Abstract
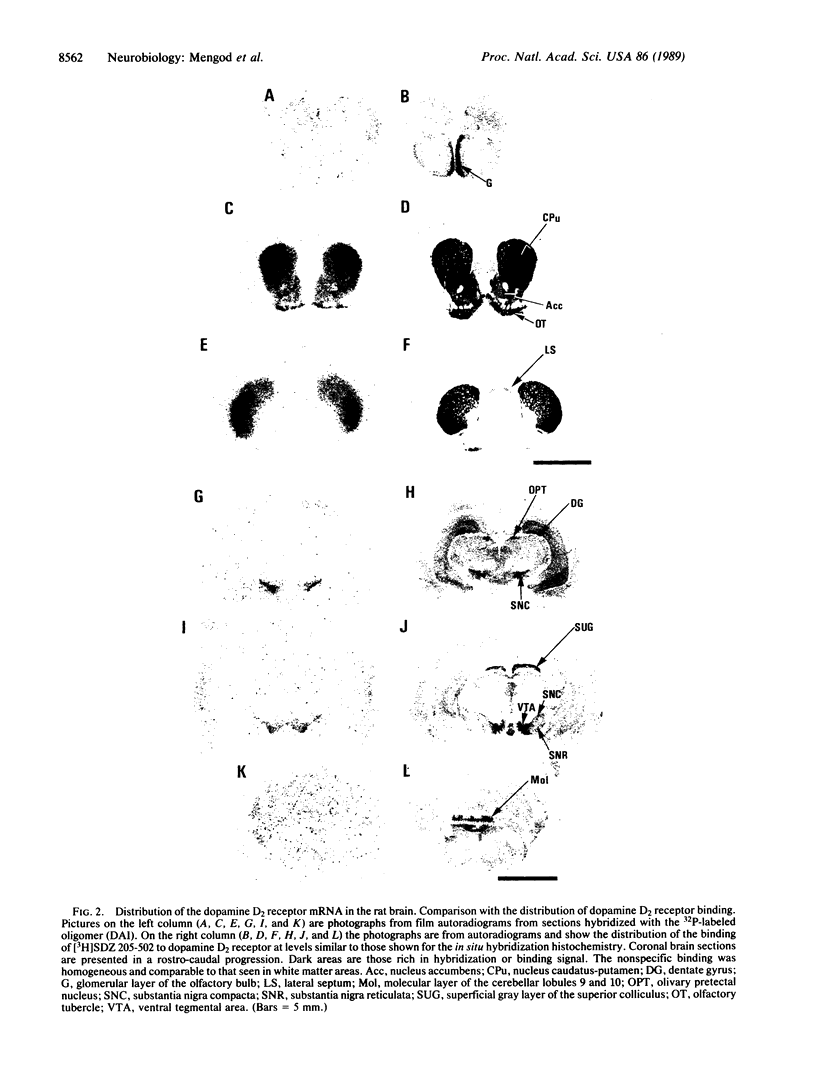
32P-labeled oligonucleotides derived from the coding region of rat dopamine D2 receptor cDNA were used as probes to localize cells in the rat brain that contain the mRNA coding for this receptor by using in situ hybridization histochemistry. The highest level of hybridization was found in the intermediate lobe of the pituitary gland. High mRNA content was observed in the anterior lobe of the pituitary gland, the nuclei caudate-putamen and accumbens, and the olfactory tubercle. Lower levels were seen in the substantia nigra pars compacta and the ventral tegmental area, as well as in the lateral mammillary body. In these areas the distribution was comparable to that of the dopamine D2 receptor binding sites as visualized by autoradiography using [3H]SDZ 205-502 as a ligand. However, in some areas such as the olfactory bulb, neocortex, hippocampus, superior colliculus, and cerebellum, D2 receptors have been visualized but no significant hybridization signal could be detected. The mRNA coding for these receptors in these areas could be contained in cells outside those brain regions, be different from the one recognized by our probes, or be present at levels below the detection limits of our procedure. The possibility of visualizing and quantifying the mRNA coding for dopamine D2 receptor at the microscopic level will yield more information about the in vivo regulation of the synthesis of these receptors and their alteration following selective lesions or drug treatments.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bunzow J. R., Van Tol H. H., Grandy D. K., Albert P., Salon J., Christie M., Machida C. A., Neve K. A., Civelli O. Cloning and expression of a rat D2 dopamine receptor cDNA. Nature. 1988 Dec 22;336(6201):783–787. doi: 10.1038/336783a0. [DOI] [PubMed] [Google Scholar]
- Chan-Palay V., Yasargil G., Hamid Q., Polak J. M., Palay S. L. Simultaneous demonstrations of neuropeptide Y gene expression and peptide storage in single neurons of the human brain. Proc Natl Acad Sci U S A. 1988 May;85(9):3213–3215. doi: 10.1073/pnas.85.9.3213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charuchinda C., Supavilai P., Karobath M., Palacios J. M. Dopamine D2 receptors in the rat brain: autoradiographic visualization using a high-affinity selective agonist ligand. J Neurosci. 1987 May;7(5):1352–1360. doi: 10.1523/JNEUROSCI.07-05-01352.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Closse A., Camps M., Wanner A., Palacios J. M. In vivo labeling of brain dopamine D2 receptors using the high-affinity specific D2 agonist [3H]CV 205-502. Brain Res. 1988 Feb 2;440(1):123–132. doi: 10.1016/0006-8993(88)91164-x. [DOI] [PubMed] [Google Scholar]
- Creese I., Snyder S. H. Chronic neuroleptic treatment and dopamine receptor regulation. Adv Biochem Psychopharmacol. 1980;24:89–94. [PubMed] [Google Scholar]
