Abstract
The centrosymmetric title cluster, hexaaquadi-μ3-carbonato-hexacyclamhexa-μ2-hydroxido-dodeca-μ2-oxido-hexamanganese(IV)hexamanganese(III) octachloride tetracosahydrate, [Mn12(CO3)2O12(OH)6(C10H24N4)6(H2O)6]Cl8·24H2O, has two μ3-CO3 groups that not only bridge octahedrally coordinated MnIII ions but also act as acceptors to two different kinds of hydrogen bonds. The carbonate anion is planar within experimental error and has an average C—O distance of 1.294 (4) Å. The crystal packing is stabilized by O—H⋯Cl, O—H⋯O, N—H⋯Cl and N—H⋯O hydrogen bonds. Two of the four independent chloride ions are disordered over five positions, and eight of the 12 independent water molecules are disordered over 21 positions.
Related literature
For the structure of an Mn9 cluster containing (μ3-CO3), see: Chakov et al. (2005 ▶). For some structures of Mn12 clusters containing MnIII/MnIV, see: Lis (1980 ▶); Aubin et al. (1996 ▶); Sun et al. (1998 ▶); Kuroda-Sowa et al. (2001 ▶); Bian et al. (2004 ▶). For a recent structure of an Ag17 cluster that has incorporated atmospheric CO2 to encapsulate a carbonate, see: Bian et al. (2009 ▶). For bond-valence sum analysis for Mn—O, see: Palenik (1997 ▶).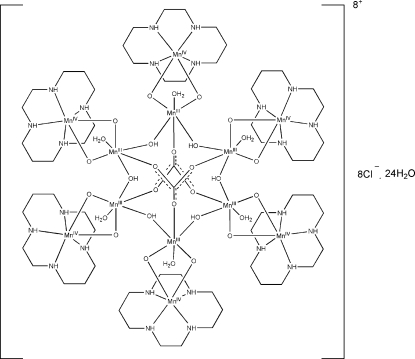
Experimental
Crystal data
[Mn12(CO3)2O12(OH)6(C10H24N4)6(H2O)6]Cl8·24H2O
M r = 3099.42
Triclinic,
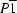
a = 15.2421 (15) Å
b = 15.5037 (15) Å
c = 17.1306 (17) Å
α = 90.707 (6)°
β = 114.523 (7)°
γ = 115.128 (7)°
V = 3245.8 (6) Å3
Z = 1
Mo Kα radiation
μ = 1.38 mm−1
T = 90 K
0.43 × 0.18 × 0.14 mm
Data collection
Bruker SMART APEXII diffractometer
Absorption correction: multi-scan (SADABS; Sheldrick, 1996 ▶) T min = 0.589, T max = 0.831
40135 measured reflections
14856 independent reflections
10648 reflections with I > 2σ(I)
R int = 0.042
Refinement
R[F 2 > 2σ(F 2)] = 0.051
wR(F 2) = 0.167
S = 1.14
14856 reflections
787 parameters
12 restraints
H atoms treated by a mixture of independent and constrained refinement
Δρmax = 1.40 e Å−3
Δρmin = −1.14 e Å−3
Data collection: APEX2 (Bruker, 2009 ▶); cell refinement: SAINT (Bruker, 2009 ▶); data reduction: SAINT; program(s) used to solve structure: SHELXS97 (Sheldrick, 2008 ▶); program(s) used to refine structure: SHELXL97 (Sheldrick, 2008 ▶); molecular graphics: SHELXTL (Sheldrick, 2008 ▶); software used to prepare material for publication: SHELXL97.
Supplementary Material
Crystal structure: contains datablocks I, global. DOI: 10.1107/S1600536810034999/ci5168sup1.cif
Structure factors: contains datablocks I. DOI: 10.1107/S1600536810034999/ci5168Isup2.hkl
Additional supplementary materials: crystallographic information; 3D view; checkCIF report
Table 1. Selected geometric parameters (Å, °).
| Mn1—O2 | 1.779 (3) |
| Mn1—O1 | 1.792 (2) |
| Mn1—N3 | 2.037 (4) |
| Mn1—N1 | 2.041 (4) |
| Mn1—N2 | 2.100 (3) |
| Mn1—N4 | 2.100 (3) |
| Mn2—O1 | 1.867 (3) |
| Mn2—O2 | 1.892 (3) |
| Mn2—O5 | 1.937 (2) |
| Mn2—O6 | 1.943 (2) |
| Mn2—O3 | 2.327 (3) |
| Mn2—O4 | 2.339 (3) |
| Mn3—O8 | 1.783 (2) |
| Mn3—O7 | 1.797 (2) |
| Mn3—N5 | 2.043 (3) |
| Mn3—N7 | 2.047 (3) |
| Mn3—N8 | 2.098 (3) |
| Mn3—N6 | 2.105 (3) |
| Mn4—O8 | 1.879 (2) |
| Mn4—O7 | 1.883 (2) |
| Mn4—O11 | 1.935 (2) |
| Mn4—O5i | 1.943 (3) |
| Mn4—O9 | 2.311 (3) |
| Mn4—O10 | 2.315 (2) |
| Mn5—O15 | 1.783 (3) |
| Mn5—O14 | 1.788 (3) |
| Mn5—N11 | 2.034 (4) |
| Mn5—N9 | 2.038 (3) |
| Mn5—N10 | 2.093 (3) |
| Mn5—N12 | 2.095 (4) |
| Mn6—O14 | 1.868 (3) |
| Mn6—O15 | 1.883 (2) |
| Mn6—O11 | 1.937 (3) |
| Mn6—O6 | 1.949 (2) |
| Mn6—O13 | 2.302 (2) |
| Mn6—O12 | 2.335 (3) |
| O3—C31 | 1.293 (4) |
| C31—O13i | 1.289 (4) |
| C31—O10 | 1.301 (4) |
| O13i—C31—O3 | 120.8 (3) |
| O13i—C31—O10 | 120.0 (3) |
| O3—C31—O10 | 119.2 (3) |
Symmetry code: (i) 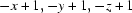 .
.
Table 2. Hydrogen-bond geometry (Å, °).
| D—H⋯A | D—H | H⋯A | D⋯A | D—H⋯A |
|---|---|---|---|---|
| N6—H6⋯Cl1 | 0.93 | 2.51 | 3.413 (3) | 163 |
| N8—H8⋯Cl1 | 0.93 | 2.27 | 3.198 (3) | 174 |
| O4—H4E⋯Cl2 | 0.82 (4) | 2.33 (3) | 3.106 (3) | 158 (4) |
| N1—H1⋯Cl2 | 0.93 | 2.49 | 3.285 (4) | 143 |
| N2—H2⋯Cl3 | 0.93 | 2.46 | 3.338 (4) | 157 |
| N4—H4⋯Cl3 | 0.93 | 2.46 | 3.384 (4) | 174 |
| N11—H11⋯Cl4 | 0.93 | 2.37 | 3.148 (5) | 141 |
| N10—H10⋯Cl5A | 0.93 | 2.68 | 3.523 (6) | 152 |
| N10—H10⋯Cl5B | 0.93 | 2.49 | 3.185 (12) | 132 |
| N5—H5⋯Cl6 | 0.93 | 2.32 | 3.138 (4) | 147 |
| O4—H4D⋯O14 | 0.82 (4) | 1.90 (2) | 2.701 (4) | 166 (4) |
| O5—H5D⋯O13 | 0.84 (4) | 1.86 (2) | 2.674 (3) | 164 (4) |
| O6—H6D⋯O10 | 0.82 (4) | 1.88 (2) | 2.681 (4) | 166 (4) |
| O9—H9E⋯O1i | 0.87 (3) | 1.83 (2) | 2.665 (4) | 161 (4) |
| O11—H11D⋯O3i | 0.83 (2) | 1.85 (2) | 2.675 (3) | 172 (4) |
| O12—H12C⋯O7 | 0.83 (5) | 1.88 (5) | 2.704 (4) | 171 (4) |
| O12—H12D⋯Cl4 | 0.82 (2) | 2.26 (2) | 3.065 (4) | 165 (4) |
| N2—H2⋯O28 | 0.93 | 2.08 | 2.952 (9) | 156 |
| N3—H3⋯O3 | 0.93 | 1.99 | 2.798 (4) | 144 |
| N7—H7⋯O10 | 0.93 | 1.99 | 2.790 (4) | 143 |
| N9—H9⋯O13 | 0.93 | 2.14 | 2.896 (4) | 138 |
| N10—H10⋯O20 | 0.93 | 2.02 | 2.912 (10) | 160 |
Symmetry code: (i) 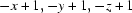 .
.
Acknowledgments
The authors thank the University of California, Davis, for the purchase of the X-ray diffractometer.
supplementary crystallographic information
Comment
Reactions of carbon dioxide with metals and metal clusters have recently attracted a great deal of interest (Bian et al., 2009). Complexes of manganese rarely exhibit such reactivity. We wish to report the fortuitous fixation of carbon dioxide as carbonate ion in a mixed valent MnIII/MnIV cluster of 12 metal ions. An interesting structural feature consists of two triply bridged carbonate anions on opposite faces of the cluster. Previously, a MnIII cluster containing carbonate was reported (Chakov et al., 2005), but it has little in common with the title compound. Many Mn12 clusters are known, some of which have mixed valent MnIII/MnIV ions (Sun et al., 1998; Kuroda-Sowa et al., 2001; Bian et al., 2004). The most famous is Mn12O12(MeCO2)16(H2O)4 (Lis, 1980; Aubin, et al., 1996), which opened up the field of single molecule magnets. These structures are unlike that of the title compound. They contain an internal cubane of four MnIV ions with µ3-O bridges and eight outer MnIII ions. The production of the title compound was unexpected. We presume that prolonged stirring of a manganese(II) chloride solution in an open vessel in the presence of base (cyclam, (1,4,8,11-tetraazacyclotetradecane)) yielded a basic MnIII/MnIV oxide which took up CO2 from the air and formed the triply bridged carbonate species of the title compound.
The cluster of the title compound has a center of symmetry. It has the overall formula {[(cyclam)MnIV(µ-O)2MnIII(H2O)(µ-OH)]6(µ3-CO3)}2}8+ with charge balanced by chloride ions. There are 24 molecules of non-coordinated water in the model, many of which are disordered. The Mn12 cluster is shown in the Scheme.
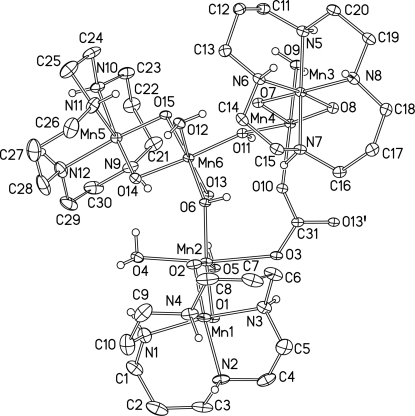
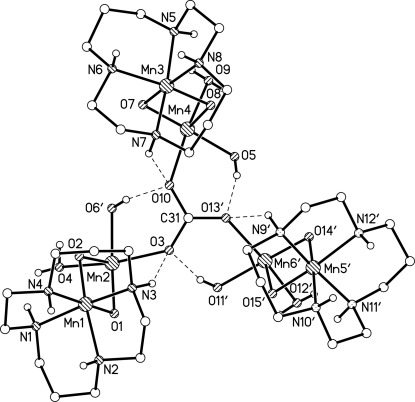
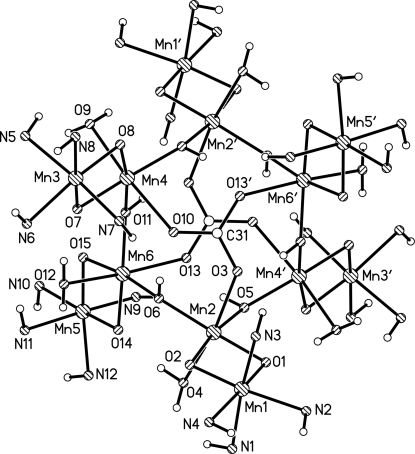
For simplicity, only one half of the cluster is depicted in Fig. 1. The atoms labeled Mn1, Mn3, and Mn5 are MnIV and those labeled Mn2, Mn4, Mn6 are MnIII. The oxidation states for the Mn ions were verified by Bond Valence Sum analysis (Palenik, 1997). Details of the bond distances and angles are given in Table 1. The asymmetric unit of the title compound contains a carbonate anion coordinated to three MnIII ions through each of its three O atoms. Within experimental error, the µ3-CO3 group is planar. The average C—O distance is 1.294 (4) Å. Each carbonate O atom accepts an intramolecular hydrogen bond from a µ-(OH) donor group that is ligated to two MnIII's. A second hydrogen bond to each carbonate oxygen is formed by donation from a N—H group of cyclam, as shown in Figure 2. A single water molecule is coordinated to each MnIII in a position trans- to the carbonate oxygen. The Mn—O(carbonate) distances, average 2.315 (8) Å, and Mn—O(H2O) distances, average 2.328 (11) Å, are long, indicating a Jahn-Teller effect of the d4 ion and strong trans-effect of carbonate. The remainder of the coordination sphere of the MnIII ion is made up of two oxo bridges, average distance 1.879 (7) Å. These bridges link the MnIII ions to MnIV ions. The average MnIV—O distance is 1.787 (6) Å. The coordination sphere of the MnIV ions is completed by four amino N atoms of the cyclam ligand. The Mn—N bond distances reflect the trans-effect of the µ-oxo bridges; the average MnIV—N(trans) distance is 2.097 (5)Å as compared to 2.040 (4)Å for MnIV—N(cis). Fig. 3 depicts the entire Mn12 cluster with cyclam CH2 groups omitted for clarity. In sum, both MnIII and MnIV have coordination number six and a pseudo- octahedral geometry. The inversion-related halves of the cluster are connected via the µ-(OH) groups. A diverse set of Mn—O bonds is exhibited in the structure, involving oxo, hydroxo, and aqua ligation to MnIII and MnIV ions as well as intramolecular hydrogen bonding. The chloride counterions are primarily nestled in cyclam cavities, hydrogen bonded to N—H donor groups of the cylam ligands as well as to non-coordinated water molecules.
