Abstract
The title compound, C20H16Cl2N4O, has two molecules in the asymmetric unit. The two five-membered rings form a dihedral angle of 54.2 (3)° in one molecule and 56.8 (3)° in the other independent molecule. The amino group of the dihydropyrazolone unit of one molecule acts as a hydrogen-bond donor to the carbonyl group of the dihydropyrazolone system of the other molecule. The resulting N—H⋯O hydrogen bonds generate a chain running along the c axis. The crystal selected was a pseudo-merohedral twin with a 44.9 (3)% twin component.
Related literature
For the crystal structure of the parent compound without the chlorine-atom substitutents, see: Bertolasi et al. (1995 ▶); Kumar et al. (1995 ▶).
Experimental
Crystal data
C20H16Cl2N4O
M r = 399.27
Monoclinic,

a = 7.8095 (2) Å
b = 20.2827 (6) Å
c = 11.5304 (3) Å
β = 90.075 (2)°
V = 1826.39 (9) Å3
Z = 4
Mo Kα radiation
μ = 0.37 mm−1
T = 100 K
0.32 × 0.08 × 0.04 mm
Data collection
Bruker SMART APEX diffractometer
Absorption correction: multi-scan (SADABS; Sheldrick, 1996 ▶) T min = 0.890, T max = 0.985
14055 measured reflections
6328 independent reflections
5280 reflections with I > 2σ(I)
R int = 0.090
Refinement
R[F 2 > 2σ(F 2)] = 0.081
wR(F 2) = 0.207
S = 1.03
6328 reflections
445 parameters
31 restraints
H-atom parameters constrained
Δρmax = 0.85 e Å−3
Δρmin = −0.65 e Å−3
Absolute structure: Flack (1983 ▶), 3812 Friedel pairs
Flack parameter: 0.0 (1)
Data collection: APEX2 (Bruker, 2008 ▶); cell refinement: SAINT (Bruker, 2008 ▶); data reduction: SAINT; program(s) used to solve structure: SHELXS97 (Sheldrick, 2008 ▶); program(s) used to refine structure: SHELXL97 (Sheldrick, 2008 ▶); molecular graphics: X-SEED (Barbour, 2001 ▶); software used to prepare material for publication: publCIF (Westrip, 2010 ▶).
Supplementary Material
Crystal structure: contains datablocks global, I. DOI: 10.1107/S1600536810035713/im2224sup1.cif
Structure factors: contains datablocks I. DOI: 10.1107/S1600536810035713/im2224Isup2.hkl
Additional supplementary materials: crystallographic information; 3D view; checkCIF report
Table 1. Hydrogen-bond geometry (Å, °).
| D—H⋯A | D—H | H⋯A | D⋯A | D—H⋯A |
|---|---|---|---|---|
| N2—H2⋯O2i | 0.88 | 1.92 | 2.65 (1) | 139 |
| N6—H6⋯O1 | 0.88 | 2.03 | 2.76 (1) | 140 |
Symmetry code: (i)  .
.
Acknowledgments
We thank the Higher Education Commission of Pakistan and the University of Malaya for supporting this study.
supplementary crystallographic information
Comment
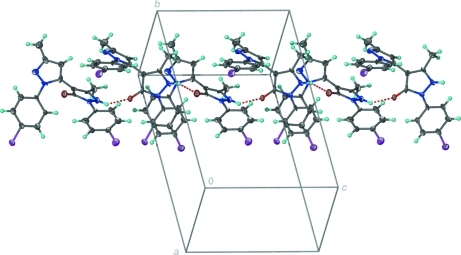
We are interested in studying the biological properties of derivatives of 1-phenyl-3-methyl-4(1-phenyl-3-methyl-1H-pyrazol-5-yl)-2H-3-pyrazolin-5-one. The crystal structure of the parent compound was reported in the context of understanding how amino as well as carbonyl groups connected to a π-system influence hydrogen bonding (Bertolasi et al., 1995; Kumar et al., 1995). The chloro-substituted compound (Scheme I, Fig. 1) crystallizes with two independant molecules in the asymmetric unit that display similar bond dimensions. Molecules are linked by N—H···O hydrogen bonds to generate a linear chain running along the c-axis of the monoclinic unit cell (Fig. 2).
The crystal studied is a racemic twin; the monoclinic unit cell, having a β-angle that is almost a right angle, emulates an orthorhombic unit cell.
Experimental
1-[1-(4-Chlorophenyl)-3-methyl-5-oxo-4,5-dihydro-1H-pyrazol-4-yl]butane-1,3-dione (0.20 g, 0.7 mmol) and 4-chlorophenylhydrazine (0.20 g, 0.14 mmol) were heated in dimethoxyethane and dilute hydrochloric acid for 4 h (synthesis of the dione will be reported elsewhere.). The reaction was quenched by 1 M potassium carbonate. The aqueous layer was extracted with ethyl acetate. The combined organic phases were concentrated and the crude product recrystallized from dichloromethane to give 0.19 g of the title compound in 70% yield.
Refinement
The refinement initially converged at an R index of about 20%. As the space group is not a centric space group, the structure was refined as a combination of general and racemic twinning, with the TWIN law of (-1 0 0 0 - 1 0 0 0 1) being used. The refinement on 3812 Friedel pairs gave a Flack parameter of 0.029; the portion of the twin component was 44.9%.
Carbon-bound H-atoms were placed in calculated positions (C—H 0.93 to 0.98 Å) and were included in the refinement in the riding model approximation, with U(H) set to 1.2 to 1.5U(C). Amino H-atoms were similarly generated.
