Abstract
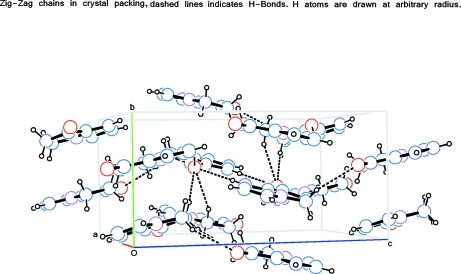
There are two nearly identical molecules in the asymmetric unit of the title compound, C7H7NO. The molecules are nearly planar (r.m.s. deviations of 0.025 and 0.017 Å) and oriented at a dihedral angle of 28.98 (3)°. The two molecules are linked by a C—H⋯O hydrogen bond. In the crystal, weak intermolecular C—H⋯O hydrogen bonds link the molecules into zigzag chains along the c axis.
Related literature
For general background to 2,3-dihydropyrrolizine derivatives and their biological activity, see: Skvortsov & Astakhova (1992 ▶). For the preparation, see: Braunholtz et al. (1962 ▶); Clemo & Ramage (1931 ▶). For natural sources, see: Meinwald & Meinwald (1965 ▶).
Experimental
Crystal data
C7H7NO
M r = 121.14
Monoclinic,
a = 11.301 (1) Å
b = 7.1730 (7) Å
c = 14.3760 (16) Å
β = 90.989 (5)°
V = 1165.2 (2) Å3
Z = 8
Mo Kα radiation
μ = 0.09 mm−1
T = 113 K
0.12 × 0.06 × 0.04 mm
Data collection
Rigaku Saturn724 CCD camera diffractometer
Absorption correction: multi-scan (CrystalClear-SM Expert; Rigaku, 2009 ▶) T min = 0.989, T max = 0.996
10183 measured reflections
2284 independent reflections
2003 reflections with I > 2σ(I)
R int = 0.051
Refinement
R[F 2 > 2σ(F 2)] = 0.057
wR(F 2) = 0.118
S = 1.16
2284 reflections
163 parameters
H-atom parameters constrained
Δρmax = 0.19 e Å−3
Δρmin = −0.30 e Å−3
Data collection: CrystalClear-SM Expert (Rigaku, 2009 ▶); cell refinement: CrystalClear-SM Expert; data reduction: CrystalClear-SM Expert; program(s) used to solve structure: SHELXS97 (Sheldrick, 2008 ▶); program(s) used to refine structure: SHELXL97 (Sheldrick, 2008 ▶); molecular graphics: ORTEP-3 (Farrugia, 1997 ▶); software used to prepare material for publication: publCIF (Westrip, 2010 ▶).
Supplementary Material
Crystal structure: contains datablocks I, global. DOI: 10.1107/S1600536810034902/bq2209sup1.cif
Structure factors: contains datablocks I. DOI: 10.1107/S1600536810034902/bq2209Isup2.hkl
Additional supplementary materials: crystallographic information; 3D view; checkCIF report
Table 1. Hydrogen-bond geometry (Å, °).
| D—H⋯A | D—H | H⋯A | D⋯A | D—H⋯A |
|---|---|---|---|---|
| C6—H6⋯O1i | 0.95 | 2.55 | 3.151 (2) | 121 |
| C7—H7⋯O2 | 0.95 | 2.55 | 3.250 (2) | 130 |
| C12—H12⋯O2ii | 0.95 | 2.51 | 3.435 (2) | 165 |
Symmetry codes: (i) 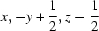 ; (ii)
; (ii) 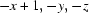 .
.
