Abstract
In the title compound, C11H8Cl2N2O2, the 2,4-dichlorophenoxy and 1H-pyrazole groups are almost planar [r.m.s. deviations of 0.0157 and 0.0008 Å, respectively] and are oriented at a dihedral angle of 64.17 (5)° with respect to one another. In the crystal, the molecules are stabilized in the form of dimers due to inversion-related C—H⋯O hydrogen bonds, with R 2 2(10) ring motifs.
Related literature
Aryloxyacetic acid and its various derivatives are used as herbicides and pesticides, see: Crafts (1957 ▶). For our work on the synthesis of heterocyclic compounds, see: Khan et al. (2009 ▶). For a related structure, see: Wang et al. (2009 ▶). For graph-set notation, see: Bernstein et al. (1995 ▶).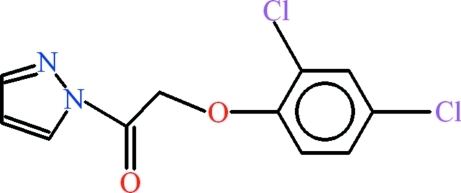
Experimental
Crystal data
C11H8Cl2N2O2
M r = 271.09
Triclinic,

a = 4.2030 (1) Å
b = 10.3074 (3) Å
c = 13.4966 (4) Å
α = 87.510 (2)°
β = 83.774 (1)°
γ = 88.335 (1)°
V = 580.53 (3) Å3
Z = 2
Mo Kα radiation
μ = 0.55 mm−1
T = 296 K
0.30 × 0.22 × 0.18 mm
Data collection
Bruker Kappa APEXII CCD diffractometer
Absorption correction: multi-scan (SADABS; Bruker, 2005 ▶) T min = 0.982, T max = 0.988
10353 measured reflections
2861 independent reflections
2232 reflections with I > 2σ(I)
R int = 0.025
Refinement
R[F 2 > 2σ(F 2)] = 0.035
wR(F 2) = 0.093
S = 1.04
2861 reflections
154 parameters
H-atom parameters constrained
Δρmax = 0.25 e Å−3
Δρmin = −0.29 e Å−3
Data collection: APEX2 (Bruker, 2009 ▶); cell refinement: SAINT (Bruker, 2009 ▶); data reduction: SAINT; program(s) used to solve structure: SHELXS97 (Sheldrick, 2008 ▶); program(s) used to refine structure: SHELXL97 (Sheldrick, 2008 ▶); molecular graphics: ORTEP-3 for Windows (Farrugia, 1997 ▶) and PLATON (Spek, 2009 ▶); software used to prepare material for publication: WinGX (Farrugia, 1999 ▶) and PLATON.
Supplementary Material
Crystal structure: contains datablocks global, I. DOI: 10.1107/S1600536810035087/si2292sup1.cif
Structure factors: contains datablocks I. DOI: 10.1107/S1600536810035087/si2292Isup2.hkl
Additional supplementary materials: crystallographic information; 3D view; checkCIF report
Table 1. Hydrogen-bond geometry (Å, °).
| D—H⋯A | D—H | H⋯A | D⋯A | D—H⋯A |
|---|---|---|---|---|
| C11—H11⋯O2i | 0.93 | 2.42 | 3.339 (2) | 170 |
Symmetry code: (i)  .
.
Acknowledgments
The authors acknowledge the provision of funds for the purchase of diffractometer and encouragement by Dr Muhammad Akram Chaudhary, Vice Chancellor, University of Sargodha, Pakistan.
supplementary crystallographic information
Comment
Aryloxyacetic acid and its various derivatives are used as herbicides and pesticides (Crafts, 1957). During our research on the synthesis of heterocyclic compounds in our laboratories (Khan et al., 2009), we have isolated the title compound (I, Fig. 1).
The crystal structure of 5-(2,4-dichlorophenoxymethyl)-1,3,4-thiadiazol-2-amine has been published (Wang et al., 2009) which is related to the title compound.
In the title compound, 2,4-dichlorophenoxy group A (O1/C1—C6/CL1/CL2) and 1H-pyrazole group B (N1/N2/C9–C11) are planar with r. m. s. deviations of 0.0157 and 0.0008 Å, respectively. The dihedral angle between A/B is 64.17 (5)°. The central group C (C7/C8/O2) is of course planar. The dihedral angle between A/C and B/C is 69.23 (8) and 5.07 (25)°, respectively. The molecules are stabilized in the form of dimers (Table 1, Fig. 2) due to inversion related C—H···O type of H-bondings with R22(10) ring motifs (Bernstein et al., 1995).
