1%. Energy transfer was evident within 3 min after warming cells to 37 degrees C. Energy transfer was not detected between Ti-CD3 and the abundantly expressed leukocyte common antigen (CD45). Of greater significance was our observation that hybridomas infected with a truncated CD4 gene lacking the cytoplasmic domain failed to transfer energy despite the fact that CD4 was expressed on the cell surface at levels equivalent to or greater than the wild type. These studies suggest that after crosslinking of the Ti-CD3 on CD4+ T cells, a physical association occurs between the antigen receptor complex and CD4 and that the association is dependent upon the presence of the cytoplasmic domain of CD4.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bank I., Chess L. Perturbation of the T4 molecule transmits a negative signal to T cells. J Exp Med. 1985 Oct 1;162(4):1294–1303. doi: 10.1084/jem.162.4.1294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eichmann K., Jönsson J. I., Falk I., Emmrich F. Effective activation of resting mouse T lymphocytes by cross-linking submitogenic concentrations of the T cell antigen receptor with either Lyt-2 or L3T4. Eur J Immunol. 1987 May;17(5):643–650. doi: 10.1002/eji.1830170510. [DOI] [PubMed] [Google Scholar]
- Fleischer B., Schrezenmeier H. Do CD4 or CD8 molecules provide a regulatory signal in T-cell activation? Immunol Today. 1988 May;9(5):132–134. doi: 10.1016/0167-5699(88)91198-X. [DOI] [PubMed] [Google Scholar]
- Fung B. K., Stryer L. Surface density determination in membranes by fluorescence energy transfer. Biochemistry. 1978 Nov 28;17(24):5241–5248. doi: 10.1021/bi00617a025. [DOI] [PubMed] [Google Scholar]
- Greenstein J. L., Kappler J., Marrack P., Burakoff S. J. The role of L3T4 in recognition of Ia by a cytotoxic, H-2Dd-specific T cell hybridoma. J Exp Med. 1984 Apr 1;159(4):1213–1224. doi: 10.1084/jem.159.4.1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janeway C. A., Jr T-cell development. Accessories or coreceptors? Nature. 1988 Sep 15;335(6187):208–210. doi: 10.1038/335208a0. [DOI] [PubMed] [Google Scholar]
- Krangel M. S. Endocytosis and recycling of the T3-T cell receptor complex. The role of T3 phosphorylation. J Exp Med. 1987 Apr 1;165(4):1141–1159. doi: 10.1084/jem.165.4.1141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ledbetter J. A., June C. H., Rabinovitch P. S., Grossmann A., Tsu T. T., Imboden J. B. Signal transduction through CD4 receptors: stimulatory vs. inhibitory activity is regulated by CD4 proximity to the CD3/T cell receptor. Eur J Immunol. 1988 Apr;18(4):525–532. doi: 10.1002/eji.1830180406. [DOI] [PubMed] [Google Scholar]
- Marrack P., Endres R., Shimonkevitz R., Zlotnik A., Dialynas D., Fitch F., Kappler J. The major histocompatibility complex-restricted antigen receptor on T cells. II. Role of the L3T4 product. J Exp Med. 1983 Oct 1;158(4):1077–1091. doi: 10.1084/jem.158.4.1077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rojo J. M., Saizawa K., Janeway C. A., Jr Physical association of CD4 and the T-cell receptor can be induced by anti-T-cell receptor antibodies. Proc Natl Acad Sci U S A. 1989 May;86(9):3311–3315. doi: 10.1073/pnas.86.9.3311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudd C. E., Trevillyan J. M., Dasgupta J. D., Wong L. L., Schlossman S. F. The CD4 receptor is complexed in detergent lysates to a protein-tyrosine kinase (pp58) from human T lymphocytes. Proc Natl Acad Sci U S A. 1988 Jul;85(14):5190–5194. doi: 10.1073/pnas.85.14.5190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samelson L. E., O'Shea J. J., Luong H., Ross P., Urdahl K. B., Klausner R. D., Bluestone J. T cell antigen receptor phosphorylation induced by an anti-receptor antibody. J Immunol. 1987 Oct 15;139(8):2708–2714. [PubMed] [Google Scholar]
- Sleckman B. P., Peterson A., Jones W. K., Foran J. A., Greenstein J. L., Seed B., Burakoff S. J. Expression and function of CD4 in a murine T-cell hybridoma. Nature. 1987 Jul 23;328(6128):351–353. doi: 10.1038/328351a0. [DOI] [PubMed] [Google Scholar]
- Stryer L. Fluorescence energy transfer as a spectroscopic ruler. Annu Rev Biochem. 1978;47:819–846. doi: 10.1146/annurev.bi.47.070178.004131. [DOI] [PubMed] [Google Scholar]
- Szöllösi J., Mátyus L., Trón L., Balázs M., Ember I., Fulwyler M. J., Damjanovich S. Flow cytometric measurements of fluorescence energy transfer using single laser excitation. Cytometry. 1987 Mar;8(2):120–128. doi: 10.1002/cyto.990080204. [DOI] [PubMed] [Google Scholar]
- Szöllösi J., Trón L., Damjanovich S., Helliwell S. H., Arndt-Jovin D., Jovin T. M. Fluorescence energy transfer measurements on cell surfaces: a critical comparison of steady-state fluorimetric and flow cytometric methods. Cytometry. 1984 Mar;5(2):210–216. doi: 10.1002/cyto.990050216. [DOI] [PubMed] [Google Scholar]
- Trón L., Szöllósi J., Damjanovich S., Helliwell S. H., Arndt-Jovin D. J., Jovin T. M. Flow cytometric measurement of fluorescence resonance energy transfer on cell surfaces. Quantitative evaluation of the transfer efficiency on a cell-by-cell basis. Biophys J. 1984 May;45(5):939–946. doi: 10.1016/S0006-3495(84)84240-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veillette A., Bookman M. A., Horak E. M., Bolen J. B. The CD4 and CD8 T cell surface antigens are associated with the internal membrane tyrosine-protein kinase p56lck. Cell. 1988 Oct 21;55(2):301–308. doi: 10.1016/0092-8674(88)90053-0. [DOI] [PubMed] [Google Scholar]
- Veillette A., Bookman M. A., Horak E. M., Samelson L. E., Bolen J. B. Signal transduction through the CD4 receptor involves the activation of the internal membrane tyrosine-protein kinase p56lck. Nature. 1989 Mar 16;338(6212):257–259. doi: 10.1038/338257a0. [DOI] [PubMed] [Google Scholar]