Experimental
To a mixture of MnCl2.4H2O (136 mg, 687 mmol), cyclam (1,4,8,11-tetraazacyclotetradecane) (144 mg, 722 mmol), and sodium tetraphenylborate (289 mg, 844 mmol) in a 200 ml round bottom flask was added 150 ml of acetonitrile. The reaction was continuously stirred for 5 days over which time it turned from pale yellow to dark brown to dark olive green and a solid material was formed. The solid was filtered and redissolved in a 1:2 mixture of H2O:acetonitrile and placed in upcapped 5 mm diameter tubes in the refrigerator. After 2 weeks, black plates formed. The crystal selected for data collection was cut from a large plate.
Refinement
Hydrogen atoms on water O and aza-N atoms were located in a difference map and subsequently refined with Uiso = 1.2Ueq(N or O) and distance restraints of 0.84 (1) Å for O—H, 0.93 Å for N—H and H···H of 1.32 (3) Å for water. The C—H geometry was determined by idealized geometry and a C—H distance of 0.99 Å. The C—H and N—H H atoms were refined as riding on the parent atoms. There are seven different positions for the four chloride ions in the asymmetric unit. Of these, Cl1 and Cl2 are included at full occupancy while Cl3, Cl4, and Cl6 are at half occupancy and Cl5A/Cl5B respresent a split position of occupancy 0.40/0.10 occupancy. These disordered chlorides were selected based on longer hydrogen bonding distances and reasonable distribution within the structure. Four hydrate water O atoms, O16, O17, O18, and O19, were in sites of full occupancy and were refined with anisotropic thermal parameters. The remainder were refined with isotropic thermal parameters and fixed occupancies that were determined by an ad hoc method. Most of the hydrogen atoms were not reliably located for the hydrate molecules and none were included in the structure factor calculation. The final difference map contains a number of peaks in the region of the chloride ions and solvate water molecules that are possibly additional minor water sites or part of disordered chloride sites.
Figures
Fig. 1.
A drawing of the asymmetric unit of the title compound. Thermal ellipsoids are drawn at the 30% probability level. Hydrogen atoms bonded to carbon, chloride counterions, and hydrate molecules have been omitted for clarity. One of the oxygen atoms (O13') is shown at its symmetry position, ' = 1 - x, 1 - y, 1 - z, in order to show the complete carbonate anion.
Fig. 2.
A view of one-half of the cluster normal to the triply bridging carbonate group. A portion of the hydrogen bonding is also depicted. Symmetry code: ' = 1 - x, 1 - y, 1 - z.
Fig. 3.
A view of the Mn12 cluster; CH2 groups have been omitted for clarity. Symmetry code: ' = 1 - x, 1 - y, 1 - z.
Crystal data
| [Mn12(CO3)2O12(OH)6(C10H24N4)6(H2O)6]Cl8·24H2O | Z = 1 |
| Mr = 3099.42 | F(000) = 1618 |
| Triclinic, P1 | Dx = 1.586 Mg m−3 |
| Hall symbol: -P 1 | Mo Kα radiation, λ = 0.71073 Å |
| a = 15.2421 (15) Å | Cell parameters from 9868 reflections |
| b = 15.5037 (15) Å | θ = 2.6–29.4° |
| c = 17.1306 (17) Å | µ = 1.38 mm−1 |
| α = 90.707 (6)° | T = 90 K |
| β = 114.523 (7)° | Parallelepiped, black |
| γ = 115.128 (7)° | 0.43 × 0.18 × 0.14 mm |
| V = 3245.8 (6) Å3 |
Data collection
| Bruker SMART APEXII diffractometer | 14856 independent reflections |
| Radiation source: fine-focus sealed tube | 10648 reflections with I > 2σ(I) |
| graphite | Rint = 0.042 |
| Detector resolution: 8.3 pixels mm-1 | θmax = 27.5°, θmin = 2.7° |
| ω scans | h = −19→19 |
| Absorption correction: multi-scan (SADABS; Sheldrick, 1996) | k = −20→20 |
| Tmin = 0.589, Tmax = 0.831 | l = −22→22 |
| 40135 measured reflections |
Refinement
| Refinement on F2 | Primary atom site location: structure-invariant direct methods |
| Least-squares matrix: full | Secondary atom site location: difference Fourier map |
| R[F2 > 2σ(F2)] = 0.051 | Hydrogen site location: difference Fourier map |
| wR(F2) = 0.167 | H atoms treated by a mixture of independent and constrained refinement |
| S = 1.14 | w = 1/[σ2(Fo2) + (0.0959P)2] where P = (Fo2 + 2Fc2)/3 |
| 14856 reflections | (Δ/σ)max = 0.006 |
| 787 parameters | Δρmax = 1.40 e Å−3 |
| 12 restraints | Δρmin = −1.14 e Å−3 |
Special details
| Geometry. All e.s.d.'s (except the e.s.d. in the dihedral angle between two l.s. planes) are estimated using the full covariance matrix. The cell e.s.d.'s are taken into account individually in the estimation of e.s.d.'s in distances, angles and torsion angles; correlations between e.s.d.'s in cell parameters are only used when they are defined by crystal symmetry. An approximate (isotropic) treatment of cell e.s.d.'s is used for estimating e.s.d.'s involving l.s. planes. |
| Refinement. Refinement of F2 against ALL reflections. The weighted R-factor wR and goodness of fit S are based on F2, conventional R-factors R are based on F, with F set to zero for negative F2. The threshold expression of F2 > σ(F2) is used only for calculating R-factors(gt) etc. and is not relevant to the choice of reflections for refinement. R-factors based on F2 are statistically about twice as large as those based on F, and R- factors based on ALL data will be even larger. |
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2)
| x | y | z | Uiso*/Ueq | Occ. (<1) | |
| Mn1 | 0.61048 (5) | 0.82243 (4) | 0.74029 (4) | 0.02005 (14) | |
| Mn2 | 0.48190 (5) | 0.66143 (4) | 0.60353 (4) | 0.01677 (13) | |
| Mn3 | 0.68442 (4) | 0.74479 (4) | 0.31273 (4) | 0.01583 (13) | |
| Mn4 | 0.56057 (4) | 0.57784 (4) | 0.34706 (3) | 0.01455 (13) | |
| Mn5 | 0.10236 (4) | 0.49328 (4) | 0.28193 (4) | 0.01948 (14) | |
| Mn6 | 0.32112 (4) | 0.55349 (4) | 0.36584 (3) | 0.01522 (13) | |
| O1 | 0.5427 (2) | 0.69222 (18) | 0.72622 (16) | 0.0195 (5) | |
| O2 | 0.5479 (2) | 0.79948 (18) | 0.62323 (17) | 0.0235 (6) | |
| O3 | 0.6380 (2) | 0.65305 (17) | 0.62194 (16) | 0.0174 (5) | |
| C31 | 0.6579 (3) | 0.6311 (2) | 0.5602 (2) | 0.0160 (7) | |
| O4 | 0.3075 (2) | 0.6456 (2) | 0.55926 (19) | 0.0259 (6) | |
| H4D | 0.279 (3) | 0.631 (3) | 0.5055 (13) | 0.031* | |
| H4E | 0.310 (4) | 0.696 (2) | 0.578 (3) | 0.031* | |
| O5 | 0.4100 (2) | 0.52276 (17) | 0.59896 (16) | 0.0156 (5) | |
| H5D | 0.365 (3) | 0.495 (3) | 0.5456 (14) | 0.019* | |
| O6 | 0.4395 (2) | 0.64189 (17) | 0.47837 (16) | 0.0157 (5) | |
| H6D | 0.500 (2) | 0.658 (3) | 0.481 (3) | 0.019* | |
| O7 | 0.5504 (2) | 0.68689 (17) | 0.30572 (16) | 0.0171 (5) | |
| O8 | 0.6949 (2) | 0.63902 (17) | 0.34421 (17) | 0.0180 (5) | |
| O9 | 0.4763 (2) | 0.46920 (19) | 0.21367 (17) | 0.0217 (6) | |
| H9D | 0.433 (3) | 0.457 (3) | 0.1600 (14) | 0.026* | |
| H9E | 0.457 (3) | 0.4095 (17) | 0.221 (3) | 0.026* | |
| O10 | 0.62797 (19) | 0.66251 (17) | 0.48852 (16) | 0.0161 (5) | |
| O11 | 0.41448 (19) | 0.52118 (17) | 0.33573 (16) | 0.0153 (5) | |
| H11D | 0.395 (3) | 0.4645 (17) | 0.344 (3) | 0.018* | |
| O12 | 0.3795 (2) | 0.6940 (2) | 0.31251 (18) | 0.0225 (6) | |
| H12C | 0.431 (3) | 0.695 (3) | 0.306 (3) | 0.027* | |
| H12D | 0.337 (3) | 0.700 (3) | 0.2668 (19) | 0.027* | |
| O13 | 0.2941 (2) | 0.42210 (17) | 0.43119 (16) | 0.0168 (5) | |
| O14 | 0.2164 (2) | 0.56917 (18) | 0.38531 (17) | 0.0207 (6) | |
| O15 | 0.1984 (2) | 0.47938 (18) | 0.25634 (16) | 0.0186 (5) | |
| N1 | 0.4899 (3) | 0.8385 (2) | 0.7517 (3) | 0.0329 (9) | |