Acknowledgments
YA is grateful to the Pakistan Council of Scientific & Industrial Research, Ministry of Science & Technology, Government of Pakistan, for financial support. PY is grateful to Tianjin University of Science & Technology for research funding (research grant No. 2009 0431).
supplementary crystallographic information
Comment
Derivatives of 2,3-dihydropyrrolizine became known through studies of their synthesis (Clemo et al., 1931) and isolation from natural source (Meinwald et al., 1965). Synthetic dihydropyrrolizines that are of interest as pharmaceuticals have been reported. The most important of these, Ketorolac, is a non steroid analgesic. Depending on their structure, derivatives of 2,3-dihydropyrrolizine have shown merit as analgesics, anti-inflammatory agents, myorelaxants, inhibitors of thrombocyte aggregation, fibrinolytics, temperature-lowering substances and drugs for the treatment of glaucoma and conjunctivitis (Skvortsov et al., 1992).
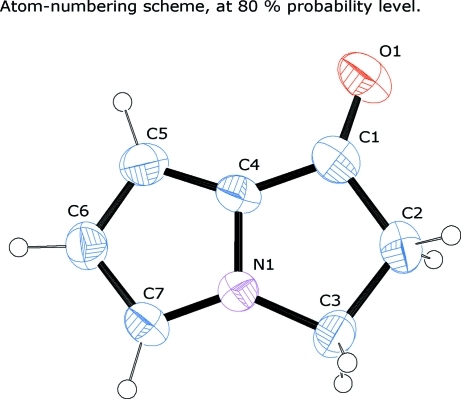
The ORTEP (Farrugia, 1997) drawing of the molecule is shown in Fig. 1. The sums of the three angles at N1 and C4 are 359.93 and 359.96 respectively, indicating that two rings are almost planer with an r.m.s. deviation of 0.05 Å. Molecules are held together in crystal packing by weak C—H···O hydrogen bonds (Table 1), in the form of zigzag infinite one dimensional polymeric chains (Fig. 2.).
Experimental
The preparation of title compound was carried out as described in the procedure reported in literature (Braunholtz et al., 1962). Purified by Flash Column Chromatography, Petroleum Ether:Ethyl Acetate = 3:1.
Refinement
All H atoms were placed in geometrically idealized positions and constrained to ride on their parent atoms with C—H = 0.95 and 0.99Å for aromatic and methylene respectively. Uiso(H) values were taken to be equal to 1.2 Ueq(C) for all hydrogen atoms.
Figures
Fig. 1.
Displacement ellipsoid plot (80% probability level) showing atom numbering scheme.
Fig. 2.
The packing showing the zigzg chains. Dashed lines indicate hydrogen bonds
Crystal data
| C7H7NO | F(000) = 512 |
| Mr = 121.14 | Dx = 1.381 Mg m−3 |
| Monoclinic, P21/c | Melting point: 327(1) K |
| Hall symbol: -P 2ybc | Mo Kα radiation, λ = 0.71075 Å |
| a = 11.301 (1) Å | Cell parameters from 3403 reflections |
| b = 7.1730 (7) Å | θ = 1.8–28.1° |
| c = 14.3760 (16) Å | µ = 0.09 mm−1 |
| β = 90.989 (5)° | T = 113 K |
| V = 1165.2 (2) Å3 | Prism, colorless |
| Z = 8 | 0.12 × 0.06 × 0.04 mm |
Data collection