Some of the diffraction spots of the second and third domain are quite close to those of the main domain affecting the refinement so that the ellipsoids of some atoms are observed to be significantly elongated. The anisotropic temperature factors of five carbon atoms (C1, C3, C10, C21 and C21) were therefore tightly restrained to be nearly isotropic. We have used a very tight restraint (ISOR 0.005). Even with ISOR 0.01, these atoms turn non-positive definite. Phenylene rings were restrained as rigid hexagons with carbon carbon bonds of 1.39 Å each.
A somewhat large WGHT was used that is almost the default value; the Goodness-of-Fit was not much different from the default value of 1.
Figures
Fig. 1.
Thermal ellipsoid plot (Barbour, 2001) plot of the two molecules of C20H16ClN4O at the 70% probability level; hydrogen atoms are drawn as spheres of arbitrary radius.
Fig. 2.
Thermal ellipsoid plot (Barbour, 2001) of the hydrogen-bonded chain structure.
Crystal data
| C20H16Cl2N4O | F(000) = 824 |
| Mr = 399.27 | Dx = 1.452 Mg m−3 |
| Monoclinic, P21 | Mo Kα radiation, λ = 0.71073 Å |
| Hall symbol: P 2yb | Cell parameters from 2188 reflections |
| a = 7.8095 (2) Å | θ = 2.6–22.1° |
| b = 20.2827 (6) Å | µ = 0.37 mm−1 |
| c = 11.5304 (3) Å | T = 100 K |
| β = 90.075 (2)° | Plate, colorless |
| V = 1826.39 (9) Å3 | 0.32 × 0.08 × 0.04 mm |
| Z = 4 |
Data collection
| Bruker SMART APEX diffractometer | 6328 independent reflections |
| Radiation source: fine-focus sealed tube | 5280 reflections with I > 2σ(I) |
| graphite | Rint = 0.090 |
| ω scans | θmax = 25.0°, θmin = 1.0° |
| Absorption correction: multi-scan (SADABS; Sheldrick, 1996) | h = −9→9 |
| Tmin = 0.890, Tmax = 0.985 | k = −24→24 |
| 14055 measured reflections | l = −13→13 |
Refinement
| Refinement on F2 | Secondary atom site location: difference Fourier map |
| Least-squares matrix: full | Hydrogen site location: inferred from neighbouring sites |
| R[F2 > 2σ(F2)] = 0.081 | H-atom parameters constrained |
| wR(F2) = 0.207 | w = 1/[σ2(Fo2) + (0.1148P)2] where P = (Fo2 + 2Fc2)/3 |
| S = 1.03 | (Δ/σ)max = 0.001 |
| 6328 reflections | Δρmax = 0.85 e Å−3 |
| 445 parameters | Δρmin = −0.65 e Å−3 |
| 31 restraints | Absolute structure: Flack (1983), 3812 Friedel pairs |
| Primary atom site location: structure-invariant direct methods | Flack parameter: 0.0 (1) |
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2)
| x | y | z | Uiso*/Ueq | ||
| Cl1 | 0.7639 (3) | 0.50000 (12) | 1.1115 (2) | 0.0254 (5) | |
| Cl2 | 0.2433 (3) | 0.73300 (12) | 0.5009 (2) | 0.0276 (5) | |
| Cl3 | 0.6738 (3) | 0.50272 (11) | 0.6071 (2) | 0.0279 (5) | |
| Cl4 | 1.3049 (3) | 0.73174 (12) | −0.0046 (2) | 0.0236 (5) | |
| O1 | 0.8167 (9) | 0.8010 (3) | 0.8422 (5) | 0.0189 (14) | |
| O2 | 0.7406 (9) | 0.8012 (3) | 0.3352 (6) | 0.0244 (16) | |
| N1 | 0.7364 (10) | 0.7877 (4) | 1.0350 (6) | 0.0149 (16) | |
| N2 | 0.6547 (9) | 0.8275 (4) | 1.1178 (7) | 0.0196 (16) | |
| H2 | 0.6287 | 0.8163 | 1.1893 | 0.023* | |
| N3 | 0.6031 (10) | 0.9429 (4) | 0.7631 (7) | 0.0177 (16) | |
| N4 | 0.6322 (10) | 1.0009 (4) | 0.7076 (7) | 0.0225 (17) | |
| N5 | 0.8150 (10) | 0.7850 (4) | 0.5275 (6) | 0.0166 (17) | |