| H1 | 0.4326 | 0.8173 | 0.6946 | 0.039* | |
| N2 | 0.6871 (3) | 0.8367 (2) | 0.8772 (2) | 0.0299 (8) | |
| H2 | 0.7216 | 0.9024 | 0.9049 | 0.036* | |
| N3 | 0.7501 (3) | 0.8279 (2) | 0.7488 (2) | 0.0277 (8) | |
| H3 | 0.7309 | 0.7636 | 0.7277 | 0.033* | |
| N4 | 0.6771 (3) | 0.9739 (2) | 0.7492 (2) | 0.0308 (8) | |
| H4 | 0.7301 | 1.0060 | 0.8071 | 0.037* | |
| N5 | 0.6293 (3) | 0.6958 (2) | 0.1816 (2) | 0.0220 (7) | |
| H5 | 0.6090 | 0.6294 | 0.1742 | 0.026* | |
| N6 | 0.6648 (3) | 0.8692 (2) | 0.2847 (2) | 0.0198 (7) | |
| H6 | 0.7222 | 0.9115 | 0.2747 | 0.024* | |
| N7 | 0.7583 (3) | 0.8187 (2) | 0.4409 (2) | 0.0197 (7) | |
| H7 | 0.7066 | 0.7878 | 0.4604 | 0.024* | |
| N8 | 0.8384 (3) | 0.7991 (2) | 0.3170 (2) | 0.0222 (7) | |
| H8 | 0.8620 | 0.8644 | 0.3140 | 0.027* | |
| N9 | 0.0652 (3) | 0.3703 (2) | 0.3302 (2) | 0.0280 (8) | |
| H9 | 0.1263 | 0.3867 | 0.3841 | 0.034* | |
| N10 | −0.0219 (3) | 0.4080 (3) | 0.1564 (2) | 0.0276 (8) | |
| H10 | −0.0890 | 0.3919 | 0.1544 | 0.033* | |
| N11 | 0.1099 (3) | 0.6064 (3) | 0.2213 (3) | 0.0349 (9) | |
| H11 | 0.1700 | 0.6246 | 0.2111 | 0.042* | |
| N12 | −0.0018 (3) | 0.5104 (3) | 0.3234 (3) | 0.0381 (9) | |
| H12 | −0.0707 | 0.4860 | 0.2751 | 0.046* | |
| C1 | 0.4406 (4) | 0.7784 (4) | 0.8054 (3) | 0.0433 (12) | |
| H1A | 0.4079 | 0.7080 | 0.7793 | 0.052* | |
| H1B | 0.3815 | 0.7913 | 0.8033 | 0.052* | |
| C2 | 0.5257 (5) | 0.8021 (4) | 0.9006 (4) | 0.0575 (16) | |
| H2A | 0.5589 | 0.8729 | 0.9252 | 0.069* | |
| H2B | 0.4879 | 0.7665 | 0.9342 | 0.069* | |
| C3 | 0.6154 (4) | 0.7779 (4) | 0.9158 (3) | 0.0444 (13) | |
| H3A | 0.5829 | 0.7076 | 0.8898 | 0.053* | |
| H3B | 0.6608 | 0.7894 | 0.9801 | 0.053* | |
| C4 | 0.7723 (4) | 0.8054 (3) | 0.8941 (3) | 0.0413 (12) | |
| H4A | 0.7376 | 0.7333 | 0.8752 | 0.050* | |
| H4B | 0.8241 | 0.8252 | 0.9579 | 0.050* | |
| C5 | 0.8319 (4) | 0.8533 (4) | 0.8431 (3) | 0.0423 (12) | |
| H5A | 0.8724 | 0.9252 | 0.8659 | 0.051* | |
| H5B | 0.8852 | 0.8299 | 0.8494 | 0.051* | |
| C6 | 0.7960 (4) | 0.8854 (3) | 0.6931 (3) | 0.0396 (12) | |
| H6A | 0.7425 | 0.8548 | 0.6302 | 0.048* | |
| H6B | 0.8635 | 0.8823 | 0.7036 | 0.048* | |
| C7 | 0.8227 (4) | 0.9928 (3) | 0.7118 (3) | 0.0455 (14) | |
| H7A | 0.8752 | 1.0225 | 0.7750 | 0.055* | |
| H7B | 0.8593 | 1.0275 | 0.6772 | 0.055* | |
| C8 | 0.7266 (5) | 1.0086 (3) | 0.6908 (3) | 0.0465 (14) | |
| H8A | 0.7496 | 1.0794 | 0.6956 | 0.056* | |
| H8B | 0.6709 | 0.9741 | 0.6291 | 0.056* | |
| C9 | 0.5843 (4) | 0.9973 (3) | 0.7282 (4) | 0.0476 (14) | |
| H9A | 0.6131 | 1.0687 | 0.7463 | 0.057* | |
| H9B | 0.5341 | 0.9757 | 0.6640 | 0.057* | |
| C10 | 0.5250 (4) | 0.9442 (4) | 0.7779 (4) | 0.0535 (15) | |
| H10A | 0.5739 | 0.9690 | 0.8421 | 0.064* | |
| H10B | 0.4606 | 0.9543 | 0.7633 | 0.064* | |
| C11 | 0.5300 (3) | 0.7012 (3) | 0.1180 (3) | 0.0270 (9) | |
| H11A | 0.4682 | 0.6607 | 0.1297 | 0.032* | |
| H11B | 0.5108 | 0.6727 | 0.0575 | 0.032* | |
| C12 | 0.5439 (3) | 0.8035 (3) | 0.1223 (3) | 0.0275 (9) | |
| H12A | 0.4782 | 0.8016 | 0.0737 | 0.033* | |
| H12B | 0.6075 | 0.8445 | 0.1128 | 0.033* | |
| C13 | 0.5608 (3) | 0.8510 (3) | 0.2084 (3) | 0.0244 (8) | |
| H13A | 0.5596 | 0.9141 | 0.2030 | 0.029* | |
| H13B | 0.4991 | 0.8084 | 0.2195 | 0.029* | |
| C14 | 0.6760 (3) | 0.9175 (3) | 0.3670 (3) | 0.0234 (8) | |
| H14A | 0.6088 | 0.8804 | 0.3730 | 0.028* | |
| H14B | 0.6870 | 0.9849 | 0.3645 | 0.028* | |
| C15 | 0.7724 (3) | 0.9199 (3) | 0.4441 (3) | 0.0248 (9) | |
| H15A | 0.8407 | 0.9634 | 0.4417 | 0.030* | |
| H15B | 0.7774 | 0.9457 | 0.4998 | 0.030* | |
| C16 | 0.8569 (3) | 0.8134 (3) | 0.5043 (3) | 0.0246 (8) | |
| H16A | 0.8366 | 0.7444 | 0.5077 | 0.030* | |
| H16B | 0.8832 | 0.8512 | 0.5634 | 0.030* | |
| C17 | 0.9504 (3) | 0.8531 (3) | 0.4794 (3) | 0.0266 (9) | |
| H17A | 0.9682 | 0.9212 | 0.4734 | 0.032* | |
| H17B | 1.0160 | 0.8550 | 0.5280 | 0.032* | |
| C18 | 0.9251 (3) | 0.7943 (3) | 0.3954 (3) | 0.0247 (8) | |
| H18A | 0.9924 | 0.8187 | 0.3886 | 0.030* | |
| H18B | 0.9020 | 0.7251 | 0.3994 | 0.030* | |
| C19 | 0.8205 (3) | 0.7441 (3) | 0.2352 (3) | 0.0272 (9) | |
| H19A | 0.8085 | 0.6770 | 0.2407 | 0.033* | |
| H19B | 0.8856 | 0.7774 | 0.2255 | 0.033* | |
| C20 | 0.7220 (3) | 0.7402 (3) | 0.1592 (3) | 0.0296 (9) | |
| H20A | 0.7044 | 0.6997 | 0.1044 | 0.035* | |
| H20B | 0.7360 | 0.8068 | 0.1503 | 0.035* | |
| C21 | 0.0490 (4) | 0.2806 (3) | 0.2803 (3) | 0.0384 (11) | |
| H21A | 0.0326 | 0.2275 | 0.3118 | 0.046* | |
| H21B | 0.1180 | 0.2943 | 0.2790 | 0.046* | |
| C22 | −0.0434 (4) | 0.2451 (3) | 0.1854 (3) | 0.0376 (11) | |
| H22A | −0.0541 | 0.1822 | 0.1587 | 0.045* | |
| H22B | −0.1121 | 0.2327 | 0.1867 | 0.045* | |
| C23 | −0.0229 (4) | 0.3161 (3) | 0.1279 (3) | 0.0334 (10) | |
| H23A | 0.0483 | 0.3325 | 0.1298 | 0.040* | |
| H23B | −0.0806 | 0.2846 | 0.0662 | 0.040* | |
| C24 | −0.0075 (4) | 0.4717 (4) | 0.0938 (3) | 0.0409 (12) | |
| H24A | −0.0740 | 0.4424 | 0.0360 | 0.049* | |
| H24B | 0.0553 | 0.4785 | 0.0852 | 0.049* | |
| C25 | 0.0127 (4) | 0.5694 (4) | 0.1313 (4) | 0.0518 (14) | |
| H25A | −0.0522 | 0.5636 | 0.1357 | 0.062* | |
| H25B | 0.0279 | 0.6151 | 0.0932 | 0.062* | |
| C26 | 0.1297 (4) | 0.6964 (4) | 0.2709 (4) | 0.0506 (14) | |
| H26A | 0.1339 | 0.7455 | 0.2343 | 0.061* | |
| H26B | 0.2013 | 0.7233 | 0.3245 | 0.061* | |
| C27 | 0.0424 (5) | 0.6807 (4) | 0.2976 (5) | 0.0619 (17) | |
| H27A | −0.0293 | 0.6516 | 0.2439 | 0.074* | |
| H27B | 0.0563 | 0.7453 | 0.3241 | 0.074* | |
| C28 | 0.0351 (4) | 0.6150 (4) | 0.3628 (4) | 0.0562 (16) | |
| H28A | 0.1078 | 0.6414 | 0.4151 | 0.067* | |
| H28B | −0.0169 | 0.6162 | 0.3826 | 0.067* | |
| C29 | −0.0100 (4) | 0.4490 (4) | 0.3874 (3) | 0.0488 (14) | |
| H29A | −0.0731 | 0.4392 | 0.3969 | 0.059* | |
| H29B | 0.0573 | 0.4817 | 0.4446 | 0.059* | |
| C30 | −0.0244 (4) | 0.3517 (4) | 0.3525 (3) | 0.0454 (13) | |
| H30A | −0.0218 | 0.3122 | 0.3976 | 0.055* | |
| H30B | −0.0962 | 0.3151 | 0.2994 | 0.055* | |
| Cl1 | 0.91039 (8) | 1.01855 (7) | 0.29167 (7) | 0.0268 (2) | |
| Cl2 | 0.24589 (13) | 0.79935 (12) | 0.60522 (13) | 0.0769 (6) | |
| Cl3 | 0.86737 (18) | 1.07361 (15) | 0.96304 (14) | 0.0367 (5) | 0.50 |
| Cl4 | 0.2516 (2) | 0.7178 (3) | 0.1287 (2) | 0.0673 (10) | 0.50 |
| Cl5A | −0.2407 (3) | 0.4394 (3) | 0.1490 (3) | 0.0730 (12) | 0.40 |
| Cl5B | −0.2790 (9) | 0.2686 (11) | 0.0485 (8) | 0.045 (3) | 0.10 |
| Cl6 | 0.5772 (2) | 0.49181 (16) | 0.08903 (17) | 0.0465 (6) | 0.50 |
| O16 | 0.2661 (3) | 0.9080 (3) | 0.7683 (3) | 0.0518 (10) | |
| O17 | 1.0355 (3) | 1.1982 (3) | 0.8871 (2) | 0.0498 (9) | |
| O18 | −0.2086 (3) | 0.6154 (3) | 0.0340 (2) | 0.0507 (9) | |
| O19 | 0.8974 (3) | 0.9445 (3) | 0.1130 (3) | 0.0585 (10) | |
| O20 | −0.2526 (7) | 0.3240 (7) | 0.1109 (6) | 0.068 (2)* | 0.50 |
| O21A | 0.2119 (5) | 0.4846 (5) | 0.6239 (4) | 0.0420 (15)* | 0.55 |
| O21B | 0.1753 (6) | 0.4493 (6) | 0.5731 (5) | 0.0388 (17)* | 0.45 |
| O22A | 0.4103 (9) | 0.9540 (8) | 0.9533 (7) | 0.061 (3)* | 0.45 |
| O22B | 0.3601 (10) | 0.9330 (9) | 0.9500 (8) | 0.049 (3)* | 0.35 |
| O22C | 0.3772 (14) | 0.8915 (13) | 0.9507 (11) | 0.037 (4)* | 0.20 |
| O23A | 0.2723 (4) | 0.4768 (4) | 0.1348 (4) | 0.0357 (12)* | 0.60 |
| O23B | 0.2584 (7) | 0.4259 (7) | 0.1386 (5) | 0.0369 (19)* | 0.40 |
| O24A | 0.4901 (5) | 0.8550 (4) | 0.4605 (4) | 0.0360 (13)* | 0.60 |
