Abstract
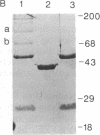
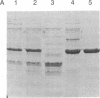
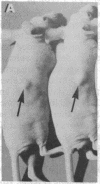
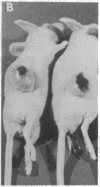
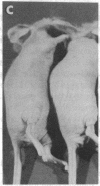
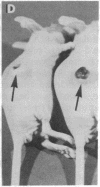
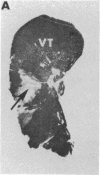
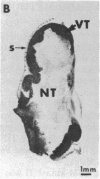
LysPE40 is a modified form of Pseudomonas exotoxin that lacks the cell-binding domain and has a chemically reactive lysine residue near the amino terminus. LysPE40 is made in Escherichia coli and secreted into the medium from which it is readily purified. Two immunotoxins were constructed by coupling LysPE40 to an antibody to the human transferrin receptor (TFR) or to an antibody to the human interleukin-2 receptor. These immunotoxins were selectively cytotoxic to receptor-bearing cells in tissue culture. Anti-TFR-LysPE40 given intraperitoneally to mice appeared rapidly in the blood and caused regression of A431 tumors growing as subcutaneous xenografts. These results show that it is possible to cause regression of a solid carcinoma by an immunotoxin if proper targeting can be achieved.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aboud-Pirak E., Hurwitz E., Bellot F., Schlessinger J., Sela M. Inhibition of human tumor growth in nude mice by a conjugate of doxorubicin with monoclonal antibodies to epidermal growth factor receptor. Proc Natl Acad Sci U S A. 1989 May;86(10):3778–3781. doi: 10.1073/pnas.86.10.3778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blakey D. C., Watson G. J., Knowles P. P., Thorpe P. E. Effect of chemical deglycosylation of ricin A chain on the in vivo fate and cytotoxic activity of an immunotoxin composed of ricin A chain and anti-Thy 1.1 antibody. Cancer Res. 1987 Feb 15;47(4):947–952. [PubMed] [Google Scholar]
- Bourrie B. J., Casellas P., Blythman H. E., Jansen F. K. Study of the plasma clearance of antibody--ricin-A-chain immunotoxins. Evidence for specific recognition sites on the A chain that mediate rapid clearance of the immunotoxin. Eur J Biochem. 1986 Feb 17;155(1):1–10. doi: 10.1111/j.1432-1033.1986.tb09451.x. [DOI] [PubMed] [Google Scholar]
- Bumol T. F., Wang Q. C., Reisfeld R. A., Kaplan N. O. Monoclonal antibody and an antibody-toxin conjugate to a cell surface proteoglycan of melanoma cells suppress in vivo tumor growth. Proc Natl Acad Sci U S A. 1983 Jan;80(2):529–533. doi: 10.1073/pnas.80.2.529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaudhary V. K., FitzGerald D. J., Adhya S., Pastan I. Activity of a recombinant fusion protein between transforming growth factor type alpha and Pseudomonas toxin. Proc Natl Acad Sci U S A. 1987 Jul;84(13):4538–4542. doi: 10.1073/pnas.84.13.4538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaudhary V. K., Xu Y. H., FitzGerald D., Adhya S., Pastan I. Role of domain II of Pseudomonas exotoxin in the secretion of proteins into the periplasm and medium by Escherichia coli. Proc Natl Acad Sci U S A. 1988 May;85(9):2939–2943. doi: 10.1073/pnas.85.9.2939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiou R. K., Vessella R. L., Limas C., Shafer R. B., Elson M. K., Arfman E. W., Lange P. H. Monoclonal antibody-targeted radiotherapy of renal cell carcinoma using a nude mouse model. Cancer. 