| N6 | 0.8991 (10) | 0.8225 (4) | 0.6125 (6) | 0.0188 (16) | |
| H6 | 0.9250 | 0.8089 | 0.6829 | 0.023* | |
| N7 | 0.9652 (10) | 0.9418 (3) | 0.2675 (7) | 0.0172 (16) | |
| N8 | 0.9481 (10) | 1.0025 (4) | 0.2169 (7) | 0.0218 (17) | |
| C1 | 0.7499 (13) | 0.8238 (5) | 0.9318 (8) | 0.021 (2) | |
| C2 | 0.6771 (12) | 0.8870 (5) | 0.9542 (8) | 0.0174 (19) | |
| C3 | 0.6235 (12) | 0.8862 (4) | 1.0671 (8) | 0.0157 (19) | |
| C4 | 0.5388 (13) | 0.9395 (4) | 1.1409 (8) | 0.024 (2) | |
| H4A | 0.4354 | 0.9215 | 1.1772 | 0.036* | |
| H4B | 0.5079 | 0.9770 | 1.0916 | 0.036* | |
| H4C | 0.6188 | 0.9540 | 1.2012 | 0.036* | |
| C5 | 0.7512 (8) | 0.7199 (2) | 1.0537 (5) | 0.0164 (19) | |
| C6 | 0.8415 (7) | 0.6801 (3) | 0.9770 (4) | 0.018 (2) | |
| H6A | 0.8992 | 0.6993 | 0.9128 | 0.022* | |
| C7 | 0.8476 (7) | 0.6124 (2) | 0.9944 (4) | 0.0188 (19) | |
| H7 | 0.9093 | 0.5852 | 0.9420 | 0.023* | |
| C8 | 0.7633 (8) | 0.5844 (2) | 1.0884 (5) | 0.020 (2) | |
| C9 | 0.6729 (7) | 0.6242 (3) | 1.1650 (4) | 0.022 (2) | |
| H9 | 0.6153 | 0.6051 | 1.2292 | 0.026* | |
| C10 | 0.6669 (7) | 0.6920 (2) | 1.1476 (4) | 0.0184 (19) | |
| H10 | 0.6051 | 0.7192 | 1.2000 | 0.022* | |
| C11 | 0.6723 (11) | 0.9425 (4) | 0.8734 (8) | 0.0156 (19) | |
| C12 | 0.7453 (12) | 1.0042 (5) | 0.8902 (8) | 0.021 (2) | |
| H12 | 0.8011 | 1.0203 | 0.9577 | 0.026* | |
| C13 | 0.7176 (13) | 1.0376 (4) | 0.7838 (8) | 0.020 (2) | |
| C14 | 0.7813 (15) | 1.1062 (5) | 0.7499 (9) | 0.031 (3) | |
| H14A | 0.7385 | 1.1174 | 0.6725 | 0.047* | |
| H14B | 0.9068 | 1.1065 | 0.7495 | 0.047* | |
| H14C | 0.7395 | 1.1386 | 0.8061 | 0.047* | |
| C15 | 0.5111 (7) | 0.8935 (2) | 0.7027 (5) | 0.019 (2) | |
| C16 | 0.5119 (7) | 0.8944 (3) | 0.5821 (5) | 0.025 (2) | |
| H16 | 0.5709 | 0.9284 | 0.5420 | 0.030* | |
| C17 | 0.4262 (8) | 0.8457 (3) | 0.5203 (4) | 0.020 (2) | |
| H17 | 0.4268 | 0.8463 | 0.4379 | 0.024* | |
| C18 | 0.3398 (8) | 0.7959 (3) | 0.5790 (5) | 0.022 (2) | |
| C19 | 0.3391 (8) | 0.7950 (2) | 0.6995 (5) | 0.022 (2) | |
| H19 | 0.2800 | 0.7610 | 0.7396 | 0.027* | |
| C20 | 0.4247 (8) | 0.8437 (3) | 0.7614 (4) | 0.020 (2) | |
| H20 | 0.4242 | 0.8431 | 0.8437 | 0.024* | |
| C21 | 0.8081 (12) | 0.8223 (4) | 0.4247 (7) | 0.0133 (18) | |
| C22 | 0.8897 (13) | 0.8838 (4) | 0.4551 (8) | 0.020 (2) | |
| C23 | 0.9332 (12) | 0.8821 (4) | 0.5691 (8) | 0.017 (2) | |
| C24 | 1.0216 (13) | 0.9322 (4) | 0.6447 (8) | 0.023 (2) | |
| H24A | 0.9802 | 0.9280 | 0.7245 | 0.034* | |
| H24B | 0.9967 | 0.9766 | 0.6158 | 0.034* | |
| H24C | 1.1455 | 0.9246 | 0.6429 | 0.034* | |
| C25 | 0.7768 (8) | 0.7182 (2) | 0.5441 (5) | 0.0152 (19) | |
| C26 | 0.8020 (8) | 0.6725 (3) | 0.4558 (4) | 0.028 (2) | |
| H26 | 0.8412 | 0.6868 | 0.3819 | 0.034* | |
| C27 | 0.7700 (9) | 0.6061 (2) | 0.4755 (4) | 0.025 (2) | |
| H27 | 0.7872 | 0.5749 | 0.4152 | 0.030* | |
| C28 | 0.7126 (9) | 0.5852 (2) | 0.5836 (5) | 0.019 (2) | |
| C29 | 0.6874 (9) | 0.6308 (3) | 0.6718 (4) | 0.024 (2) | |
| H29 | 0.6482 | 0.6166 | 0.7457 | 0.029* | |
| C30 | 0.7194 (8) | 0.6973 (3) | 0.6521 (4) | 0.024 (2) | |
| H30 | 0.7022 | 0.7285 | 0.7124 | 0.029* | |