| O24B | 0.4420 (7) | 0.8431 (6) | 0.4547 (5) | 0.0337 (19)* | 0.40 |
| O25A | 0.6386 (9) | 1.0576 (7) | 0.4969 (6) | 0.037 (2)* | 0.35 |
| O25B | 0.5869 (11) | 1.0364 (9) | 0.4906 (8) | 0.030 (3)* | 0.25 |
| O25C | 0.7427 (14) | 1.1185 (12) | 0.5456 (11) | 0.038 (4)* | 0.20 |
| O25D | 0.7994 (10) | 1.1247 (9) | 0.5422 (8) | 0.017 (3)* | 0.20 |
| O26A | 0.3055 (10) | 0.8377 (9) | 0.0711 (8) | 0.045 (3)* | 0.31 |
| O26B | 0.3085 (13) | 0.8749 (13) | 0.0392 (11) | 0.075 (5)* | 0.30 |
| O27 | 0.8491 (6) | 1.1410 (5) | 0.9027 (5) | 0.0325 (16)* | 0.42 |
| O28 | 0.7746 (6) | 1.0212 (6) | 1.0010 (5) | 0.0361 (18)* | 0.40 |
| O29 | 0.2422 (9) | 0.6563 (8) | 0.1076 (7) | 0.049 (3)* | 0.40 |
| O30 | 0.2682 (14) | 0.6482 (13) | 0.0730 (12) | 0.091 (5)* | 0.33 |
| O31 | 0.7693 (9) | 0.4686 (9) | 0.2254 (8) | 0.054 (3)* | 0.34 |
Atomic displacement parameters (Å2)
| U11 | U22 | U33 | U12 | U13 | U23 | |
| Mn1 | 0.0274 (3) | 0.0092 (3) | 0.0139 (3) | 0.0021 (2) | 0.0082 (2) | −0.0014 (2) |
| Mn2 | 0.0219 (3) | 0.0074 (2) | 0.0125 (3) | 0.0006 (2) | 0.0073 (2) | −0.0006 (2) |
| Mn3 | 0.0165 (3) | 0.0086 (3) | 0.0168 (3) | 0.0003 (2) | 0.0087 (2) | 0.0013 (2) |
| Mn4 | 0.0145 (3) | 0.0078 (2) | 0.0147 (3) | −0.0003 (2) | 0.0068 (2) | 0.0009 (2) |
| Mn5 | 0.0152 (3) | 0.0159 (3) | 0.0197 (3) | 0.0015 (2) | 0.0077 (2) | 0.0024 (2) |
| Mn6 | 0.0143 (3) | 0.0104 (3) | 0.0128 (3) | 0.0001 (2) | 0.0053 (2) | −0.0018 (2) |
| O1 | 0.0248 (14) | 0.0118 (12) | 0.0140 (13) | 0.0025 (10) | 0.0086 (11) | 0.0008 (10) |
| O2 | 0.0320 (15) | 0.0104 (12) | 0.0139 (13) | 0.0036 (11) | 0.0052 (12) | −0.0005 (10) |
| O3 | 0.0203 (13) | 0.0113 (12) | 0.0119 (12) | 0.0011 (10) | 0.0067 (10) | −0.0018 (9) |
| C31 | 0.0131 (16) | 0.0070 (15) | 0.0147 (18) | −0.0051 (13) | 0.0053 (14) | −0.0015 (13) |
| O4 | 0.0355 (16) | 0.0237 (15) | 0.0235 (15) | 0.0149 (13) | 0.0171 (14) | 0.0052 (12) |
| O5 | 0.0163 (12) | 0.0103 (12) | 0.0109 (12) | 0.0005 (10) | 0.0043 (10) | −0.0005 (9) |
| O6 | 0.0150 (12) | 0.0112 (12) | 0.0121 (12) | −0.0002 (10) | 0.0054 (10) | −0.0021 (9) |
| O7 | 0.0181 (12) | 0.0107 (12) | 0.0167 (13) | 0.0028 (10) | 0.0073 (11) | 0.0030 (10) |
| O8 | 0.0170 (12) | 0.0098 (12) | 0.0213 (14) | 0.0015 (10) | 0.0088 (11) | 0.0026 (10) |
| O9 | 0.0269 (15) | 0.0164 (13) | 0.0145 (13) | 0.0051 (11) | 0.0088 (12) | 0.0012 (11) |
| O10 | 0.0156 (12) | 0.0107 (11) | 0.0160 (13) | 0.0008 (10) | 0.0076 (10) | 0.0015 (10) |
| O11 | 0.0162 (12) | 0.0077 (11) | 0.0160 (13) | 0.0009 (10) | 0.0072 (10) | 0.0018 (10) |
| O12 | 0.0239 (15) | 0.0227 (14) | 0.0207 (14) | 0.0105 (12) | 0.0107 (12) | 0.0062 (11) |
| O13 | 0.0172 (12) | 0.0118 (12) | 0.0137 (13) | 0.0012 (10) | 0.0065 (10) | 0.0000 (10) |
| O14 | 0.0188 (13) | 0.0166 (13) | 0.0210 (14) | 0.0036 (11) | 0.0095 (11) | −0.0010 (11) |
| O15 | 0.0151 (12) | 0.0180 (13) | 0.0121 (12) | 0.0002 (10) | 0.0053 (10) | −0.0005 (10) |
| N1 | 0.033 (2) | 0.0193 (18) | 0.040 (2) | 0.0093 (15) | 0.0152 (18) | −0.0016 (16) |
| N2 | 0.037 (2) | 0.0174 (17) | 0.0154 (17) | 0.0010 (15) | 0.0069 (15) | −0.0011 (13) |
| N3 | 0.0266 (18) | 0.0146 (16) | 0.0287 (19) | −0.0007 (14) | 0.0125 (15) | −0.0042 (14) |
| N4 | 0.041 (2) | 0.0092 (15) | 0.0228 (18) | 0.0010 (14) | 0.0093 (16) | −0.0027 (13) |
| N5 | 0.0236 (17) | 0.0156 (15) | 0.0230 (17) | 0.0049 (13) | 0.0122 (14) | 0.0021 (13) |
| N6 | 0.0211 (16) | 0.0117 (14) | 0.0229 (17) | 0.0015 (12) | 0.0134 (14) | 0.0027 (13) |
| N7 | 0.0196 (16) | 0.0129 (15) | 0.0174 (16) | −0.0001 (12) | 0.0087 (13) | 0.0007 (12) |
| N8 | 0.0222 (16) | 0.0138 (15) | 0.0291 (19) | 0.0031 (13) | 0.0161 (15) | 0.0045 (13) |
| N9 | 0.0241 (17) | 0.0188 (17) | 0.0214 (18) | −0.0022 (14) | 0.0065 (15) | 0.0041 (14) |
| N10 | 0.0178 (16) | 0.0301 (19) | 0.0185 (17) | 0.0007 (14) | 0.0053 (14) | 0.0044 (14) |
| N11 | 0.030 (2) | 0.0240 (19) | 0.040 (2) | 0.0112 (16) | 0.0080 (17) | 0.0096 (17) |
| N12 | 0.0235 (19) | 0.040 (2) | 0.041 (2) | 0.0077 (17) | 0.0141 (18) | −0.0052 (18) |
| C1 | 0.050 (3) | 0.038 (3) | 0.045 (3) | 0.016 (2) | 0.030 (3) | 0.002 (2) |
| C2 | 0.074 (4) | 0.045 (3) | 0.047 (3) | 0.012 (3) | 0.041 (3) | −0.005 (3) |
| C3 | 0.061 (3) | 0.031 (3) | 0.023 (2) | 0.003 (2) | 0.023 (2) | 0.0019 (19) |
| C4 | 0.039 (3) | 0.028 (2) | 0.023 (2) | 0.005 (2) | −0.003 (2) | −0.0016 (19) |
| C5 | 0.028 (2) | 0.031 (3) | 0.039 (3) | −0.001 (2) | 0.007 (2) | −0.006 (2) |
| C6 | 0.042 (3) | 0.025 (2) | 0.037 (3) | −0.003 (2) | 0.025 (2) | −0.0042 (19) |
| C7 | 0.052 (3) | 0.020 (2) | 0.039 (3) | −0.011 (2) | 0.028 (3) | −0.0087 (19) |
| C8 | 0.068 (4) | 0.016 (2) | 0.027 (3) | 0.002 (2) | 0.017 (2) | 0.0015 (18) |
| C9 | 0.049 (3) | 0.020 (2) | 0.047 (3) | 0.014 (2) | 0.002 (2) | 0.001 (2) |
| C10 | 0.042 (3) | 0.026 (3) | 0.079 (4) | 0.012 (2) | 0.021 (3) | −0.010 (3) |
| C11 | 0.028 (2) | 0.025 (2) | 0.020 (2) | 0.0079 (17) | 0.0090 (18) | 0.0011 (16) |
| C12 | 0.028 (2) | 0.029 (2) | 0.025 (2) | 0.0144 (18) | 0.0115 (18) | 0.0074 (18) |
| C13 | 0.028 (2) | 0.0187 (19) | 0.027 (2) | 0.0111 (17) | 0.0125 (18) | 0.0076 (16) |
| C14 | 0.029 (2) | 0.0136 (18) | 0.032 (2) | 0.0077 (16) | 0.0201 (18) | 0.0043 (16) |
| C15 | 0.029 (2) | 0.0116 (17) | 0.026 (2) | 0.0009 (16) | 0.0152 (18) | −0.0032 (15) |
| C16 | 0.0218 (19) | 0.0180 (19) | 0.022 (2) | 0.0020 (15) | 0.0077 (16) | 0.0012 (15) |
| C17 | 0.0167 (18) | 0.0174 (19) | 0.030 (2) | −0.0013 (15) | 0.0075 (17) | 0.0018 (16) |
| C18 | 0.0199 (19) | 0.0191 (19) | 0.031 (2) | 0.0050 (16) | 0.0123 (17) | 0.0070 (17) |
| C19 | 0.034 (2) | 0.025 (2) | 0.030 (2) | 0.0135 (18) | 0.0213 (19) | 0.0072 (17) |
| C20 | 0.034 (2) | 0.030 (2) | 0.026 (2) | 0.0116 (19) | 0.0189 (19) | 0.0066 (18) |
| C21 | 0.036 (3) | 0.016 (2) | 0.034 (3) | −0.0011 (18) | 0.005 (2) | 0.0042 (18) |
| C22 | 0.028 (2) | 0.021 (2) | 0.035 (3) | −0.0041 (18) | 0.006 (2) | −0.0037 (19) |
| C23 | 0.026 (2) | 0.028 (2) | 0.021 (2) | −0.0017 (18) | 0.0049 (18) | −0.0031 (18) |
| C24 | 0.031 (2) | 0.045 (3) | 0.022 (2) | 0.006 (2) | 0.0033 (19) | 0.016 (2) |
| C25 | 0.043 (3) | 0.047 (3) | 0.047 (3) | 0.019 (3) | 0.008 (3) | 0.023 (3) |
| C26 | 0.045 (3) | 0.026 (3) | 0.069 (4) | 0.017 (2) | 0.016 (3) | 0.008 (3) |
| C27 | 0.051 (3) | 0.044 (3) | 0.092 (5) | 0.030 (3) | 0.027 (3) | 0.002 (3) |
| C28 | 0.037 (3) | 0.052 (3) | 0.075 (4) | 0.017 (3) | 0.027 (3) | −0.018 (3) |
| C29 | 0.034 (3) | 0.061 (4) | 0.041 (3) | 0.004 (2) | 0.028 (2) | −0.001 (3) |
| C30 | 0.036 (3) | 0.043 (3) | 0.033 (3) | −0.004 (2) | 0.019 (2) | 0.005 (2) |
| Cl1 | 0.0260 (5) | 0.0144 (4) | 0.0314 (5) | 0.0009 (4) | 0.0150 (4) | 0.0040 (4) |
| Cl2 | 0.0516 (9) | 0.0594 (10) | 0.0962 (13) | 0.0178 (8) | 0.0241 (9) | −0.0287 (9) |
| Cl3 | 0.0369 (12) | 0.0217 (10) | 0.0243 (11) | −0.0075 (9) | 0.0134 (9) | −0.0130 (8) |
| Cl4 | 0.0354 (14) | 0.076 (2) | 0.0549 (19) | 0.0091 (15) | 0.0080 (13) | 0.0296 (17) |
| Cl5A | 0.0283 (17) | 0.079 (3) | 0.078 (3) | 0.0061 (17) | 0.0153 (18) | 0.008 (2) |
| Cl5B | 0.020 (5) | 0.065 (8) | 0.041 (7) | 0.017 (5) | 0.009 (5) | 0.016 (6) |
| Cl6 | 0.0596 (16) | 0.0195 (10) | 0.0430 (14) | 0.0106 (10) | 0.0176 (12) | −0.0006 (10) |
| O16 | 0.043 (2) | 0.044 (2) | 0.077 (3) | 0.0195 (18) | 0.037 (2) | 0.028 (2) |
| O17 | 0.048 (2) | 0.046 (2) | 0.035 (2) | 0.0090 (17) | 0.0154 (17) | −0.0040 (16) |