- Cross A. J., Crow T. J., Owen F. 3H-Flupenthixol binding in post-mortem brains of schizophrenics: evidence for a selective increase in dopamine D2 receptors. Psychopharmacology (Berl) 1981;74(2):122–124. doi: 10.1007/BF00432676. [DOI] [PubMed] [Google Scholar]
- Farde L., Wiesel F. A., Hall H., Halldin C., Stone-Elander S., Sedvall G. No D2 receptor increase in PET study of schizophrenia. Arch Gen Psychiatry. 1987 Jul;44(7):671–672. doi: 10.1001/archpsyc.1987.01800190091013. [DOI] [PubMed] [Google Scholar]
- Kebabian J. W., Calne D. B. Multiple receptors for dopamine. Nature. 1979 Jan 11;277(5692):93–96. doi: 10.1038/277093a0. [DOI] [PubMed] [Google Scholar]
- Martres M. P., Bouthenet M. L., Sales N., Sokoloff P., Schwartz J. C. Widespread distribution of brain dopamine receptors evidenced with [125I]iodosulpride, a highly selective ligand. Science. 1985 May 10;228(4700):752–755. doi: 10.1126/science.3838821. [DOI] [PubMed] [Google Scholar]
- McMaster G. K., Carmichael G. G. Analysis of single- and double-stranded nucleic acids on polyacrylamide and agarose gels by using glyoxal and acridine orange. Proc Natl Acad Sci U S A. 1977 Nov;74(11):4835–4838. doi: 10.1073/pnas.74.11.4835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palacios J. M. Neurobiology. Dopamine receptor disputes. Nature. 1986 Sep 18;323(6085):205–205. doi: 10.1038/323205a0. [DOI] [PubMed] [Google Scholar]
- Pazos A., Stoeckel M. E., Hindelang C., Palacios J. M. Autoradiographic studies on dopamine D2 receptors in rat pituitary: influence of hormonal states. Neurosci Lett. 1985 Aug 16;59(1):1–7. doi: 10.1016/0304-3940(85)90206-x. [DOI] [PubMed] [Google Scholar]
- Savasta M., Ruberte E., Palacios J. M., Mengod G. The colocalization of cholecystokinin and tyrosine hydroxylase mRNAs in mesencephalic dopaminergic neurons in the rat brain examined by in situ hybridization. Neuroscience. 1989;29(2):363–369. doi: 10.1016/0306-4522(89)90063-8. [DOI] [PubMed] [Google Scholar]
- Schwarcz R., Creese I., Coyle J. T., Snyder S. H. Dopamine receptors localised on cerebral cortical afferents to rat corpus striatum. Nature. 1978 Feb 23;271(5647):766–768. doi: 10.1038/271766a0. [DOI] [PubMed] [Google Scholar]
- Trugman J. M., Geary W. A., 2nd, Wooten G. F. Localization of D-2 dopamine receptors to intrinsic striatal neurones by quantitative autoradiography. Nature. 1986 Sep 18;323(6085):267–269. doi: 10.1038/323267a0. [DOI] [PubMed] [Google Scholar]
- Wagner H. N., Jr, Burns H. D., Dannals R. F., Wong D. F., Langstrom B., Duelfer T., Frost J. J., Ravert H. T., Links J. M., Rosenbloom S. B. Imaging dopamine receptors in the human brain by positron tomography. Science. 1983 Sep 23;221(4617):1264–1266. doi: 10.1126/science.6604315. [DOI] [PubMed] [Google Scholar]
- Wong D. F., Wagner H. N., Jr, Tune L. E., Dannals R. F., Pearlson G. D., Links J. M., Tamminga C. A., Broussolle E. P., Ravert H. T., Wilson A. A. Positron emission tomography reveals elevated D2 dopamine receptors in drug-naive schizophrenics. Science. 1986 Dec 19;234(4783):1558–1563. doi: 10.1126/science.2878495. [DOI] [PubMed] [Google Scholar]