| Rigaku Saturn724 CCD camera diffractometer | 2284 independent reflections |
| Radiation source: rotating anode | 2003 reflections with I > 2σ(I) |
| multilayer | Rint = 0.051 |
| ω scans | θmax = 26.0°, θmin = 1.8° |
| Absorption correction: multi-scan (CrystalClear-SM Expert; Rigaku, 2009) | h = −13→13 |
| Tmin = 0.989, Tmax = 0.996 | k = −8→8 |
| 10183 measured reflections | l = −17→15 |
Refinement
| Refinement on F2 | Primary atom site location: structure-invariant direct methods |
| Least-squares matrix: full | Secondary atom site location: difference Fourier map |
| R[F2 > 2σ(F2)] = 0.057 | Hydrogen site location: inferred from neighbouring sites |
| wR(F2) = 0.118 | H-atom parameters constrained |
| S = 1.16 | w = 1/[σ2(Fo2) + (0.043P)2 + 0.3505P] where P = (Fo2 + 2Fc2)/3 |
| 2284 reflections | (Δ/σ)max < 0.001 |
| 163 parameters | Δρmax = 0.19 e Å−3 |
| 0 restraints | Δρmin = −0.30 e Å−3 |
Special details
| Experimental. Single crystals suitable for X-ray crystallography were grown by slow cooling of a hot saturated solution of Petroleum Ether. |
| Geometry. All e.s.d.'s (except the e.s.d. in the dihedral angle between two l.s. planes) are estimated using the full covariance matrix. The cell e.s.d.'s are taken into account individually in the estimation of e.s.d.'s in distances, angles and torsion angles; correlations between e.s.d.'s in cell parameters are only used when they are defined by crystal symmetry. An approximate (isotropic) treatment of cell e.s.d.'s is used for estimating e.s.d.'s involving l.s. planes. |
| Refinement. Refinement of F2 against ALL reflections. The weighted RSHELXS-97 -factor wR and goodness of fit S are based on F2, conventional R-factors R are based on F, with F set to zero for negative F2. The threshold expression of F2 > σ(F2) is used only for calculating R-factors(gt) etc. and is not relevant to the choice of reflections for refinement. R-factors based on F2 are statistically about twice as large as those based on F, and R- factors based on ALL data will be even larger. |
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2)
| x | y | z | Uiso*/Ueq | ||
| O1 | 0.84120 (12) | 0.0832 (2) | 0.57308 (9) | 0.0293 (4) | |
| O2 | 0.49964 (11) | 0.0828 (2) | 0.17318 (10) | 0.0329 (4) | |
| N1 | 0.72173 (13) | 0.1521 (2) | 0.35032 (11) | 0.0181 (4) | |
| N2 | 0.20229 (13) | 0.1557 (2) | 0.11650 (11) | 0.0190 (4) | |
| C1 | 0.78107 (17) | 0.0987 (3) | 0.50200 (13) | 0.0214 (4) | |
| C2 | 0.64640 (16) | 0.0776 (3) | 0.49734 (13) | 0.0229 (5) | |
| H2A | 0.6086 | 0.1646 | 0.5413 | 0.027* | |