| C31 | 0.8965 (12) | 0.9420 (5) | 0.3771 (8) | 0.019 (2) | |
| C32 | 0.8358 (13) | 1.0035 (5) | 0.3962 (8) | 0.024 (2) | |
| H32 | 0.7826 | 1.0189 | 0.4651 | 0.029* | |
| C33 | 0.8663 (12) | 1.0403 (5) | 0.2950 (8) | 0.023 (2) | |
| C34 | 0.8278 (15) | 1.1108 (5) | 0.2670 (9) | 0.029 (2) | |
| H34A | 0.8228 | 1.1165 | 0.1827 | 0.044* | |
| H34B | 0.9181 | 1.1391 | 0.2991 | 0.044* | |
| H34C | 0.7174 | 1.1231 | 0.3011 | 0.044* | |
| C35 | 1.0548 (7) | 0.8927 (2) | 0.2047 (4) | 0.0170 (19) | |
| C36 | 1.0520 (7) | 0.8956 (2) | 0.0843 (5) | 0.020 (2) | |
| H36 | 0.9962 | 0.9312 | 0.0462 | 0.024* | |
| C37 | 1.1310 (8) | 0.8465 (3) | 0.0195 (3) | 0.021 (2) | |
| H37 | 1.1290 | 0.8484 | −0.0628 | 0.026* | |
| C38 | 1.2127 (7) | 0.7945 (3) | 0.0752 (4) | 0.0174 (19) | |
| C39 | 1.2155 (8) | 0.7916 (2) | 0.1957 (5) | 0.026 (2) | |
| H39 | 1.2714 | 0.7560 | 0.2337 | 0.031* | |
| C40 | 1.1366 (9) | 0.8407 (3) | 0.2604 (3) | 0.021 (2) | |
| H40 | 1.1385 | 0.8387 | 0.3427 | 0.025* |
Atomic displacement parameters (Å2)
| U11 | U22 | U33 | U12 | U13 | U23 | |
| Cl1 | 0.0274 (13) | 0.0186 (11) | 0.0301 (14) | −0.0004 (12) | 0.0030 (11) | 0.0032 (11) |
| Cl2 | 0.0312 (13) | 0.0280 (12) | 0.0238 (12) | −0.0023 (13) | −0.0073 (12) | −0.0023 (12) |
| Cl3 | 0.0309 (14) | 0.0219 (12) | 0.0307 (13) | −0.0064 (13) | −0.0026 (11) | 0.0031 (11) |
| Cl4 | 0.0220 (12) | 0.0288 (11) | 0.0202 (11) | 0.0040 (12) | 0.0030 (11) | −0.0016 (10) |
| O1 | 0.021 (3) | 0.026 (3) | 0.010 (3) | 0.002 (3) | 0.000 (3) | 0.001 (3) |
| O2 | 0.033 (4) | 0.024 (4) | 0.016 (3) | −0.002 (3) | 0.001 (3) | −0.003 (3) |
| N1 | 0.016 (4) | 0.019 (4) | 0.010 (4) | 0.006 (3) | −0.002 (3) | −0.004 (3) |
| N2 | 0.018 (4) | 0.027 (4) | 0.014 (4) | 0.001 (3) | 0.006 (3) | −0.005 (3) |
| N3 | 0.010 (4) | 0.021 (4) | 0.022 (4) | 0.002 (3) | 0.005 (3) | 0.001 (4) |
| N4 | 0.023 (4) | 0.019 (4) | 0.025 (4) | 0.001 (4) | −0.008 (3) | 0.001 (4) |
| N5 | 0.021 (4) | 0.020 (4) | 0.008 (4) | 0.007 (3) | −0.004 (3) | −0.003 (3) |
| N6 | 0.022 (4) | 0.029 (4) | 0.006 (4) | 0.001 (3) | −0.006 (3) | −0.004 (3) |
| N7 | 0.012 (4) | 0.018 (4) | 0.022 (4) | 0.002 (3) | 0.001 (3) | −0.007 (3) |
| N8 | 0.017 (4) | 0.021 (4) | 0.027 (4) | −0.001 (4) | −0.001 (3) | 0.001 (4) |
| C1 | 0.028 (4) | 0.023 (4) | 0.013 (4) | −0.005 (3) | −0.003 (3) | 0.002 (3) |
| C2 | 0.011 (5) | 0.026 (5) | 0.015 (4) | −0.004 (4) | −0.002 (4) | 0.002 (4) |
| C3 | 0.013 (4) | 0.019 (3) | 0.015 (4) | 0.000 (3) | 0.007 (3) | −0.003 (3) |
| C4 | 0.022 (5) | 0.024 (5) | 0.025 (5) | 0.002 (4) | 0.004 (4) | −0.002 (4) |
| C5 | 0.014 (4) | 0.027 (5) | 0.008 (4) | −0.003 (4) | 0.001 (3) | −0.003 (4) |
| C6 | 0.017 (5) | 0.028 (5) | 0.010 (5) | 0.003 (4) | −0.003 (4) | −0.006 (4) |
| C7 | 0.011 (4) | 0.031 (5) | 0.014 (4) | −0.001 (4) | 0.001 (4) | 0.001 (4) |
| C8 | 0.005 (4) | 0.036 (5) | 0.018 (5) | −0.002 (4) | 0.000 (4) | −0.009 (4) |
| C9 | 0.017 (5) | 0.028 (5) | 0.021 (5) | 0.005 (4) | 0.000 (4) | −0.005 (4) |
| C10 | 0.013 (3) | 0.028 (4) | 0.014 (4) | −0.002 (3) | 0.005 (3) | −0.001 (3) |
| C11 | 0.014 (5) | 0.023 (5) | 0.010 (4) | 0.001 (4) | 0.003 (4) | 0.001 (4) |