Experimental
A mixture of 2,4-dichlorophenoxyacetic acid (0.5 g; 2.25 mmole) and 1 ml of thionyl chloride was heated under reflux for 1 h. Then an excess of pyrazole (0.5 g) in 5 ml of chloroform was added to the refluxing mixture and heated for a further period of 1.5 h. The solvents were removed and the residue dissolved in chloroform and washed with saturated sodium bicarbonate, dried and let crystallize to give pale brown prisms of (I).
Yield, 84%.
Refinement
The H-atoms were positioned geometrically (C–H = 0.93–0.97 Å) and were included in the refinement in the riding model approximation, with Uiso(H) = xUeq(C), where x = 1.2 for all H-atoms.
Figures
Fig. 1.
View of the title compound with the atom numbering scheme. The thermal ellipsoids are drawn at the 50% probability level. H-atoms are shown as small spheres of arbitrary radii.
Fig. 2.
Packing section of the title compound (PLATON: Spek, 2009) showing that molecules are stabilized in the form of dimers.
Crystal data
| C11H8Cl2N2O2 | Z = 2 |
| Mr = 271.09 | F(000) = 276 |
| Triclinic, P1 | Dx = 1.551 Mg m−3 |
| Hall symbol: -P 1 | Mo Kα radiation, λ = 0.71073 Å |
| a = 4.2030 (1) Å | Cell parameters from 2920 reflections |
| b = 10.3074 (3) Å | θ = 2.6–27.9° |
| c = 13.4966 (4) Å | µ = 0.55 mm−1 |
| α = 87.510 (2)° | T = 296 K |
| β = 83.774 (1)° | Prismatic, pale brown |
| γ = 88.335 (1)° | 0.30 × 0.22 × 0.18 mm |
| V = 580.53 (3) Å3 |
Data collection
| Bruker Kappa APEXII CCD diffractometer | 2861 independent reflections |
| Radiation source: fine-focus sealed tube | 2232 reflections with I > 2σ(I) |
| graphite | Rint = 0.025 |
| Detector resolution: 7.50 pixels mm-1 | θmax = 28.4°, θmin = 2.0° |
| ω scans | h = −5→5 |
| Absorption correction: multi-scan (SADABS; Bruker, 2005) | k = −13→13 |
| Tmin = 0.982, Tmax = 0.988 | l = −17→17 |
| 10353 measured reflections |
Refinement
| Refinement on F2 | Primary atom site location: structure-invariant direct methods |
| Least-squares matrix: full | Secondary atom site location: difference Fourier map |
| R[F2 > 2σ(F2)] = 0.035 | Hydrogen site location: inferred from neighbouring sites |
| wR(F2) = 0.093 | H-atom parameters constrained |
| S = 1.04 | w = 1/[σ2(Fo2) + (0.0378P)2 + 0.1536P] where P = (Fo2 + 2Fc2)/3 |
| 2861 reflections | (Δ/σ)max = 0.001 |
| 154 parameters | Δρmax = 0.25 e Å−3 |
| 0 restraints | Δρmin = −0.29 e Å−3 |
Special details
| Geometry. Bond distances, angles etc. have been calculated using the rounded fractional coordinates. All su's are estimated from the variances of the (full) variance-covariance matrix. The cell e.s.d.'s are taken into account in the estimation of distances, angles and torsion angles |