| H2B | 0.6233 | −0.0513 | 0.5136 | 0.027* | |
| C3 | 0.60795 (16) | 0.1231 (3) | 0.39650 (13) | 0.0222 (4) | |
| H3A | 0.5634 | 0.0183 | 0.3680 | 0.027* | |
| H3B | 0.5586 | 0.2371 | 0.3938 | 0.027* | |
| C4 | 0.81910 (16) | 0.1386 (3) | 0.40853 (13) | 0.0186 (4) | |
| C5 | 0.91883 (17) | 0.1737 (3) | 0.35603 (13) | 0.0226 (4) | |
| H5 | 0.9988 | 0.1728 | 0.3775 | 0.027* | |
| C6 | 0.87834 (16) | 0.2109 (3) | 0.26502 (13) | 0.0218 (4) | |
| H6 | 0.9265 | 0.2405 | 0.2136 | 0.026* | |
| C7 | 0.75535 (16) | 0.1968 (3) | 0.26317 (13) | 0.0209 (4) | |
| H7 | 0.7046 | 0.2152 | 0.2106 | 0.025* | |
| C8 | 0.39292 (16) | 0.1181 (3) | 0.16955 (14) | 0.0219 (4) | |
| C9 | 0.32016 (16) | 0.1840 (3) | 0.25181 (13) | 0.0223 (4) | |
| H9A | 0.3490 | 0.3065 | 0.2743 | 0.027* | |
| H9B | 0.3260 | 0.0934 | 0.3036 | 0.027* | |
| C10 | 0.19132 (16) | 0.1993 (3) | 0.21584 (13) | 0.0208 (4) | |
| H10A | 0.1395 | 0.1084 | 0.2472 | 0.025* | |
| H10B | 0.1596 | 0.3265 | 0.2250 | 0.025* | |
| C11 | 0.31432 (15) | 0.1065 (3) | 0.09001 (13) | 0.0186 (4) | |
| C12 | 0.30999 (17) | 0.0643 (3) | −0.00456 (14) | 0.0223 (4) | |
| H12 | 0.3741 | 0.0258 | −0.0419 | 0.027* | |
| C13 | 0.19207 (17) | 0.0901 (3) | −0.03344 (14) | 0.0239 (5) | |
| H13 | 0.1615 | 0.0721 | −0.0948 | 0.029* | |
| C14 | 0.12721 (17) | 0.1468 (3) | 0.04322 (14) | 0.0238 (5) | |
| H14 | 0.0450 | 0.1741 | 0.0435 | 0.029* |
Atomic displacement parameters (Å2)
| U11 | U22 | U33 | U12 | U13 | U23 | |
| O1 | 0.0349 (8) | 0.0343 (9) | 0.0185 (8) | 0.0044 (6) | −0.0039 (6) | −0.0005 (6) |
| O2 | 0.0195 (7) | 0.0467 (10) | 0.0324 (9) | 0.0055 (6) | −0.0011 (6) | −0.0079 (7) |
| N1 | 0.0169 (7) | 0.0209 (8) | 0.0165 (8) | 0.0002 (6) | 0.0003 (6) | −0.0004 (7) |
| N2 | 0.0187 (8) | 0.0195 (9) | 0.0188 (9) | 0.0013 (6) | 0.0020 (7) | −0.0012 (7) |
| C1 | 0.0263 (10) | 0.0175 (10) | 0.0203 (11) | 0.0021 (8) | −0.0006 (8) | −0.0020 (8) |
| C2 | 0.0280 (10) | 0.0213 (10) | 0.0196 (10) | −0.0017 (8) | 0.0044 (8) | 0.0000 (8) |
| C3 | 0.0179 (9) | 0.0247 (10) | 0.0241 (11) | −0.0019 (8) | 0.0032 (8) | 0.0006 (8) |
| C4 | 0.0198 (9) | 0.0198 (10) | 0.0160 (10) | 0.0012 (7) | −0.0031 (8) | −0.0022 (8) |
| C5 | 0.0189 (9) | 0.0254 (10) | 0.0233 (11) | −0.0002 (8) | −0.0016 (8) | −0.0033 (9) |
| C6 | 0.0224 (10) | 0.0237 (10) | 0.0193 (10) | −0.0013 (8) | 0.0032 (8) | −0.0003 (8) |
| C7 | 0.0238 (10) | 0.0227 (10) | 0.0161 (10) | 0.0015 (8) | −0.0015 (8) | 0.0016 (8) |
| C8 | 0.0207 (9) | 0.0200 (10) | 0.0249 (11) | −0.0004 (8) | 0.0007 (8) | 0.0004 (8) |