| C12 | 0.023 (5) | 0.026 (5) | 0.015 (5) | 0.009 (5) | 0.008 (4) | 0.003 (4) |
| C13 | 0.027 (5) | 0.015 (4) | 0.019 (5) | 0.000 (4) | 0.001 (4) | −0.003 (4) |
| C14 | 0.040 (7) | 0.023 (5) | 0.031 (6) | −0.001 (4) | −0.009 (5) | 0.001 (4) |
| C15 | 0.030 (6) | 0.015 (4) | 0.013 (4) | 0.010 (4) | 0.009 (4) | 0.006 (4) |
| C16 | 0.023 (5) | 0.030 (5) | 0.021 (5) | 0.002 (4) | −0.005 (4) | 0.008 (5) |
| C17 | 0.018 (5) | 0.034 (5) | 0.007 (4) | 0.006 (4) | 0.003 (4) | 0.001 (4) |
| C18 | 0.022 (5) | 0.028 (5) | 0.015 (5) | −0.002 (4) | −0.005 (4) | 0.003 (4) |
| C19 | 0.020 (5) | 0.019 (4) | 0.027 (5) | −0.004 (4) | −0.002 (4) | 0.005 (4) |
| C20 | 0.013 (5) | 0.032 (5) | 0.015 (5) | 0.003 (4) | 0.001 (4) | −0.002 (4) |
| C21 | 0.017 (4) | 0.015 (3) | 0.008 (3) | 0.002 (3) | 0.004 (3) | 0.000 (3) |
| C22 | 0.025 (6) | 0.011 (4) | 0.023 (5) | 0.008 (4) | 0.009 (4) | 0.004 (4) |
| C23 | 0.019 (5) | 0.010 (4) | 0.023 (5) | 0.006 (4) | −0.004 (4) | 0.006 (4) |
| C24 | 0.020 (5) | 0.038 (6) | 0.011 (5) | −0.003 (4) | −0.002 (4) | −0.005 (4) |
| C25 | 0.018 (4) | 0.017 (4) | 0.011 (3) | 0.005 (3) | −0.001 (3) | 0.005 (3) |
| C26 | 0.031 (6) | 0.026 (6) | 0.028 (6) | 0.008 (4) | 0.000 (5) | −0.004 (4) |
| C27 | 0.028 (5) | 0.025 (5) | 0.022 (5) | 0.006 (4) | 0.005 (4) | 0.001 (4) |
| C28 | 0.017 (5) | 0.025 (5) | 0.017 (5) | 0.004 (4) | −0.002 (4) | 0.003 (4) |
| C29 | 0.028 (5) | 0.024 (5) | 0.021 (5) | 0.003 (4) | 0.002 (4) | 0.000 (4) |
| C30 | 0.016 (5) | 0.039 (6) | 0.017 (5) | −0.001 (4) | 0.004 (4) | −0.012 (4) |
| C31 | 0.019 (5) | 0.027 (5) | 0.012 (5) | −0.004 (4) | −0.004 (4) | 0.005 (4) |
| C32 | 0.032 (5) | 0.024 (5) | 0.015 (5) | 0.007 (5) | −0.002 (4) | −0.004 (4) |
| C33 | 0.017 (5) | 0.033 (5) | 0.019 (5) | −0.008 (4) | 0.003 (4) | −0.009 (4) |
| C34 | 0.038 (6) | 0.022 (5) | 0.027 (6) | 0.005 (5) | 0.000 (5) | 0.001 (4) |
| C35 | 0.015 (5) | 0.024 (5) | 0.012 (4) | 0.000 (4) | 0.008 (4) | −0.005 (4) |
| C36 | 0.020 (5) | 0.022 (5) | 0.018 (5) | −0.006 (4) | −0.001 (4) | 0.003 (4) |
| C37 | 0.015 (5) | 0.031 (5) | 0.018 (5) | −0.003 (4) | 0.006 (4) | 0.002 (4) |
| C38 | 0.010 (4) | 0.027 (5) | 0.015 (5) | −0.001 (4) | 0.003 (3) | 0.000 (4) |
| C39 | 0.027 (6) | 0.032 (6) | 0.018 (5) | 0.006 (5) | 0.000 (4) | 0.004 (4) |
| C40 | 0.030 (6) | 0.026 (5) | 0.008 (4) | 0.011 (4) | 0.001 (4) | 0.003 (4) |
Geometric parameters (Å, °)
| Cl1—C8 | 1.732 (5) | C14—H14B | 0.9800 |
| Cl2—C18 | 1.734 (5) | C14—H14C | 0.9800 |
| Cl3—C28 | 1.722 (5) | C15—C16 | 1.3900 |
| Cl4—C38 | 1.728 (4) | C15—C20 | 1.3900 |
| O1—C1 | 1.247 (11) | C16—C17 | 1.3900 |
| O2—C21 | 1.234 (10) | C16—H16 | 0.9500 |
| N1—C5 | 1.395 (9) | C17—C18 | 1.3900 |
| N1—C1 | 1.401 (12) | C17—H17 | 0.9500 |
| N1—N2 | 1.405 (10) | C18—C19 | 1.3900 |
| N2—C3 | 1.348 (12) | C19—C20 | 1.3900 |
| N2—H2 | 0.8800 | C19—H19 | 0.9500 |
| N3—N4 | 1.358 (11) | C20—H20 | 0.9500 |
| N3—C11 | 1.381 (12) | C21—C22 | 1.445 (13) |
| N3—C15 | 1.417 (8) | C22—C23 | 1.358 (12) |
| N4—C13 | 1.330 (12) | C22—C31 | 1.485 (12) |
| N5—C25 | 1.402 (9) | C23—C24 | 1.506 (12) |
| N5—N6 | 1.403 (10) | C24—H24A | 0.9800 |
| N5—C21 | 1.407 (11) | C24—H24B | 0.9800 |
| N6—C23 | 1.337 (11) | C24—H24C | 0.9800 |