| Refinement. Refinement of F2 against ALL reflections. The weighted R-factor wR and goodness of fit S are based on F2, conventional R-factors R are based on F, with F set to zero for negative F2. The threshold expression of F2 > σ(F2) is used only for calculating R-factors(gt) etc. and is not relevant to the choice of reflections for refinement. R-factors based on F2 are statistically about twice as large as those based on F, and R- factors based on ALL data will be even larger. |
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2)
| x | y | z | Uiso*/Ueq | ||
| Cl1 | 0.18429 (10) | 1.01977 (4) | 0.34325 (3) | 0.0568 (2) | |
| Cl2 | 0.65932 (16) | 0.65004 (6) | 0.58253 (4) | 0.0775 (2) | |
| O1 | 0.5309 (3) | 0.88439 (11) | 0.18509 (8) | 0.0484 (4) | |
| O2 | 0.4918 (3) | 0.66285 (12) | 0.08285 (9) | 0.0547 (4) | |
| N1 | 0.8767 (3) | 0.72296 (12) | −0.03954 (9) | 0.0409 (4) | |
| N2 | 1.0808 (3) | 0.81744 (14) | −0.07931 (11) | 0.0508 (5) | |
| C1 | 0.5754 (3) | 0.82450 (15) | 0.27460 (11) | 0.0409 (5) | |
| C2 | 0.4121 (3) | 0.87940 (15) | 0.35859 (12) | 0.0414 (5) | |
| C3 | 0.4354 (4) | 0.82649 (16) | 0.45278 (12) | 0.0484 (5) | |
| C4 | 0.6248 (4) | 0.71690 (17) | 0.46376 (13) | 0.0499 (5) | |
| C5 | 0.7924 (4) | 0.66131 (17) | 0.38241 (13) | 0.0534 (6) | |
| C6 | 0.7676 (4) | 0.71501 (17) | 0.28800 (13) | 0.0498 (5) | |
| C7 | 0.7588 (4) | 0.86184 (16) | 0.10197 (12) | 0.0456 (5) | |
| C8 | 0.6884 (4) | 0.74046 (15) | 0.05091 (11) | 0.0404 (5) | |
| C9 | 1.2064 (5) | 0.77078 (19) | −0.16381 (13) | 0.0589 (6) | |
| C10 | 1.0901 (5) | 0.6484 (2) | −0.17990 (13) | 0.0609 (7) | |
| C11 | 0.8813 (4) | 0.62017 (17) | −0.09989 (13) | 0.0523 (6) | |
| H3 | 0.32501 | 0.86416 | 0.50819 | 0.0580* | |
| H5 | 0.92191 | 0.58786 | 0.39101 | 0.0641* | |
| H6 | 0.88072 | 0.67744 | 0.23299 | 0.0597* | |
| H7A | 0.97086 | 0.85358 | 0.12400 | 0.0547* | |
| H7B | 0.75702 | 0.93557 | 0.05484 | 0.0547* | |
| H9 | 1.35614 | 0.81406 | −0.20817 | 0.0706* | |
| H10 | 1.14575 | 0.59735 | −0.23456 | 0.0731* | |
| H11 | 0.76304 | 0.54516 | −0.08799 | 0.0627* |
Atomic displacement parameters (Å2)
| U11 | U22 | U33 | U12 | U13 | U23 | |
| Cl1 | 0.0547 (3) | 0.0481 (2) | 0.0649 (3) | 0.0094 (2) | 0.0060 (2) | −0.0084 (2) |
| Cl2 | 0.1045 (4) | 0.0712 (3) | 0.0552 (3) | 0.0028 (3) | −0.0074 (3) | 0.0102 (2) |
| O1 | 0.0497 (6) | 0.0498 (7) | 0.0447 (6) | 0.0070 (5) | −0.0004 (5) | −0.0064 (5) |
| O2 | 0.0566 (7) | 0.0542 (7) | 0.0528 (7) | −0.0212 (6) | 0.0022 (5) | −0.0033 (5) |
| N1 | 0.0431 (7) | 0.0386 (7) | 0.0406 (7) | −0.0048 (5) | −0.0022 (5) | −0.0017 (5) |
| N2 | 0.0541 (8) | 0.0445 (8) | 0.0516 (8) | −0.0087 (6) | 0.0033 (6) | 0.0038 (6) |
| C1 | 0.0385 (8) | 0.0399 (8) | 0.0448 (8) | −0.0053 (6) | −0.0030 (6) | −0.0079 (6) |