| C9 | 0.0230 (10) | 0.0251 (10) | 0.0187 (10) | −0.0004 (8) | 0.0007 (8) | −0.0008 (8) |
| C10 | 0.0222 (10) | 0.0228 (10) | 0.0177 (10) | 0.0015 (8) | 0.0043 (8) | −0.0020 (8) |
| C11 | 0.0190 (9) | 0.0183 (10) | 0.0184 (10) | 0.0016 (7) | 0.0030 (8) | 0.0000 (8) |
| C12 | 0.0267 (10) | 0.0199 (10) | 0.0204 (10) | 0.0004 (8) | 0.0039 (8) | −0.0003 (8) |
| C13 | 0.0307 (11) | 0.0233 (11) | 0.0176 (10) | 0.0011 (8) | −0.0024 (8) | 0.0005 (8) |
| C14 | 0.0222 (10) | 0.0244 (11) | 0.0246 (11) | 0.0008 (8) | −0.0044 (8) | 0.0001 (9) |
Geometric parameters (Å, °)
| O1—C1 | 1.222 (2) | C5—H5 | 0.9500 |
| O2—C8 | 1.232 (2) | C6—C7 | 1.393 (2) |
| N1—C7 | 1.354 (2) | C6—H6 | 0.9500 |
| N1—C4 | 1.374 (2) | C7—H7 | 0.9500 |
| N1—C3 | 1.472 (2) | C8—C11 | 1.438 (3) |
| N2—C14 | 1.343 (2) | C8—C9 | 1.527 (3) |
| N2—C11 | 1.374 (2) | C9—C10 | 1.541 (3) |
| N2—C10 | 1.469 (2) | C9—H9A | 0.9900 |
| C1—C4 | 1.446 (3) | C9—H9B | 0.9900 |
| C1—C2 | 1.530 (3) | C10—H10A | 0.9900 |
| C2—C3 | 1.541 (3) | C10—H10B | 0.9900 |
| C2—H2A | 0.9900 | C11—C12 | 1.393 (3) |
| C2—H2B | 0.9900 | C12—C13 | 1.401 (3) |
| C3—H3A | 0.9900 | C12—H12 | 0.9500 |
| C3—H3B | 0.9900 | C13—C14 | 1.395 (3) |
| C4—C5 | 1.390 (3) | C13—H13 | 0.9500 |
| C5—C6 | 1.404 (3) | C14—H14 | 0.9500 |
| C7—N1—C4 | 110.21 (16) | N1—C7—C6 | 107.17 (17) |
| C7—N1—C3 | 135.39 (16) | N1—C7—H7 | 126.4 |
| C4—N1—C3 | 114.33 (15) | C6—C7—H7 | 126.4 |
| C14—N2—C11 | 110.07 (16) | O2—C8—C11 | 127.76 (18) |
| C14—N2—C10 | 135.23 (16) | O2—C8—C9 | 124.78 (18) |
| C11—N2—C10 | 114.68 (15) | C11—C8—C9 | 107.47 (15) |
| O1—C1—C4 | 128.66 (18) | C8—C9—C10 | 106.29 (15) |
| O1—C1—C2 | 124.46 (18) | C8—C9—H9A | 110.5 |
| C4—C1—C2 | 106.88 (16) | C10—C9—H9A | 110.5 |
| C1—C2—C3 | 106.52 (15) | C8—C9—H9B | 110.5 |
| C1—C2—H2A | 110.4 | C10—C9—H9B | 110.5 |
| C3—C2—H2A | 110.4 | H9A—C9—H9B | 108.7 |
| C1—C2—H2B | 110.4 | N2—C10—C9 | 102.47 (14) |
| C3—C2—H2B | 110.4 | N2—C10—H10A | 111.3 |
| H2A—C2—H2B | 108.6 | C9—C10—H10A | 111.3 |
| N1—C3—C2 | 102.70 (14) | N2—C10—H10B | 111.3 |
| N1—C3—H3A | 111.2 | C9—C10—H10B | 111.3 |
| C2—C3—H3A | 111.2 | H10A—C10—H10B | 109.2 |
| N1—C3—H3B | 111.2 | N2—C11—C12 | 108.01 (16) |
| C2—C3—H3B | 111.2 | N2—C11—C8 | 108.92 (16) |
| H3A—C3—H3B | 109.1 | C12—C11—C8 | 143.08 (18) |
| N1—C4—C5 | 107.75 (16) | C11—C12—C13 | 106.13 (17) |
| N1—C4—C1 | 109.38 (16) | C11—C12—H12 | 126.9 |
| C5—C4—C1 | 142.83 (17) | C13—C12—H12 | 126.9 |
| C4—C5—C6 | 106.62 (16) | C14—C13—C12 | 108.33 (17) |
| C4—C5—H5 | 126.7 | C14—C13—H13 | 125.8 |