| O18 | 0.049 (2) | 0.049 (2) | 0.038 (2) | 0.0144 (18) | 0.0151 (17) | −0.0024 (17) |
| O19 | 0.072 (3) | 0.054 (2) | 0.047 (2) | 0.023 (2) | 0.033 (2) | 0.0101 (19) |
Geometric parameters (Å, °)
| Mn1—O2 | 1.779 (3) | C1—C2 | 1.517 (8) |
| Mn1—O1 | 1.792 (2) | C1—H1A | 0.99 |
| Mn1—N3 | 2.037 (4) | C1—H1B | 0.99 |
| Mn1—N1 | 2.041 (4) | C2—C3 | 1.489 (8) |
| Mn1—N2 | 2.100 (3) | C2—H2A | 0.99 |
| Mn1—N4 | 2.100 (3) | C2—H2B | 0.99 |
| Mn1—Mn2 | 2.7295 (8) | C3—H3A | 0.99 |
| Mn2—O1 | 1.867 (3) | C3—H3B | 0.99 |
| Mn2—O2 | 1.892 (3) | C4—C5 | 1.497 (7) |
| Mn2—O5 | 1.937 (2) | C4—H4A | 0.99 |
| Mn2—O6 | 1.943 (2) | C4—H4B | 0.99 |
| Mn2—O3 | 2.327 (3) | C5—H5A | 0.99 |
| Mn2—O4 | 2.339 (3) | C5—H5B | 0.99 |
| Mn3—O8 | 1.783 (2) | C6—C7 | 1.531 (7) |
| Mn3—O7 | 1.797 (2) | C6—H6A | 0.99 |
| Mn3—N5 | 2.043 (3) | C6—H6B | 0.99 |
| Mn3—N7 | 2.047 (3) | C7—C8 | 1.484 (8) |
| Mn3—N8 | 2.098 (3) | C7—H7A | 0.99 |
| Mn3—N6 | 2.105 (3) | C7—H7B | 0.99 |
| Mn3—Mn4 | 2.7237 (8) | C8—H8A | 0.99 |
| Mn4—O8 | 1.879 (2) | C8—H8B | 0.99 |
| Mn4—O7 | 1.883 (2) | C9—C10 | 1.498 (8) |
| Mn4—O11 | 1.935 (2) | C9—H9A | 0.99 |
| Mn4—O5i | 1.943 (3) | C9—H9B | 0.99 |
| Mn4—O9 | 2.311 (3) | C10—H10A | 0.99 |
| Mn4—O10 | 2.315 (2) | C10—H10B | 0.99 |
| Mn5—O15 | 1.783 (3) | C11—C12 | 1.505 (6) |
| Mn5—O14 | 1.788 (3) | C11—H11A | 0.99 |
| Mn5—N11 | 2.034 (4) | C11—H11B | 0.99 |
| Mn5—N9 | 2.038 (3) | C12—C13 | 1.510 (6) |
| Mn5—N10 | 2.093 (3) | C12—H12A | 0.99 |
| Mn5—N12 | 2.095 (4) | C12—H12B | 0.99 |
| Mn5—Mn6 | 2.7204 (9) | C13—H13A | 0.99 |
| Mn6—O14 | 1.868 (3) | C13—H13B | 0.99 |
| Mn6—O15 | 1.883 (2) | C14—C15 | 1.498 (6) |
| Mn6—O11 | 1.937 (3) | C14—H14A | 0.99 |
| Mn6—O6 | 1.949 (2) | C14—H14B | 0.99 |
| Mn6—O13 | 2.302 (2) | C15—H15A | 0.99 |
| Mn6—O12 | 2.335 (3) | C15—H15B | 0.99 |
| O3—C31 | 1.293 (4) | C16—C17 | 1.535 (5) |
| C31—O13i | 1.289 (4) | C16—H16A | 0.99 |
| C31—O10 | 1.301 (4) | C16—H16B | 0.99 |
| O4—H4D | 0.82 (4) | C17—C18 | 1.503 (6) |
| O4—H4E | 0.82 (4) | C17—H17A | 0.99 |
| O5—Mn4i | 1.943 (3) | C17—H17B | 0.99 |
| O5—H5D | 0.84 (4) | C18—H18A | 0.99 |
| O6—H6D | 0.82 (4) | C18—H18B | 0.99 |
| O9—H9D | 0.84 (4) | C19—C20 | 1.500 (6) |
| O9—H9E | 0.87 (3) | C19—H19A | 0.99 |
| O11—H11D | 0.83 (4) | C19—H19B | 0.99 |
| O12—H12C | 0.83 (5) | C20—H20A | 0.99 |
| O12—H12D | 0.82 (5) | C20—H20B | 0.99 |
| O13—C31i | 1.289 (4) | C21—C22 | 1.532 (6) |
| N1—C10 | 1.486 (6) | C21—H21A | 0.99 |
| N1—C1 | 1.509 (6) | C21—H21B | 0.99 |
| N1—H1 | 0.93 | C22—C23 | 1.506 (6) |
| N2—C3 | 1.494 (6) | C22—H22A | 0.99 |
| N2—C4 | 1.495 (6) | C22—H22B | 0.99 |
| N2—H2 | 0.93 | C23—H23A | 0.99 |
| N3—C5 | 1.489 (6) | C23—H23B | 0.99 |
| N3—C6 | 1.497 (5) | C24—C25 | 1.489 (8) |
| N3—H3 | 0.93 | C24—H24A | 0.99 |
| N4—C8 | 1.468 (6) | C24—H24B | 0.99 |
| N4—C9 | 1.509 (6) | C25—H25A | 0.99 |
| N4—H4 | 0.93 | C25—H25B | 0.99 |
| N5—C11 | 1.492 (5) | C26—C27 | 1.508 (8) |
| N5—C20 | 1.501 (5) | C26—H26A | 0.99 |
| N5—H5 | 0.93 | C26—H26B | 0.99 |
| N6—C13 | 1.481 (5) | C27—C28 | 1.528 (9) |
| N6—C14 | 1.498 (5) | C27—H27A | 0.99 |
| N6—H6 | 0.93 | C27—H27B | 0.99 |
| N7—C16 | 1.482 (5) | C28—H28A | 0.99 |
| N7—C15 | 1.488 (5) | C28—H28B | 0.99 |
| N7—H7 | 0.93 | C29—C30 | 1.506 (8) |
| N8—C18 | 1.473 (5) | C29—H29A | 0.99 |
| N8—C19 | 1.490 (5) | C29—H29B | 0.99 |
| N8—H8 | 0.93 | C30—H30A | 0.99 |
| N9—C30 | 1.483 (6) | C30—H30B | 0.99 |
| N9—C21 | 1.493 (6) | Cl3—O27 | 1.507 (8) |
| N9—H9 | 0.93 | Cl3—O28 | 1.697 (8) |
| N10—C24 | 1.486 (5) | Cl4—O29 | 0.945 (10) |
| N10—C23 | 1.492 (6) | Cl4—O30 | 1.597 (19) |
| N10—H10 | 0.93 | Cl4—O26A | 2.127 (13) |
| N11—C26 | 1.470 (6) | Cl5A—O31ii | 1.304 (12) |
| N11—C25 | 1.503 (6) | Cl5A—O20 | 1.806 (11) |
| N11—H11 | 0.93 | Cl5B—O20 | 1.169 (16) |
| N12—C29 | 1.470 (7) | O29—O30 | 0.873 (17) |
| N12—C28 | 1.511 (6) | O31—Cl5Aiii | 1.304 (12) |
| N12—H12 | 0.93 | ||
| O2—Mn1—O1 | 86.28 (12) | C30—N9—H9 | 104.8 |
| O2—Mn1—N3 | 92.60 (14) | C21—N9—H9 | 104.8 |
| O1—Mn1—N3 | 94.14 (13) | Mn5—N9—H9 | 104.8 |
| O2—Mn1—N1 | 95.52 (15) | C24—N10—C23 | 109.0 (4) |
| O1—Mn1—N1 | 93.32 (13) | C24—N10—Mn5 | 106.1 (3) |
| N3—Mn1—N1 | 169.31 (15) | C23—N10—Mn5 | 115.9 (3) |
| O2—Mn1—N2 | 173.95 (14) | C24—N10—H10 | 108.5 |
| O1—Mn1—N2 | 89.08 (12) | C23—N10—H10 | 108.5 |
| N3—Mn1—N2 | 83.87 (15) | Mn5—N10—H10 | 108.5 |
| N1—Mn1—N2 | 88.61 (16) | C26—N11—C25 | 112.8 (4) |
| O2—Mn1—N4 | 90.63 (13) | C26—N11—Mn5 | 117.8 (3) |
| O1—Mn1—N4 | 175.16 (14) | C25—N11—Mn5 | 109.4 (3) |
| N3—Mn1—N4 | 89.71 (15) | C26—N11—H11 | 105.2 |
| N1—Mn1—N4 | 83.26 (15) | C25—N11—H11 | 105.2 |
| N2—Mn1—N4 | 94.25 (14) | Mn5—N11—H11 | 105.2 |
| O2—Mn1—Mn2 | 43.57 (8) | C29—N12—C28 | 111.0 (4) |
| O1—Mn1—Mn2 | 42.83 (8) | C29—N12—Mn5 | 106.2 (3) |
| N3—Mn1—Mn2 | 92.08 (10) | C28—N12—Mn5 | 114.4 (3) |
| N1—Mn1—Mn2 | 98.60 (11) | C29—N12—H12 | 108.3 |
| N2—Mn1—Mn2 | 131.44 (10) | C28—N12—H12 | 108.3 |
| N4—Mn1—Mn2 | 134.20 (10) | Mn5—N12—H12 | 108.3 |
| O1—Mn2—O2 | 81.01 (11) | N1—C1—C2 | 111.6 (4) |
| O1—Mn2—O5 | 91.51 (11) | N1—C1—H1A | 109.3 |
| O2—Mn2—O5 | 170.92 (11) | C2—C1—H1A | 109.3 |
| O1—Mn2—O6 | 171.82 (11) | N1—C1—H1B | 109.3 |
| O2—Mn2—O6 | 94.09 (11) | C2—C1—H1B | 109.3 |
| O5—Mn2—O6 | 93.92 (10) | H1A—C1—H1B | 108.0 |
| O1—Mn2—O3 | 87.64 (10) | C3—C2—C1 | 116.1 (4) |
| O2—Mn2—O3 | 94.66 (11) | C3—C2—H2A | 108.3 |
| O5—Mn2—O3 | 90.15 (10) | C1—C2—H2A | 108.3 |
| O6—Mn2—O3 | 86.24 (10) | C3—C2—H2B | 108.3 |
| O1—Mn2—O4 | 102.12 (11) | C1—C2—H2B | 108.3 |
| O2—Mn2—O4 | 92.91 (11) | H2A—C2—H2B | 107.4 |
| O5—Mn2—O4 | 83.57 (10) | C2—C3—N2 | 113.2 (4) |
| O6—Mn2—O4 | 84.58 (10) | C2—C3—H3A | 108.9 |
| O3—Mn2—O4 | 168.49 (9) | N2—C3—H3A | 108.9 |
| O1—Mn2—Mn1 | 40.72 (8) | C2—C3—H3B | 108.9 |
| O2—Mn2—Mn1 | 40.40 (8) | N2—C3—H3B | 108.9 |
| O5—Mn2—Mn1 | 132.21 (8) | H3A—C3—H3B | 107.8 |
| O6—Mn2—Mn1 | 133.67 (7) | N2—C4—C5 | 108.2 (4) |
| O3—Mn2—Mn1 | 89.21 (6) | N2—C4—H4A | 110.1 |
| O4—Mn2—Mn1 | 102.16 (8) | C5—C4—H4A | 110.1 |
| O8—Mn3—O7 | 86.63 (11) | N2—C4—H4B | 110.1 |
| O8—Mn3—N5 | 95.31 (12) | C5—C4—H4B | 110.1 |
| O7—Mn3—N5 | 94.42 (12) | H4A—C4—H4B | 108.4 |
| O8—Mn3—N7 | 92.66 (12) | N3—C5—C4 | 108.3 (4) |
| O7—Mn3—N7 | 93.53 (12) | N3—C5—H5A | 110.0 |
| N5—Mn3—N7 | 169.05 (12) | C4—C5—H5A | 110.0 |
| O8—Mn3—N8 | 88.84 (12) | N3—C5—H5B | 110.0 |
| O7—Mn3—N8 | 174.58 (12) | C4—C5—H5B | 110.0 |
| N5—Mn3—N8 | 83.04 (13) | H5A—C5—H5B | 108.4 |
| N7—Mn3—N8 | 89.64 (13) | N3—C6—C7 | 113.1 (4) |
| O8—Mn3—N6 | 173.89 (12) | N3—C6—H6A | 109.0 |
| O7—Mn3—N6 | 88.92 (12) | C7—C6—H6A | 109.0 |
| N5—Mn3—N6 | 89.20 (13) | N3—C6—H6B | 109.0 |
| N7—Mn3—N6 | 83.44 (12) | C7—C6—H6B | 109.0 |
| N8—Mn3—N6 | 95.81 (12) | H6A—C6—H6B | 107.8 |
| O8—Mn3—Mn4 | 43.30 (8) | C8—C7—C6 | 114.8 (4) |
| O7—Mn3—Mn4 | 43.48 (8) | C8—C7—H7A | 108.6 |
| N5—Mn3—Mn4 | 99.53 (9) | C6—C7—H7A | 108.6 |
| N7—Mn3—Mn4 | 91.41 (9) | C8—C7—H7B | 108.6 |
| N8—Mn3—Mn4 | 132.13 (9) | C6—C7—H7B | 108.6 |
| N6—Mn3—Mn4 | 131.84 (9) | H7A—C7—H7B | 107.5 |
| O8—Mn4—O7 | 81.51 (11) | N4—C8—C7 | 112.9 (4) |
| O8—Mn4—O11 | 172.17 (11) | N4—C8—H8A | 109.0 |
| O7—Mn4—O11 | 92.09 (11) | C7—C8—H8A | 109.0 |
| O8—Mn4—O5i | 93.45 (11) | N4—C8—H8B | 109.0 |
| O7—Mn4—O5i | 172.62 (11) | C7—C8—H8B | 109.0 |
| O11—Mn4—O5i | 93.34 (10) | H8A—C8—H8B | 107.8 |