| N6—H6 | 0.8800 | C25—C26 | 1.3900 |
| N7—N8 | 1.368 (11) | C25—C30 | 1.3900 |
| N7—C31 | 1.373 (12) | C26—C27 | 1.3900 |
| N7—C35 | 1.416 (8) | C26—H26 | 0.9500 |
| N8—C33 | 1.345 (12) | C27—C28 | 1.3900 |
| C1—C2 | 1.426 (14) | C27—H27 | 0.9500 |
| C2—C3 | 1.367 (12) | C28—C29 | 1.3900 |
| C2—C11 | 1.462 (13) | C29—C30 | 1.3900 |
| C3—C4 | 1.527 (12) | C29—H29 | 0.9500 |
| C4—H4A | 0.9800 | C30—H30 | 0.9500 |
| C4—H4B | 0.9800 | C31—C32 | 1.352 (14) |
| C4—H4C | 0.9800 | C32—C33 | 1.406 (14) |
| C5—C6 | 1.3900 | C32—H32 | 0.9500 |
| C5—C10 | 1.3900 | C33—C34 | 1.496 (13) |
| C6—C7 | 1.3900 | C34—H34A | 0.9800 |
| C6—H6A | 0.9500 | C34—H34B | 0.9800 |
| C7—C8 | 1.3900 | C34—H34C | 0.9800 |
| C7—H7 | 0.9500 | C35—C36 | 1.3900 |
| C8—C9 | 1.3900 | C35—C40 | 1.3900 |
| C9—C10 | 1.3900 | C36—C37 | 1.3900 |
| C9—H9 | 0.9500 | C36—H36 | 0.9500 |
| C10—H10 | 0.9500 | C37—C38 | 1.3900 |
| C11—C12 | 1.387 (15) | C37—H37 | 0.9500 |
| C12—C13 | 1.418 (13) | C38—C39 | 1.3900 |
| C12—H12 | 0.9500 | C39—C40 | 1.3900 |
| C13—C14 | 1.527 (13) | C39—H39 | 0.9500 |
| C14—H14A | 0.9800 | C40—H40 | 0.9500 |
| C5—N1—C1 | 129.8 (7) | C16—C17—H17 | 120.0 |
| C5—N1—N2 | 119.9 (7) | C17—C18—C19 | 120.0 |
| C1—N1—N2 | 108.1 (7) | C17—C18—Cl2 | 119.5 (3) |
| C3—N2—N1 | 107.2 (7) | C19—C18—Cl2 | 120.4 (3) |
| C3—N2—H2 | 126.4 | C18—C19—C20 | 120.0 |
| N1—N2—H2 | 126.4 | C18—C19—H19 | 120.0 |
| N4—N3—C11 | 111.9 (8) | C20—C19—H19 | 120.0 |
| N4—N3—C15 | 117.8 (7) | C19—C20—C15 | 120.0 |
| C11—N3—C15 | 130.3 (7) | C19—C20—H20 | 120.0 |
| C13—N4—N3 | 104.9 (7) | C15—C20—H20 | 120.0 |
| C25—N5—N6 | 121.9 (6) | O2—C21—N5 | 122.3 (8) |
| C25—N5—C21 | 128.8 (6) | O2—C21—C22 | 133.6 (8) |
| N6—N5—C21 | 108.4 (7) | N5—C21—C22 | 104.1 (7) |
| C23—N6—N5 | 108.7 (7) | C23—C22—C21 | 108.8 (8) |
| C23—N6—H6 | 125.6 | C23—C22—C31 | 126.7 (9) |
| N5—N6—H6 | 125.6 | C21—C22—C31 | 123.8 (8) |
| N8—N7—C31 | 110.6 (7) | N6—C23—C22 | 109.7 (8) |
| N8—N7—C35 | 117.5 (7) | N6—C23—C24 | 119.0 (8) |
| C31—N7—C35 | 131.8 (7) | C22—C23—C24 | 131.1 (9) |
| C33—N8—N7 | 105.9 (8) | C23—C24—H24A | 109.5 |
| O1—C1—N1 | 122.8 (8) | C23—C24—H24B | 109.5 |
| O1—C1—C2 | 130.6 (9) | H24A—C24—H24B | 109.5 |
| N1—C1—C2 | 106.6 (8) | C23—C24—H24C | 109.5 |
| C3—C2—C1 | 106.5 (8) | H24A—C24—H24C | 109.5 |
| C3—C2—C11 | 127.5 (9) | H24B—C24—H24C | 109.5 |
| C1—C2—C11 | 125.9 (8) | C26—C25—C30 | 120.0 |
| N2—C3—C2 | 111.6 (8) | C26—C25—N5 | 120.9 (5) |
| N2—C3—C4 | 117.5 (8) | C30—C25—N5 | 119.0 (4) |
| C2—C3—C4 | 130.9 (9) | C25—C26—C27 | 120.0 |
| C3—C4—H4A | 109.5 | C25—C26—H26 | 120.0 |
| C3—C4—H4B | 109.5 | C27—C26—H26 | 120.0 |
| H4A—C4—H4B | 109.5 | C28—C27—C26 | 120.0 |
| C3—C4—H4C | 109.5 | C28—C27—H27 | 120.0 |
| H4A—C4—H4C | 109.5 | C26—C27—H27 | 120.0 |
| H4B—C4—H4C | 109.5 | C29—C28—C27 | 120.0 |
| C6—C5—C10 | 120.0 | C29—C28—Cl3 | 120.4 (3) |
| C6—C5—N1 | 121.0 (4) | C27—C28—Cl3 | 119.6 (3) |
| C10—C5—N1 | 118.9 (4) | C28—C29—C30 | 120.0 |
| C5—C6—C7 | 120.0 | C28—C29—H29 | 120.0 |
| C5—C6—H6A | 120.0 | C30—C29—H29 | 120.0 |
| C7—C6—H6A | 120.0 | C29—C30—C25 | 120.0 |
| C6—C7—C8 | 120.0 | C29—C30—H30 | 120.0 |