1988 May 1;61(9):1766–1775. doi: 10.1002/1097-0142(19880501)61:9<1766::aid-cncr2820610908>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- FitzGerald D. J., Willingham M. C., Pastan I. Antitumor effects of an immunotoxin made with Pseudomonas exotoxin in a nude mouse model of human ovarian cancer. Proc Natl Acad Sci U S A. 1986 Sep;83(17):6627–6630. doi: 10.1073/pnas.83.17.6627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haynes B. F., Hemler M., Cotner T., Mann D. L., Eisenbarth G. S., Strominger J. L., Fauci A. S. Characterization of a monoclonal antibody (5E9) that defines a human cell surface antigen of cell activation. J Immunol. 1981 Jul;127(1):347–351. [PubMed] [Google Scholar]
- Hwang J., Fitzgerald D. J., Adhya S., Pastan I. Functional domains of Pseudomonas exotoxin identified by deletion analysis of the gene expressed in E. coli. Cell. 1987 Jan 16;48(1):129–136. doi: 10.1016/0092-8674(87)90363-1. [DOI] [PubMed] [Google Scholar]
- Kondo T., FitzGerald D., Chaudhary V. K., Adhya S., Pastan I. Activity of immunotoxins constructed with modified Pseudomonas exotoxin A lacking the cell recognition domain. J Biol Chem. 1988 Jul 5;263(19):9470–9475. [PubMed] [Google Scholar]
- Masui H., Kamrath H., Apell G., Houston L. L., Mendelsohn J. Cytotoxicity against human tumor cells mediated by the conjugate of anti-epidermal growth factor receptor monoclonal antibody to recombinant ricin A chain. Cancer Res. 1989 Jul 1;49(13):3482–3488. [PubMed] [Google Scholar]
- Pastan I., Willingham M. C., FitzGerald D. J. Immunotoxins. Cell. 1986 Dec 5;47(5):641–648. doi: 10.1016/0092-8674(86)90506-4. [DOI] [PubMed] [Google Scholar]
- Pirker R., FitzGerald D. J., Hamilton T. C., Ozols R. F., Willingham M. C., Pastan I. Anti-transferrin receptor antibody linked to Pseudomonas exotoxin as a model immunotoxin in human ovarian carcinoma cell lines. Cancer Res. 1985 Feb;45(2):751–757. [PubMed] [Google Scholar]
- Scott C. F., Jr, Goldmacher V. S., Lambert J. M., Jackson J. V., McIntyre G. D. An immunotoxin composed of a monoclonal antitransferrin receptor antibody linked by a disulfide bond to the ribosome-inactivating protein gelonin: potent in vitro and in vivo effects against human tumors. J Natl Cancer Inst. 1987 Nov;79(5):1163–1172. [PubMed] [Google Scholar]
- Singh M., Kralovec J., Mezei M., Ghose T. Inhibition of human renal cancer by methotrexate linked to a monoclonal antibody. J Urol. 1989 Feb;141(2):428–431. doi: 10.1016/s0022-5347(17)40787-7. [DOI] [PubMed] [Google Scholar]
- Thorpe P. E., Wallace P. M., Knowles P. P., Relf M. G., Brown A. N., Watson G. J., Blakey D. C., Newell D. R. Improved antitumor effects of immunotoxins prepared with deglycosylated ricin A-chain and hindered disulfide linkages. Cancer Res. 1988 Nov 15;48(22):6396–6403. [PubMed] [Google Scholar]
- Uchiyama T., Broder S., Waldmann T. A. A monoclonal antibody (anti-Tac) reactive with activated and functionally mature human T cells. I. Production of anti-Tac monoclonal antibody and distribution of Tac (+) cells. J Immunol. 1981 Apr;126(4):1393–1397. [PubMed] [Google Scholar]
- Vitetta E. S., Fulton R. J., May R. D., Till M., Uhr J. W. Redesigning nature's poisons to create anti-tumor reagents. Science. 1987 Nov 20;238(4830):1098–1104. doi: 10.1126/science.3317828. [DOI] [PubMed] [Google Scholar]
- Willingham M. C., FitzGerald D. J., Pastan I. Pseudomonas exotoxin coupled to a monoclonal antibody against ovarian cancer inhibits the growth of human ovarian cancer cells in a mouse model. Proc Natl Acad Sci U S A. 1987 Apr;84(8):2474–2478. doi: 10.1073/pnas.84.8.2474. [DOI] [PMC free article] [PubMed] [Google Scholar]