| O8—Mn4—O9 | 92.53 (10) | C10—C9—N4 | 107.4 (4) |
| O7—Mn4—O9 | 99.40 (10) | C10—C9—H9A | 110.2 |
| O11—Mn4—O9 | 84.01 (10) | N4—C9—H9A | 110.2 |
| O5i—Mn4—O9 | 86.15 (10) | C10—C9—H9B | 110.2 |
| O8—Mn4—O10 | 95.15 (10) | N4—C9—H9B | 110.2 |
| O7—Mn4—O10 | 88.53 (10) | H9A—C9—H9B | 108.5 |
| O11—Mn4—O10 | 89.17 (10) | N1—C10—C9 | 107.5 (4) |
| O5i—Mn4—O10 | 86.55 (10) | N1—C10—H10A | 110.2 |
| O9—Mn4—O10 | 169.69 (9) | C9—C10—H10A | 110.2 |
| O8—Mn4—Mn3 | 40.60 (8) | N1—C10—H10B | 110.2 |
| O7—Mn4—Mn3 | 41.04 (8) | C9—C10—H10B | 110.2 |
| O11—Mn4—Mn3 | 133.13 (8) | H10A—C10—H10B | 108.5 |
| O5i—Mn4—Mn3 | 133.36 (8) | N5—C11—C12 | 113.8 (3) |
| O9—Mn4—Mn3 | 100.44 (7) | N5—C11—H11A | 108.8 |
| O10—Mn4—Mn3 | 89.86 (6) | C12—C11—H11A | 108.8 |
| O15—Mn5—O14 | 86.53 (11) | N5—C11—H11B | 108.8 |
| O15—Mn5—N11 | 92.78 (14) | C12—C11—H11B | 108.8 |
| O14—Mn5—N11 | 94.52 (14) | H11A—C11—H11B | 107.7 |
| O15—Mn5—N9 | 94.10 (13) | C11—C12—C13 | 114.0 (3) |
| O14—Mn5—N9 | 93.53 (13) | C11—C12—H12A | 108.7 |
| N11—Mn5—N9 | 169.71 (15) | C13—C12—H12A | 108.7 |
| O15—Mn5—N10 | 89.18 (13) | C11—C12—H12B | 108.7 |
| O14—Mn5—N10 | 175.15 (13) | C13—C12—H12B | 108.7 |
| N11—Mn5—N10 | 83.40 (14) | H12A—C12—H12B | 107.6 |
| N9—Mn5—N10 | 89.04 (14) | N6—C13—C12 | 113.4 (3) |
| O15—Mn5—N12 | 175.04 (14) | N6—C13—H13A | 108.9 |
| O14—Mn5—N12 | 89.28 (14) | C12—C13—H13A | 108.9 |
| N11—Mn5—N12 | 90.20 (17) | N6—C13—H13B | 108.9 |
| N9—Mn5—N12 | 83.51 (16) | C12—C13—H13B | 108.9 |
| N10—Mn5—N12 | 95.11 (15) | H13A—C13—H13B | 107.7 |
| O15—Mn5—Mn6 | 43.54 (8) | N6—C14—C15 | 108.3 (3) |
| O14—Mn5—Mn6 | 43.05 (8) | N6—C14—H14A | 110.0 |
| N11—Mn5—Mn6 | 96.77 (11) | C15—C14—H14A | 110.0 |
| N9—Mn5—Mn6 | 93.48 (10) | N6—C14—H14B | 110.0 |
| N10—Mn5—Mn6 | 132.72 (10) | C15—C14—H14B | 110.0 |
| N12—Mn5—Mn6 | 132.11 (11) | H14A—C14—H14B | 108.4 |
| O14—Mn6—O15 | 81.47 (11) | N7—C15—C14 | 108.6 (3) |
| O14—Mn6—O11 | 172.64 (11) | N7—C15—H15A | 110.0 |
| O15—Mn6—O11 | 92.11 (11) | C14—C15—H15A | 110.0 |
| O14—Mn6—O6 | 92.30 (11) | N7—C15—H15B | 110.0 |
| O15—Mn6—O6 | 172.26 (11) | C14—C15—H15B | 110.0 |
| O11—Mn6—O6 | 94.39 (11) | H15A—C15—H15B | 108.4 |
| O14—Mn6—O13 | 89.99 (10) | N7—C16—C17 | 113.3 (3) |
| O15—Mn6—O13 | 93.85 (10) | N7—C16—H16A | 108.9 |
| O11—Mn6—O13 | 86.85 (9) | C17—C16—H16A | 108.9 |
| O6—Mn6—O13 | 90.71 (10) | N7—C16—H16B | 108.9 |
| O14—Mn6—O12 | 97.18 (11) | C17—C16—H16B | 108.9 |
| O15—Mn6—O12 | 93.50 (10) | H16A—C16—H16B | 107.7 |
| O11—Mn6—O12 | 86.74 (10) | C18—C17—C16 | 114.1 (3) |
| O6—Mn6—O12 | 82.68 (10) | C18—C17—H17A | 108.7 |
| O13—Mn6—O12 | 170.42 (10) | C16—C17—H17A | 108.7 |
| O14—Mn6—Mn5 | 40.82 (8) | C18—C17—H17B | 108.7 |
| O15—Mn6—Mn5 | 40.70 (8) | C16—C17—H17B | 108.7 |
| O11—Mn6—Mn5 | 132.53 (8) | H17A—C17—H17B | 107.6 |
| O6—Mn6—Mn5 | 133.08 (8) | N8—C18—C17 | 112.7 (3) |
| O13—Mn6—Mn5 | 90.94 (6) | N8—C18—H18A | 109.0 |
| O12—Mn6—Mn5 | 98.65 (7) | C17—C18—H18A | 109.0 |
| Mn1—O1—Mn2 | 96.45 (12) | N8—C18—H18B | 109.0 |
| Mn1—O2—Mn2 | 96.04 (12) | C17—C18—H18B | 109.0 |
| C31—O3—Mn2 | 126.7 (2) | H18A—C18—H18B | 107.8 |
| O13i—C31—O3 | 120.8 (3) | N8—C19—C20 | 108.2 (3) |
| O13i—C31—O10 | 120.0 (3) | N8—C19—H19A | 110.1 |
| O3—C31—O10 | 119.2 (3) | C20—C19—H19A | 110.1 |
| Mn2—O4—H4D | 103 (3) | N8—C19—H19B | 110.1 |
| Mn2—O4—H4E | 112 (3) | C20—C19—H19B | 110.1 |
| H4D—O4—H4E | 113 (4) | H19A—C19—H19B | 108.4 |
| Mn2—O5—Mn4i | 141.16 (14) | C19—C20—N5 | 106.9 (3) |
| Mn2—O5—H5D | 106 (3) | C19—C20—H20A | 110.3 |
| Mn4i—O5—H5D | 106 (3) | N5—C20—H20A | 110.3 |
| Mn2—O6—Mn6 | 140.94 (13) | C19—C20—H20B | 110.3 |
| Mn2—O6—H6D | 99 (3) | N5—C20—H20B | 110.3 |
| Mn6—O6—H6D | 111 (3) | H20A—C20—H20B | 108.6 |
| Mn3—O7—Mn4 | 95.48 (12) | N9—C21—C22 | 113.5 (4) |
| Mn3—O8—Mn4 | 96.10 (12) | N9—C21—H21A | 108.9 |
| Mn4—O9—H9D | 144 (3) | C22—C21—H21A | 108.9 |
| Mn4—O9—H9E | 110 (3) | N9—C21—H21B | 108.9 |
| H9D—O9—H9E | 99 (3) | C22—C21—H21B | 108.9 |
| C31—O10—Mn4 | 126.1 (2) | H21A—C21—H21B | 107.7 |
| Mn4—O11—Mn6 | 141.70 (13) | C23—C22—C21 | 113.9 (3) |
| Mn4—O11—H11D | 110 (3) | C23—C22—H22A | 108.8 |
| Mn6—O11—H11D | 100 (3) | C21—C22—H22A | 108.8 |
| Mn6—O12—H12C | 103 (3) | C23—C22—H22B | 108.8 |
| Mn6—O12—H12D | 121 (3) | C21—C22—H22B | 108.8 |
| H12C—O12—H12D | 109 (3) | H22A—C22—H22B | 107.7 |
| C31i—O13—Mn6 | 125.7 (2) | N10—C23—C22 | 112.2 (4) |
| Mn5—O14—Mn6 | 96.13 (12) | N10—C23—H23A | 109.2 |
| Mn5—O15—Mn6 | 95.76 (12) | C22—C23—H23A | 109.2 |
| C10—N1—C1 | 112.6 (4) | N10—C23—H23B | 109.2 |
| C10—N1—Mn1 | 109.8 (3) | C22—C23—H23B | 109.2 |
| C1—N1—Mn1 | 117.9 (3) | H23A—C23—H23B | 107.9 |
| C10—N1—H1 | 105.1 | N10—C24—C25 | 108.3 (4) |
| C1—N1—H1 | 105.1 | N10—C24—H24A | 110.0 |
| Mn1—N1—H1 | 105.1 | C25—C24—H24A | 110.0 |
| C3—N2—C4 | 109.0 (4) | N10—C24—H24B | 110.0 |
| C3—N2—Mn1 | 116.4 (3) | C25—C24—H24B | 110.0 |
| C4—N2—Mn1 | 105.0 (3) | H24A—C24—H24B | 108.4 |
| C3—N2—H2 | 108.7 | C24—C25—N11 | 107.2 (4) |
| C4—N2—H2 | 108.7 | C24—C25—H25A | 110.3 |
| Mn1—N2—H2 | 108.7 | N11—C25—H25A | 110.3 |
| C5—N3—C6 | 113.7 (3) | C24—C25—H25B | 110.3 |
| C5—N3—Mn1 | 109.6 (3) | N11—C25—H25B | 110.3 |
| C6—N3—Mn1 | 117.2 (3) | H25A—C25—H25B | 108.5 |
| C5—N3—H3 | 105.0 | N11—C26—C27 | 113.2 (4) |
| C6—N3—H3 | 105.0 | N11—C26—H26A | 108.9 |
| Mn1—N3—H3 | 105.0 | C27—C26—H26A | 108.9 |
| C8—N4—C9 | 110.4 (4) | N11—C26—H26B | 108.9 |
| C8—N4—Mn1 | 114.8 (3) | C27—C26—H26B | 108.9 |
| C9—N4—Mn1 | 105.9 (3) | H26A—C26—H26B | 107.8 |
| C8—N4—H4 | 108.5 | C26—C27—C28 | 114.8 (5) |
| C9—N4—H4 | 108.5 | C26—C27—H27A | 108.6 |
| Mn1—N4—H4 | 108.5 | C28—C27—H27A | 108.6 |
| C11—N5—C20 | 112.7 (3) | C26—C27—H27B | 108.6 |
| C11—N5—Mn3 | 117.3 (2) | C28—C27—H27B | 108.6 |
| C20—N5—Mn3 | 110.1 (2) | H27A—C27—H27B | 107.6 |
| C11—N5—H5 | 105.2 | N12—C28—C27 | 112.5 (5) |
| C20—N5—H5 | 105.2 | N12—C28—H28A | 109.1 |
| Mn3—N5—H5 | 105.2 | C27—C28—H28A | 109.1 |
| C13—N6—C14 | 110.1 (3) | N12—C28—H28B | 109.1 |
| C13—N6—Mn3 | 116.1 (2) | C27—C28—H28B | 109.1 |
| C14—N6—Mn3 | 105.7 (2) | H28A—C28—H28B | 107.8 |
| C13—N6—H6 | 108.2 | N12—C29—C30 | 109.0 (4) |
| C14—N6—H6 | 108.2 | N12—C29—H29A | 109.9 |
| Mn3—N6—H6 | 108.2 | C30—C29—H29A | 109.9 |
| C16—N7—C15 | 113.8 (3) | N12—C29—H29B | 109.9 |
| C16—N7—Mn3 | 117.5 (2) | C30—C29—H29B | 109.9 |
| C15—N7—Mn3 | 110.0 (2) | H29A—C29—H29B | 108.3 |
| C16—N7—H7 | 104.7 | N9—C30—C29 | 108.1 (4) |
| C15—N7—H7 | 104.7 | N9—C30—H30A | 110.1 |
| Mn3—N7—H7 | 104.7 | C29—C30—H30A | 110.1 |
| C18—N8—C19 | 110.7 (3) | N9—C30—H30B | 110.1 |
| C18—N8—Mn3 | 116.4 (2) | C29—C30—H30B | 110.1 |
| C19—N8—Mn3 | 106.3 (2) | H30A—C30—H30B | 108.4 |
| C18—N8—H8 | 107.7 | O27—Cl3—O28 | 116.6 (4) |
| C19—N8—H8 | 107.7 | O29—Cl4—O26A | 120.7 (8) |
| Mn3—N8—H8 | 107.7 | O30—Cl4—O26A | 94.1 (7) |
| C30—N9—C21 | 113.1 (3) | O31ii—Cl5A—O20 | 125.5 (7) |
| C30—N9—Mn5 | 110.0 (3) | Cl5B—O20—Cl5A | 144.3 (10) |
| C21—N9—Mn5 | 118.0 (3) | ||
| O2—Mn1—Mn2—O1 | −174.57 (19) | N3—Mn1—N1—C10 | 37.6 (10) |
| N3—Mn1—Mn2—O1 | 93.85 (16) | N2—Mn1—N1—C10 | 82.9 (4) |
| N1—Mn1—Mn2—O1 | −85.59 (17) | N4—Mn1—N1—C10 | −11.6 (4) |
| N2—Mn1—Mn2—O1 | 10.20 (19) | Mn2—Mn1—N1—C10 | −145.4 (3) |
| N4—Mn1—Mn2—O1 | −174.5 (2) | O2—Mn1—N1—C1 | 127.8 (3) |
| O1—Mn1—Mn2—O2 | 174.57 (19) | O1—Mn1—N1—C1 | 41.2 (3) |
| N3—Mn1—Mn2—O2 | −91.58 (17) | N3—Mn1—N1—C1 | −93.0 (8) |