| C6—C7—H7 | 120.0 | C25—C30—H30 | 120.0 |
| C8—C7—H7 | 120.0 | C32—C31—N7 | 106.9 (8) |
| C7—C8—C9 | 120.0 | C32—C31—C22 | 128.4 (9) |
| C7—C8—Cl1 | 121.5 (3) | N7—C31—C22 | 124.7 (8) |
| C9—C8—Cl1 | 118.5 (3) | C31—C32—C33 | 107.2 (9) |
| C10—C9—C8 | 120.0 | C31—C32—H32 | 126.4 |
| C10—C9—H9 | 120.0 | C33—C32—H32 | 126.4 |
| C8—C9—H9 | 120.0 | N8—C33—C32 | 109.5 (9) |
| C9—C10—C5 | 120.0 | N8—C33—C34 | 119.8 (9) |
| C9—C10—H10 | 120.0 | C32—C33—C34 | 130.7 (9) |
| C5—C10—H10 | 120.0 | C33—C34—H34A | 109.5 |
| N3—C11—C12 | 106.5 (8) | C33—C34—H34B | 109.5 |
| N3—C11—C2 | 126.9 (8) | H34A—C34—H34B | 109.5 |
| C12—C11—C2 | 126.5 (8) | C33—C34—H34C | 109.5 |
| C11—C12—C13 | 104.4 (8) | H34A—C34—H34C | 109.5 |
| C11—C12—H12 | 127.8 | H34B—C34—H34C | 109.5 |
| C13—C12—H12 | 127.8 | C36—C35—C40 | 120.0 |
| N4—C13—C12 | 112.3 (8) | C36—C35—N7 | 118.3 (5) |
| N4—C13—C14 | 120.3 (8) | C40—C35—N7 | 121.6 (5) |
| C12—C13—C14 | 127.4 (9) | C35—C36—C37 | 120.0 |
| C13—C14—H14A | 109.5 | C35—C36—H36 | 120.0 |
| C13—C14—H14B | 109.5 | C37—C36—H36 | 120.0 |
| H14A—C14—H14B | 109.5 | C38—C37—C36 | 120.0 |
| C13—C14—H14C | 109.5 | C38—C37—H37 | 120.0 |
| H14A—C14—H14C | 109.5 | C36—C37—H37 | 120.0 |
| H14B—C14—H14C | 109.5 | C37—C38—C39 | 120.0 |
| C16—C15—C20 | 120.0 | C37—C38—Cl4 | 120.3 (3) |
| C16—C15—N3 | 118.6 (5) | C39—C38—Cl4 | 119.7 (3) |
| C20—C15—N3 | 121.4 (5) | C38—C39—C40 | 120.0 |
| C15—C16—C17 | 120.0 | C38—C39—H39 | 120.0 |
| C15—C16—H16 | 120.0 | C40—C39—H39 | 120.0 |
| C17—C16—H16 | 120.0 | C39—C40—C35 | 120.0 |
| C18—C17—C16 | 120.0 | C39—C40—H40 | 120.0 |
| C18—C17—H17 | 120.0 | C35—C40—H40 | 120.0 |
| C5—N1—N2—C3 | −166.1 (7) | Cl2—C18—C19—C20 | 176.5 (5) |
| C1—N1—N2—C3 | −1.3 (9) | C18—C19—C20—C15 | 0.0 |
| C11—N3—N4—C13 | −1.0 (10) | C16—C15—C20—C19 | 0.0 |
| C15—N3—N4—C13 | 179.3 (7) | N3—C15—C20—C19 | −178.4 (6) |
| C25—N5—N6—C23 | 174.0 (8) | C25—N5—C21—O2 | 11.0 (15) |
| C21—N5—N6—C23 | 4.1 (10) | N6—N5—C21—O2 | −180.0 (8) |
| C31—N7—N8—C33 | −0.8 (10) | C25—N5—C21—C22 | −170.0 (8) |
| C35—N7—N8—C33 | −177.4 (7) | N6—N5—C21—C22 | −1.0 (10) |
| C5—N1—C1—O1 | −16.9 (15) | O2—C21—C22—C23 | 176.4 (10) |
| N2—N1—C1—O1 | −179.7 (8) | N5—C21—C22—C23 | −2.3 (11) |
| C5—N1—C1—C2 | 163.7 (8) | O2—C21—C22—C31 | 5.4 (17) |
| N2—N1—C1—C2 | 0.9 (10) | N5—C21—C22—C31 | −173.3 (8) |
| O1—C1—C2—C3 | −179.5 (10) | N5—N6—C23—C22 | −5.7 (10) |
| N1—C1—C2—C3 | −0.2 (10) | N5—N6—C23—C24 | 179.2 (8) |
| O1—C1—C2—C11 | −3.1 (17) | C21—C22—C23—N6 | 5.0 (11) |
| N1—C1—C2—C11 | 176.1 (8) | C31—C22—C23—N6 | 175.7 (9) |
| N1—N2—C3—C2 | 1.2 (10) | C21—C22—C23—C24 | 179.4 (9) |
| N1—N2—C3—C4 | −178.4 (7) | C31—C22—C23—C24 | −9.9 (17) |
| C1—C2—C3—N2 | −0.6 (11) | N6—N5—C25—C26 | −137.2 (6) |
| C11—C2—C3—N2 | −176.9 (8) | C21—N5—C25—C26 | 30.5 (11) |
| C1—C2—C3—C4 | 178.9 (9) | N6—N5—C25—C30 | 40.2 (10) |
| C11—C2—C3—C4 | 2.6 (16) | C21—N5—C25—C30 | −152.1 (7) |
| C1—N1—C5—C6 | 23.4 (11) | C30—C25—C26—C27 | 0.0 |
| N2—N1—C5—C6 | −175.6 (5) | N5—C25—C26—C27 | 177.3 (7) |
| C1—N1—C5—C10 | −153.6 (8) | C25—C26—C27—C28 | 0.0 |
| N2—N1—C5—C10 | 7.5 (8) | C26—C27—C28—C29 | 0.0 |
| C10—C5—C6—C7 | 0.0 | C26—C27—C28—Cl3 | −179.9 (5) |