| C6—C5—H5 | 126.7 | C12—C13—H13 | 125.8 |
| C7—C6—C5 | 108.24 (17) | N2—C14—C13 | 107.47 (17) |
| C7—C6—H6 | 125.9 | N2—C14—H14 | 126.3 |
| C5—C6—H6 | 125.9 | C13—C14—H14 | 126.3 |
| O1—C1—C2—C3 | 175.74 (18) | O2—C8—C9—C10 | −176.94 (19) |
| C4—C1—C2—C3 | −4.4 (2) | C11—C8—C9—C10 | 3.2 (2) |
| C7—N1—C3—C2 | −179.46 (19) | C14—N2—C10—C9 | −178.40 (19) |
| C4—N1—C3—C2 | −2.6 (2) | C11—N2—C10—C9 | 3.8 (2) |
| C1—C2—C3—N1 | 4.14 (19) | C8—C9—C10—N2 | −4.05 (19) |
| C7—N1—C4—C5 | −0.8 (2) | C14—N2—C11—C12 | 0.0 (2) |
| C3—N1—C4—C5 | −178.45 (15) | C10—N2—C11—C12 | 178.29 (15) |
| C7—N1—C4—C1 | 177.53 (15) | C14—N2—C11—C8 | 179.72 (15) |
| C3—N1—C4—C1 | −0.1 (2) | C10—N2—C11—C8 | −2.0 (2) |
| O1—C1—C4—N1 | −177.28 (18) | O2—C8—C11—N2 | 179.24 (19) |
| C2—C1—C4—N1 | 2.8 (2) | C9—C8—C11—N2 | −0.9 (2) |
| O1—C1—C4—C5 | 0.1 (4) | O2—C8—C11—C12 | −1.2 (4) |
| C2—C1—C4—C5 | −179.7 (2) | C9—C8—C11—C12 | 178.7 (2) |
| N1—C4—C5—C6 | 0.7 (2) | N2—C11—C12—C13 | 0.1 (2) |
| C1—C4—C5—C6 | −176.7 (2) | C8—C11—C12—C13 | −179.6 (2) |
| C4—C5—C6—C7 | −0.4 (2) | C11—C12—C13—C14 | −0.1 (2) |
| C4—N1—C7—C6 | 0.6 (2) | C11—N2—C14—C13 | 0.0 (2) |
| C3—N1—C7—C6 | 177.51 (19) | C10—N2—C14—C13 | −177.84 (19) |
| C5—C6—C7—N1 | −0.1 (2) | C12—C13—C14—N2 | 0.0 (2) |
Hydrogen-bond geometry (Å, °)
| D—H···A | D—H | H···A | D···A | D—H···A |
| C6—H6···O1i | 0.95 | 2.55 | 3.151 (2) | 121 |
| C7—H7···O2 | 0.95 | 2.55 | 3.250 (2) | 130 |
| C12—H12···O2ii | 0.95 | 2.51 | 3.435 (2) | 165 |
Symmetry codes: (i) x, −y+1/2, z−1/2; (ii) −x+1, −y, −z.
Footnotes
Supplementary data and figures for this paper are available from the IUCr electronic archives (Reference: BQ2209).
References
- Braunholtz, J. T., Mallion, K. B. & Frederick, G. M. (1962). J. Chem. Soc. pp. 4346–4353.
- Clemo, G. R. & Ramage, G. R. (1931). J. Chem. Soc.7, 49–55.
- Farrugia, L. J. (1997). J. Appl. Cryst.30, 565.
- Meinwald, J. & Meinwald, Y. C. (1965). J. Am. Chem. Soc.88, 1305–1310.
- Rigaku (2009). CrystalClear-SM Expert Rigaku Corporation, Tokyo, Japan.
- Sheldrick, G. M. (2008). Acta Cryst. A64, 112–122. [DOI] [PubMed]
- Skvortsov, I. M. & Astakhova, L. N. (1992). Chem. Heterocycl. Compd, 28, 117–134.
- Westrip, S. P. (2010). J. Appl. Cryst.43, 920–925.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Crystal structure: contains datablocks I, global. DOI: 10.1107/S1600536810034902/bq2209sup1.cif
Structure factors: contains datablocks I. DOI: 10.1107/S1600536810034902/bq2209Isup2.hkl
Additional supplementary materials: crystallographic information; 3D view; checkCIF report