| N1—Mn1—Mn2—O2 | 88.98 (18) | N2—Mn1—N1—C1 | −47.8 (3) |
| N2—Mn1—Mn2—O2 | −175.2 (2) | N4—Mn1—N1—C1 | −142.3 (3) |
| N4—Mn1—Mn2—O2 | 0.0 (2) | Mn2—Mn1—N1—C1 | 83.9 (3) |
| O2—Mn1—Mn2—O5 | −172.38 (17) | O1—Mn1—N2—C3 | −46.7 (3) |
| O1—Mn1—Mn2—O5 | 2.18 (16) | N3—Mn1—N2—C3 | −141.0 (3) |
| N3—Mn1—Mn2—O5 | 96.03 (15) | N1—Mn1—N2—C3 | 46.6 (3) |
| N1—Mn1—Mn2—O5 | −83.40 (15) | N4—Mn1—N2—C3 | 129.7 (3) |
| N2—Mn1—Mn2—O5 | 12.38 (18) | Mn2—Mn1—N2—C3 | −53.7 (4) |
| N4—Mn1—Mn2—O5 | −172.35 (18) | O1—Mn1—N2—C4 | 73.9 (3) |
| O2—Mn1—Mn2—O6 | 14.10 (17) | N3—Mn1—N2—C4 | −20.4 (3) |
| O1—Mn1—Mn2—O6 | −171.34 (17) | N1—Mn1—N2—C4 | 167.2 (3) |
| N3—Mn1—Mn2—O6 | −77.49 (15) | N4—Mn1—N2—C4 | −109.6 (3) |
| N1—Mn1—Mn2—O6 | 103.07 (16) | Mn2—Mn1—N2—C4 | 67.0 (3) |
| N2—Mn1—Mn2—O6 | −161.14 (18) | O2—Mn1—N3—C5 | 176.5 (3) |
| N4—Mn1—Mn2—O6 | 14.1 (2) | O1—Mn1—N3—C5 | −97.1 (3) |
| O2—Mn1—Mn2—O3 | 98.13 (15) | N1—Mn1—N3—C5 | 37.1 (9) |
| O1—Mn1—Mn2—O3 | −87.30 (14) | N2—Mn1—N3—C5 | −8.4 (3) |
| N3—Mn1—Mn2—O3 | 6.55 (12) | N4—Mn1—N3—C5 | 85.9 (3) |
| N1—Mn1—Mn2—O3 | −172.89 (13) | Mn2—Mn1—N3—C5 | −139.9 (3) |
| N2—Mn1—Mn2—O3 | −77.10 (16) | O2—Mn1—N3—C6 | 45.0 (3) |
| N4—Mn1—Mn2—O3 | 98.17 (17) | O1—Mn1—N3—C6 | 131.4 (3) |
| O2—Mn1—Mn2—O4 | −80.04 (16) | N1—Mn1—N3—C6 | −94.4 (8) |
| O1—Mn1—Mn2—O4 | 94.53 (15) | N2—Mn1—N3—C6 | −140.0 (3) |
| N3—Mn1—Mn2—O4 | −171.62 (12) | N4—Mn1—N3—C6 | −45.7 (3) |
| N1—Mn1—Mn2—O4 | 8.94 (13) | Mn2—Mn1—N3—C6 | 88.6 (3) |
| N2—Mn1—Mn2—O4 | 104.73 (16) | O2—Mn1—N4—C8 | −44.9 (3) |
| N4—Mn1—Mn2—O4 | −80.00 (17) | N3—Mn1—N4—C8 | 47.7 (3) |
| O7—Mn3—Mn4—O8 | 173.98 (17) | N1—Mn1—N4—C8 | −140.3 (4) |
| N5—Mn3—Mn4—O8 | 87.63 (15) | N2—Mn1—N4—C8 | 131.6 (3) |
| N7—Mn3—Mn4—O8 | −92.38 (15) | Mn2—Mn1—N4—C8 | −44.9 (4) |
| N8—Mn3—Mn4—O8 | −1.59 (17) | O2—Mn1—N4—C9 | 77.2 (3) |
| N6—Mn3—Mn4—O8 | −174.83 (17) | N3—Mn1—N4—C9 | 169.8 (3) |
| O8—Mn3—Mn4—O7 | −173.98 (17) | N1—Mn1—N4—C9 | −18.3 (3) |
| N5—Mn3—Mn4—O7 | −86.36 (15) | N2—Mn1—N4—C9 | −106.4 (3) |
| N7—Mn3—Mn4—O7 | 93.64 (15) | Mn2—Mn1—N4—C9 | 77.2 (3) |
| N8—Mn3—Mn4—O7 | −175.57 (17) | O8—Mn3—N5—C11 | 130.4 (3) |
| N6—Mn3—Mn4—O7 | 11.19 (16) | O7—Mn3—N5—C11 | 43.4 (3) |
| O8—Mn3—Mn4—O11 | −173.18 (16) | N7—Mn3—N5—C11 | −93.1 (7) |
| O7—Mn3—Mn4—O11 | 0.81 (15) | N8—Mn3—N5—C11 | −141.4 (3) |
| N5—Mn3—Mn4—O11 | −85.55 (14) | N6—Mn3—N5—C11 | −45.5 (3) |
| N7—Mn3—Mn4—O11 | 94.45 (14) | Mn4—Mn3—N5—C11 | 86.9 (3) |
| N8—Mn3—Mn4—O11 | −174.76 (16) | O8—Mn3—N5—C20 | −99.0 (3) |
| N6—Mn3—Mn4—O11 | 12.00 (16) | O7—Mn3—N5—C20 | 174.0 (3) |
| O8—Mn3—Mn4—O5i | 12.97 (15) | N7—Mn3—N5—C20 | 37.6 (8) |
| O7—Mn3—Mn4—O5i | −173.04 (16) | N8—Mn3—N5—C20 | −10.8 (3) |
| N5—Mn3—Mn4—O5i | 100.60 (14) | N6—Mn3—N5—C20 | 85.2 (3) |
| N7—Mn3—Mn4—O5i | −79.40 (14) | Mn4—Mn3—N5—C20 | −142.5 (2) |
| N8—Mn3—Mn4—O5i | 11.39 (17) | O7—Mn3—N6—C13 | −48.7 (3) |
| N6—Mn3—Mn4—O5i | −161.85 (15) | N5—Mn3—N6—C13 | 45.7 (3) |
| O8—Mn3—Mn4—O9 | −81.61 (14) | N7—Mn3—N6—C13 | −142.4 (3) |
| O7—Mn3—Mn4—O9 | 92.37 (14) | N8—Mn3—N6—C13 | 128.6 (3) |
| N5—Mn3—Mn4—O9 | 6.01 (12) | Mn4—Mn3—N6—C13 | −56.4 (3) |
| N7—Mn3—Mn4—O9 | −173.99 (12) | O7—Mn3—N6—C14 | 73.6 (2) |
| N8—Mn3—Mn4—O9 | −83.20 (14) | N5—Mn3—N6—C14 | 168.1 (2) |
| N6—Mn3—Mn4—O9 | 103.56 (14) | N7—Mn3—N6—C14 | −20.1 (2) |
| O8—Mn3—Mn4—O10 | 98.10 (13) | N8—Mn3—N6—C14 | −109.0 (2) |
| O7—Mn3—Mn4—O10 | −87.92 (13) | Mn4—Mn3—N6—C14 | 65.9 (3) |
| N5—Mn3—Mn4—O10 | −174.28 (11) | O8—Mn3—N7—C16 | 44.0 (3) |
| N7—Mn3—Mn4—O10 | 5.72 (11) | O7—Mn3—N7—C16 | 130.8 (2) |
| N8—Mn3—Mn4—O10 | 96.51 (14) | N5—Mn3—N7—C16 | −92.7 (7) |
| N6—Mn3—Mn4—O10 | −76.73 (13) | N8—Mn3—N7—C16 | −44.8 (3) |
| O15—Mn5—Mn6—O14 | 176.28 (17) | N6—Mn3—N7—C16 | −140.7 (3) |
| N11—Mn5—Mn6—O14 | 89.38 (17) | Mn4—Mn3—N7—C16 | 87.4 (2) |
| N9—Mn5—Mn6—O14 | −91.44 (16) | O8—Mn3—N7—C15 | 176.5 (2) |
| N10—Mn5—Mn6—O14 | 176.65 (18) | O7—Mn3—N7—C15 | −96.7 (2) |
| N12—Mn5—Mn6—O14 | −7.0 (2) | N5—Mn3—N7—C15 | 39.8 (8) |
| O14—Mn5—Mn6—O15 | −176.28 (17) | N8—Mn3—N7—C15 | 87.7 (2) |
| N11—Mn5—Mn6—O15 | −86.89 (17) | N6—Mn3—N7—C15 | −8.2 (2) |
| N9—Mn5—Mn6—O15 | 92.28 (16) | Mn4—Mn3—N7—C15 | −140.2 (2) |
| N10—Mn5—Mn6—O15 | 0.37 (17) | O8—Mn3—N8—C18 | −47.0 (3) |
| N12—Mn5—Mn6—O15 | 176.7 (2) | N5—Mn3—N8—C18 | −142.5 (3) |
| O15—Mn5—Mn6—O11 | −8.24 (15) | N7—Mn3—N8—C18 | 45.7 (3) |
| O14—Mn5—Mn6—O11 | 175.48 (16) | N6—Mn3—N8—C18 | 129.0 (3) |
| N11—Mn5—Mn6—O11 | −95.13 (16) | Mn4—Mn3—N8—C18 | −45.9 (3) |
| N9—Mn5—Mn6—O11 | 84.04 (14) | N5—Mn3—N8—C19 | −18.7 (2) |
| N10—Mn5—Mn6—O11 | −7.87 (17) | N7—Mn3—N8—C19 | 169.4 (3) |
| N12—Mn5—Mn6—O11 | 168.44 (18) | N6—Mn3—N8—C19 | −107.2 (2) |
| O15—Mn5—Mn6—O6 | 173.33 (16) | Mn4—Mn3—N8—C19 | 77.9 (3) |
| O14—Mn5—Mn6—O6 | −2.95 (16) | O15—Mn5—N9—C30 | −175.0 (3) |
| N11—Mn5—Mn6—O6 | 86.44 (16) | O14—Mn5—N9—C30 | 98.2 (3) |
| N9—Mn5—Mn6—O6 | −94.39 (15) | N11—Mn5—N9—C30 | −43.2 (10) |
| N10—Mn5—Mn6—O6 | 173.70 (16) | N10—Mn5—N9—C30 | −85.9 (3) |
| N12—Mn5—Mn6—O6 | −10.0 (2) | N12—Mn5—N9—C30 | 9.4 (3) |
| O15—Mn5—Mn6—O13 | −94.82 (13) | Mn6—Mn5—N9—C30 | 141.4 (3) |
| O14—Mn5—Mn6—O13 | 88.90 (14) | O15—Mn5—N9—C21 | −43.2 (3) |
| N11—Mn5—Mn6—O13 | 178.29 (13) | O14—Mn5—N9—C21 | −130.0 (3) |
| N9—Mn5—Mn6—O13 | −2.54 (12) | N11—Mn5—N9—C21 | 88.6 (9) |
| N10—Mn5—Mn6—O13 | −94.45 (15) | N10—Mn5—N9—C21 | 45.9 (3) |
| N12—Mn5—Mn6—O13 | 81.86 (17) | N12—Mn5—N9—C21 | 141.1 (3) |
| O15—Mn5—Mn6—O12 | 85.29 (14) | Mn6—Mn5—N9—C21 | −86.9 (3) |
| O14—Mn5—Mn6—O12 | −90.99 (14) | O15—Mn5—N10—C24 | −74.8 (3) |
| N11—Mn5—Mn6—O12 | −1.60 (14) | N11—Mn5—N10—C24 | 18.1 (3) |
| N9—Mn5—Mn6—O12 | 177.57 (12) | N9—Mn5—N10—C24 | −168.9 (3) |
| N10—Mn5—Mn6—O12 | 85.66 (15) | N12—Mn5—N10—C24 | 107.7 (3) |
| N12—Mn5—Mn6—O12 | −98.03 (17) | Mn6—Mn5—N10—C24 | −75.0 (3) |
| O2—Mn1—O1—Mn2 | 3.75 (13) | O15—Mn5—N10—C23 | 46.4 (3) |
| N3—Mn1—O1—Mn2 | −88.59 (14) | N11—Mn5—N10—C23 | 139.3 (3) |
| N1—Mn1—O1—Mn2 | 99.07 (15) | N9—Mn5—N10—C23 | −47.7 (3) |
| N2—Mn1—O1—Mn2 | −172.37 (14) | N12—Mn5—N10—C23 | −131.1 (3) |
| O2—Mn2—O1—Mn1 | −3.56 (12) | Mn6—Mn5—N10—C23 | 46.1 (3) |
| O5—Mn2—O1—Mn1 | −178.38 (12) | O15—Mn5—N11—C26 | −129.2 (3) |
| O3—Mn2—O1—Mn1 | 91.53 (11) | O14—Mn5—N11—C26 | −42.4 (3) |
| O4—Mn2—O1—Mn1 | −94.63 (12) | N9—Mn5—N11—C26 | 98.9 (9) |
| O1—Mn1—O2—Mn2 | −3.70 (13) | N10—Mn5—N11—C26 | 142.0 (4) |
| N3—Mn1—O2—Mn2 | 90.28 (14) | N12—Mn5—N11—C26 | 46.8 (3) |
| N1—Mn1—O2—Mn2 | −96.68 (14) | Mn6—Mn5—N11—C26 | −85.7 (3) |
| N4—Mn1—O2—Mn2 | −179.97 (15) | O15—Mn5—N11—C25 | 100.1 (4) |
| O1—Mn2—O2—Mn1 | 3.59 (12) | O14—Mn5—N11—C25 | −173.1 (3) |
| O6—Mn2—O2—Mn1 | −169.83 (12) | N9—Mn5—N11—C25 | −31.7 (11) |
| O3—Mn2—O2—Mn1 | −83.28 (12) | N10—Mn5—N11—C25 | 11.3 (4) |
| O4—Mn2—O2—Mn1 | 105.40 (13) | N12—Mn5—N11—C25 | −83.8 (4) |
| O1—Mn2—O3—C31 | 178.5 (3) | Mn6—Mn5—N11—C25 | 143.7 (3) |
| O2—Mn2—O3—C31 | −100.7 (3) | O14—Mn5—N12—C29 | −74.7 (3) |
| O5—Mn2—O3—C31 | 87.0 (3) | N11—Mn5—N12—C29 | −169.2 (3) |
| O6—Mn2—O3—C31 | −6.9 (3) | N9—Mn5—N12—C29 | 18.9 (3) |
| O4—Mn2—O3—C31 | 30.2 (6) | N10—Mn5—N12—C29 | 107.4 (3) |
| Mn1—Mn2—O3—C31 | −140.8 (3) | Mn6—Mn5—N12—C29 | −69.9 (3) |
| Mn2—O3—C31—O13i | −141.3 (3) | N11—Mn5—N12—C28 | −46.4 (4) |
| Mn2—O3—C31—O10 | 38.1 (4) | N9—Mn5—N12—C28 | 141.7 (4) |
| O1—Mn2—O5—Mn4i | −48.7 (2) | N10—Mn5—N12—C28 | −129.8 (4) |