| N1—C5—C6—C7 | −176.9 (6) | C27—C28—C29—C30 | 0.0 |
| C5—C6—C7—C8 | 0.0 | Cl3—C28—C29—C30 | 179.9 (5) |
| C6—C7—C8—C9 | 0.0 | C28—C29—C30—C25 | 0.0 |
| C6—C7—C8—Cl1 | 178.7 (5) | C26—C25—C30—C29 | 0.0 |
| C7—C8—C9—C10 | 0.0 | N5—C25—C30—C29 | −177.4 (6) |
| Cl1—C8—C9—C10 | −178.7 (4) | N8—N7—C31—C32 | −0.2 (10) |
| C8—C9—C10—C5 | 0.0 | C35—N7—C31—C32 | 175.7 (8) |
| C6—C5—C10—C9 | 0.0 | N8—N7—C31—C22 | 178.5 (9) |
| N1—C5—C10—C9 | 177.0 (6) | C35—N7—C31—C22 | −5.6 (15) |
| N4—N3—C11—C12 | 1.7 (10) | C23—C22—C31—C32 | −48.2 (16) |
| C15—N3—C11—C12 | −178.6 (8) | C21—C22—C31—C32 | 121.1 (11) |
| N4—N3—C11—C2 | −175.0 (8) | C23—C22—C31—N7 | 133.4 (10) |
| C15—N3—C11—C2 | 4.6 (14) | C21—C22—C31—N7 | −57.3 (14) |
| C3—C2—C11—N3 | −128.0 (11) | N7—C31—C32—C33 | 1.1 (10) |
| C1—C2—C11—N3 | 56.4 (14) | C22—C31—C32—C33 | −177.5 (10) |
| C3—C2—C11—C12 | 55.9 (14) | N7—N8—C33—C32 | 1.5 (10) |
| C1—C2—C11—C12 | −119.7 (11) | N7—N8—C33—C34 | 179.6 (9) |
| N3—C11—C12—C13 | −1.6 (9) | C31—C32—C33—N8 | −1.6 (11) |
| C2—C11—C12—C13 | 175.2 (9) | C31—C32—C33—C34 | −179.4 (10) |
| N3—N4—C13—C12 | −0.1 (10) | N8—N7—C35—C36 | −28.8 (9) |
| N3—N4—C13—C14 | 177.0 (9) | C31—N7—C35—C36 | 155.5 (8) |
| C11—C12—C13—N4 | 1.1 (11) | N8—N7—C35—C40 | 154.5 (6) |
| C11—C12—C13—C14 | −175.7 (10) | C31—N7—C35—C40 | −21.1 (11) |
| N4—N3—C15—C16 | 24.3 (9) | C40—C35—C36—C37 | 0.0 |
| C11—N3—C15—C16 | −155.4 (8) | N7—C35—C36—C37 | −176.7 (6) |
| N4—N3—C15—C20 | −157.3 (6) | C35—C36—C37—C38 | 0.0 |
| C11—N3—C15—C20 | 23.1 (11) | C36—C37—C38—C39 | 0.0 |
| C20—C15—C16—C17 | 0.0 | C36—C37—C38—Cl4 | 177.8 (5) |
| N3—C15—C16—C17 | 178.5 (6) | C37—C38—C39—C40 | 0.0 |
| C15—C16—C17—C18 | 0.0 | Cl4—C38—C39—C40 | −177.8 (5) |
| C16—C17—C18—C19 | 0.0 | C38—C39—C40—C35 | 0.0 |
| C16—C17—C18—Cl2 | −176.6 (5) | C36—C35—C40—C39 | 0.0 |
| C17—C18—C19—C20 | 0.0 | N7—C35—C40—C39 | 176.6 (6) |
Hydrogen-bond geometry (Å, °)
| D—H···A | D—H | H···A | D···A | D—H···A |
| N2—H2···O2i | 0.88 | 1.92 | 2.65 (1) | 139 |
| N6—H6···O1 | 0.88 | 2.03 | 2.76 (1) | 140 |
Symmetry codes: (i) x, y, z+1.
Footnotes
Supplementary data and figures for this paper are available from the IUCr electronic archives (Reference: IM2224).
References
- Barbour, L. J. (2001). J. Supramol. Chem.1, 189–191.
- Bertolasi, V., Gilli, P., Ferretti, V. & Gilli, G. (1995). Acta Cryst. B51, 1004–1015.
- Bruker (2008). APEX2 and SAINT Bruker AXS Inc., Madison, Wisconsin, USA.
- Flack, H. D. (1983). Acta Cryst. A39, 876–881.
- Kumar, D., Singh, S. P., Martínez, A., Fruchier, A., Elguero, J., Martinez-Ripoll, M., Carrió, J. S. & Virgili, A. (1995). Tetrahedron, 51, 4891–4896.
- Sheldrick, G. M. (1996). SADABS University of Göttingen, Germany.
- Sheldrick, G. M. (2008). Acta Cryst. A64, 112–122. [DOI] [PubMed]
- Westrip, S. P. (2010). J. Appl. Cryst.43, 920–925.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Crystal structure: contains datablocks global, I. DOI: 10.1107/S1600536810035713/im2224sup1.cif
Structure factors: contains datablocks I. DOI: 10.1107/S1600536810035713/im2224Isup2.hkl
Additional supplementary materials: crystallographic information; 3D view; checkCIF report