| O6—Mn2—O5—Mn4i | 125.2 (2) | Mn6—Mn5—N12—C28 | 52.9 (4) |
| O3—Mn2—O5—Mn4i | 38.9 (2) | C10—N1—C1—C2 | −68.1 (5) |
| O4—Mn2—O5—Mn4i | −150.8 (2) | Mn1—N1—C1—C2 | 61.3 (5) |
| Mn1—Mn2—O5—Mn4i | −50.2 (3) | N1—C1—C2—C3 | −64.4 (6) |
| O2—Mn2—O6—Mn6 | −143.2 (2) | C1—C2—C3—N2 | 65.0 (6) |
| O5—Mn2—O6—Mn6 | 32.5 (2) | C4—N2—C3—C2 | −178.6 (4) |
| O3—Mn2—O6—Mn6 | 122.4 (2) | Mn1—N2—C3—C2 | −60.1 (5) |
| O4—Mn2—O6—Mn6 | −50.7 (2) | C3—N2—C4—C5 | 171.2 (3) |
| Mn1—Mn2—O6—Mn6 | −152.32 (16) | Mn1—N2—C4—C5 | 45.8 (4) |
| O14—Mn6—O6—Mn2 | 54.3 (2) | C6—N3—C5—C4 | 169.5 (4) |
| O11—Mn6—O6—Mn2 | −122.6 (2) | Mn1—N3—C5—C4 | 36.2 (4) |
| O13—Mn6—O6—Mn2 | −35.7 (2) | N2—C4—C5—N3 | −55.4 (5) |
| O12—Mn6—O6—Mn2 | 151.3 (2) | C5—N3—C6—C7 | −71.2 (6) |
| Mn5—Mn6—O6—Mn2 | 56.2 (3) | Mn1—N3—C6—C7 | 58.4 (5) |
| O8—Mn3—O7—Mn4 | 4.13 (11) | N3—C6—C7—C8 | −64.0 (6) |
| N5—Mn3—O7—Mn4 | 99.20 (13) | C9—N4—C8—C7 | 176.7 (4) |
| N7—Mn3—O7—Mn4 | −88.33 (12) | Mn1—N4—C8—C7 | −63.7 (4) |
| N6—Mn3—O7—Mn4 | −171.69 (12) | C6—C7—C8—N4 | 67.9 (5) |
| O8—Mn4—O7—Mn3 | −3.95 (11) | C8—N4—C9—C10 | 169.7 (4) |
| O11—Mn4—O7—Mn3 | −179.41 (11) | Mn1—N4—C9—C10 | 44.9 (4) |
| O9—Mn4—O7—Mn3 | −95.13 (11) | C1—N1—C10—C9 | 173.2 (4) |
| O10—Mn4—O7—Mn3 | 91.48 (11) | Mn1—N1—C10—C9 | 39.8 (5) |
| O7—Mn3—O8—Mn4 | −4.14 (11) | N4—C9—C10—N1 | −56.6 (5) |
| N5—Mn3—O8—Mn4 | −98.28 (13) | C20—N5—C11—C12 | −68.6 (4) |
| N7—Mn3—O8—Mn4 | 89.24 (12) | Mn3—N5—C11—C12 | 60.9 (4) |
| N8—Mn3—O8—Mn4 | 178.82 (12) | N5—C11—C12—C13 | −65.1 (5) |
| O7—Mn4—O8—Mn3 | 3.99 (11) | C14—N6—C13—C12 | 179.2 (3) |
| O5i—Mn4—O8—Mn3 | −170.59 (11) | Mn3—N6—C13—C12 | −60.8 (4) |
| O9—Mn4—O8—Mn3 | 103.12 (11) | C11—C12—C13—N6 | 65.8 (4) |
| O10—Mn4—O8—Mn3 | −83.75 (11) | C13—N6—C14—C15 | 171.1 (3) |
| O13i—C31—O10—Mn4 | 36.4 (4) | Mn3—N6—C14—C15 | 44.9 (3) |
| O3—C31—O10—Mn4 | −142.9 (2) | C16—N7—C15—C14 | 169.8 (3) |
| O8—Mn4—O10—C31 | −97.8 (3) | Mn3—N7—C15—C14 | 35.5 (4) |
| O7—Mn4—O10—C31 | −179.2 (3) | N6—C14—C15—N7 | −54.1 (4) |
| O11—Mn4—O10—C31 | 88.7 (3) | C15—N7—C16—C17 | −71.1 (4) |
| O5i—Mn4—O10—C31 | −4.7 (3) | Mn3—N7—C16—C17 | 59.6 (4) |
| O9—Mn4—O10—C31 | 40.3 (7) | N7—C16—C17—C18 | −65.4 (5) |
| Mn3—Mn4—O10—C31 | −138.1 (3) | C19—N8—C18—C17 | 177.0 (3) |
| O7—Mn4—O11—Mn6 | −49.4 (2) | Mn3—N8—C18—C17 | −61.6 (4) |
| O5i—Mn4—O11—Mn6 | 125.6 (2) | C16—C17—C18—N8 | 66.8 (4) |
| O9—Mn4—O11—Mn6 | −148.7 (2) | C18—N8—C19—C20 | 172.5 (3) |
| O10—Mn4—O11—Mn6 | 39.1 (2) | Mn3—N8—C19—C20 | 45.2 (3) |
| Mn3—Mn4—O11—Mn6 | −50.0 (3) | N8—C19—C20—N5 | −55.6 (4) |
| O15—Mn6—O11—Mn4 | 140.5 (2) | C11—N5—C20—C19 | 171.3 (3) |
| O6—Mn6—O11—Mn4 | −35.3 (2) | Mn3—N5—C20—C19 | 38.2 (4) |
| O13—Mn6—O11—Mn4 | −125.7 (2) | C30—N9—C21—C22 | 71.0 (5) |
| O12—Mn6—O11—Mn4 | 47.1 (2) | Mn5—N9—C21—C22 | −59.4 (5) |
| Mn5—Mn6—O11—Mn4 | 145.89 (16) | N9—C21—C22—C23 | 64.0 (6) |
| O14—Mn6—O13—C31i | 178.3 (3) | C24—N10—C23—C22 | −176.9 (3) |
| O15—Mn6—O13—C31i | 96.8 (3) | Mn5—N10—C23—C22 | 63.5 (4) |
| O11—Mn6—O13—C31i | 4.9 (3) | C21—C22—C23—N10 | −66.7 (5) |
| O6—Mn6—O13—C31i | −89.4 (3) | C23—N10—C24—C25 | −170.3 (4) |
| Mn5—Mn6—O13—C31i | 137.5 (3) | Mn5—N10—C24—C25 | −44.8 (4) |
| O15—Mn5—O14—Mn6 | −2.57 (12) | N10—C24—C25—N11 | 56.0 (5) |
| N11—Mn5—O14—Mn6 | −95.08 (15) | C26—N11—C25—C24 | −172.1 (4) |
| N9—Mn5—O14—Mn6 | 91.33 (14) | Mn5—N11—C25—C24 | −38.8 (5) |
| N12—Mn5—O14—Mn6 | 174.78 (15) | C25—N11—C26—C27 | 68.5 (6) |
| O15—Mn6—O14—Mn5 | 2.45 (11) | Mn5—N11—C26—C27 | −60.6 (6) |
| O6—Mn6—O14—Mn5 | 177.85 (11) | N11—C26—C27—C28 | 65.0 (7) |
| O13—Mn6—O14—Mn5 | −91.44 (11) | C29—N12—C28—C27 | −178.9 (4) |
| O12—Mn6—O14—Mn5 | 94.94 (12) | Mn5—N12—C28—C27 | 61.0 (5) |
| O14—Mn5—O15—Mn6 | 2.54 (12) | C26—C27—C28—N12 | −66.4 (6) |
| N11—Mn5—O15—Mn6 | 96.91 (14) | C28—N12—C29—C30 | −168.7 (4) |
| N9—Mn5—O15—Mn6 | −90.75 (13) | Mn5—N12—C29—C30 | −43.7 (4) |
| N10—Mn5—O15—Mn6 | −179.73 (13) | C21—N9—C30—C29 | −169.8 (4) |
| O14—Mn6—O15—Mn5 | −2.46 (11) | Mn5—N9—C30—C29 | −35.6 (5) |
| O11—Mn6—O15—Mn5 | 173.93 (11) | N12—C29—C30—N9 | 53.5 (5) |
| O13—Mn6—O15—Mn5 | 86.95 (11) | O31ii—Cl5A—O20—Cl5B | 162.0 (15) |
| O12—Mn6—O15—Mn5 | −99.20 (11) | O26A—Cl4—O29—O30 | 14 (2) |
| O2—Mn1—N1—C10 | −101.6 (4) | O26A—Cl4—O30—O29 | −168 (2) |
| O1—Mn1—N1—C10 | 171.9 (4) |
Symmetry codes: (i) −x+1, −y+1, −z+1; (ii) x−1, y, z; (iii) x+1, y, z.
Hydrogen-bond geometry (Å, °)
| D—H···A | D—H | H···A | D···A | D—H···A |
| N6—H6···Cl1 | 0.93 | 2.51 | 3.413 (3) | 163 |
| N8—H8···Cl1 | 0.93 | 2.27 | 3.198 (3) | 174 |
| O4—H4E···Cl2 | 0.82 (4) | 2.33 (3) | 3.106 (3) | 158 (4) |
| N1—H1···Cl2 | 0.93 | 2.49 | 3.285 (4) | 143 |
| N2—H2···Cl3 | 0.93 | 2.46 | 3.338 (4) | 157 |
| N4—H4···Cl3 | 0.93 | 2.46 | 3.384 (4) | 174 |
| N11—H11···Cl4 | 0.93 | 2.37 | 3.148 (5) | 141 |
| N10—H10···Cl5A | 0.93 | 2.68 | 3.523 (6) | 152 |
| N10—H10···Cl5B | 0.93 | 2.49 | 3.185 (12) | 132 |
| N5—H5···Cl6 | 0.93 | 2.32 | 3.138 (4) | 147 |
| O4—H4D···O14 | 0.82 (4) | 1.90 (2) | 2.701 (4) | 166 (4) |
| O5—H5D···O13 | 0.84 (4) | 1.86 (2) | 2.674 (3) | 164 (4) |
| O6—H6D···O10 | 0.82 (4) | 1.88 (2) | 2.681 (4) | 166 (4) |
| O9—H9E···O1i | 0.87 (3) | 1.83 (2) | 2.665 (4) | 161 (4) |
| O11—H11D···O3i | 0.83 (2) | 1.85 (2) | 2.675 (3) | 172 (4) |
| O12—H12C···O7 | 0.83 (5) | 1.88 (5) | 2.704 (4) | 171 (4) |
| O12—H12D···Cl4 | 0.82 (2) | 2.26 (2) | 3.065 (4) | 165 (4) |
| N2—H2···O28 | 0.93 | 2.08 | 2.952 (9) | 156 |
| N3—H3···O3 | 0.93 | 1.99 | 2.798 (4) | 144 |
| N7—H7···O10 | 0.93 | 1.99 | 2.790 (4) | 143 |
| N9—H9···O13 | 0.93 | 2.14 | 2.896 (4) | 138 |
| N10—H10···O20 | 0.93 | 2.02 | 2.912 (10) | 160 |
Symmetry codes: (i) −x+1, −y+1, −z+1.
Footnotes
Supplementary data and figures for this paper are available from the IUCr electronic archives (Reference: CI5168).
References
- Aubin, S. M. J., Wemple, M. W., Adams, D. M., Tsai, H.-L., Christou, G. & Hendrickson, D. N. (1996). J. Am. Chem. Soc.118, 7746–7754.
- Bian, S.-D., Jia, J.-H. & Wang, Q.-M. (2009). J. Am. Chem. Soc.131, 3422–3423. [DOI] [PubMed]
- Bian, G.-Q., Kuroda-Sowa, T., Konaka, H., Hatano, M., Maekawa, M., Munakata, M., Miyasaka, H. & Yamashita, M. (2004). Inorg. Chem.43, 4790–4792. [DOI] [PubMed]
- Bruker (2009). APEX2 and SAINT Bruker AXS Inc., Madison, Wisconsin, USA.
- Chakov, N. E., Zakharov, L. N., Rheingold, A. L., Abboud, K. A. & Christou, G. (2005). Inorg. Chem.44, 4555–4567. [DOI] [PubMed]
- Kuroda-Sowa, T., Lam, M., Rheingold, A. L., Frommen, C., Reiff, W. M., Nakano, M., Yoo, J., Maniero, A. L., Brunel, L.-C. & Hendrickson, D. N. (2001). Inorg. Chem.40, 6469–6480. [DOI] [PubMed]
- Lis, T. (1980). Acta Cryst. B36, 2042–2046.
- Palenik, T. J. (1997). Inorg. Chem.36, 4888–4890. [DOI] [PubMed]
- Sheldrick, G. M. (1996). SADABS University of Göttingen, Germany.
- Sheldrick, G. M. (2008). Acta Cryst. A64, 112–122. [DOI] [PubMed]
- Sun, A., Ruiz, D., Rumberger, E., Incarvito, C. D., Folting, K., Rheingold, A. L., Christou, G. & Hendrickson, D. N. (1998). Inorg. Chem.37, 4758–4759. [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Crystal structure: contains datablocks I, global. DOI: 10.1107/S1600536810034999/ci5168sup1.cif
Structure factors: contains datablocks I. DOI: 10.1107/S1600536810034999/ci5168Isup2.hkl
Additional supplementary materials: crystallographic information; 3D view; checkCIF report