| C2 | 0.0361 (8) | 0.0366 (8) | 0.0511 (9) | −0.0043 (6) | 0.0006 (6) | −0.0085 (6) |
| C3 | 0.0473 (9) | 0.0488 (9) | 0.0476 (9) | −0.0087 (7) | 0.0058 (7) | −0.0087 (7) |
| C4 | 0.0561 (10) | 0.0465 (9) | 0.0476 (9) | −0.0078 (8) | −0.0065 (7) | −0.0006 (7) |
| C5 | 0.0583 (10) | 0.0432 (9) | 0.0600 (11) | 0.0039 (8) | −0.0119 (8) | −0.0065 (8) |
| C6 | 0.0516 (9) | 0.0471 (9) | 0.0509 (9) | 0.0043 (7) | −0.0042 (7) | −0.0130 (7) |
| C7 | 0.0496 (9) | 0.0410 (8) | 0.0451 (8) | −0.0060 (7) | 0.0028 (7) | −0.0059 (7) |
| C8 | 0.0407 (8) | 0.0400 (8) | 0.0408 (8) | −0.0041 (6) | −0.0049 (6) | −0.0005 (6) |
| C9 | 0.0600 (11) | 0.0630 (12) | 0.0498 (10) | 0.0000 (9) | 0.0086 (8) | 0.0045 (8) |
| C10 | 0.0696 (12) | 0.0647 (12) | 0.0471 (10) | 0.0031 (10) | 0.0024 (8) | −0.0134 (9) |
| C11 | 0.0586 (10) | 0.0470 (9) | 0.0524 (10) | −0.0032 (8) | −0.0067 (8) | −0.0114 (8) |
Geometric parameters (Å, °)
| Cl1—C2 | 1.7290 (15) | C4—C5 | 1.376 (2) |
| Cl2—C4 | 1.7362 (18) | C5—C6 | 1.380 (2) |
| O1—C1 | 1.3613 (18) | C7—C8 | 1.506 (2) |
| O1—C7 | 1.417 (2) | C9—C10 | 1.400 (3) |
| O2—C8 | 1.200 (2) | C10—C11 | 1.343 (3) |
| N1—N2 | 1.3695 (19) | C3—H3 | 0.9300 |
| N1—C8 | 1.397 (2) | C5—H5 | 0.9300 |
| N1—C11 | 1.363 (2) | C6—H6 | 0.9300 |
| N2—C9 | 1.309 (2) | C7—H7A | 0.9700 |
| C1—C2 | 1.393 (2) | C7—H7B | 0.9700 |
| C1—C6 | 1.386 (2) | C9—H9 | 0.9300 |
| C2—C3 | 1.374 (2) | C10—H10 | 0.9300 |
| C3—C4 | 1.375 (2) | C11—H11 | 0.9300 |
| Cl1···O1 | 2.8447 (12) | C2···C6i | 3.476 (2) |
| Cl1···C1i | 3.5263 (15) | C3···C5i | 3.474 (2) |
| Cl1···C2i | 3.5736 (14) | C5···C2viii | 3.469 (2) |
| Cl1···Cl2ii | 3.6862 (7) | C5···C3viii | 3.474 (2) |
| Cl2···Cl1ii | 3.6862 (7) | C6···O2 | 3.188 (2) |
| Cl1···H9iii | 3.0200 | C6···C1viii | 3.592 (2) |
| Cl1···H3iv | 3.0300 | C6···C2viii | 3.476 (2) |
| Cl2···H10v | 3.1400 | C6···C8 | 3.252 (2) |
| Cl2···H5vi | 3.0100 | C7···N2iii | 3.387 (2) |
| O1···Cl1 | 2.8447 (12) | C8···N2i | 3.313 (2) |
| O1···O2 | 2.7368 (17) | C8···C6 | 3.252 (2) |
| O2···N2i | 3.2665 (19) | C9···C11viii | 3.370 (3) |
| O2···O1 | 2.7368 (17) | C9···N1viii | 3.445 (2) |
| O2···N1i | 3.2466 (18) | C11···C9i | 3.370 (3) |
| O2···C1 | 3.1942 (19) | C11···O2vii | 3.339 (2) |
| O2···C6 | 3.188 (2) | C6···H7A | 2.6600 |
| O2···C11vii | 3.339 (2) | C7···H6 | 2.6200 |
| O1···H7Ai | 2.6100 | C8···H6 | 2.7200 |
| O2···H6 | 2.7500 | H3···Cl1iv | 3.0300 |
| O2···H11 | 2.7700 | H5···Cl2vi | 3.0100 |
| O2···H11vii | 2.4200 | H6···O2 | 2.7500 |
| N1···O2viii | 3.2466 (18) | H6···C7 | 2.6200 |
| N1···C9i | 3.445 (2) | H6···C8 | 2.7200 |
| N2···O2viii | 3.2665 (19) | H6···H7A | 2.3000 |
| N2···C8viii | 3.313 (2) | H7A···O1viii | 2.6100 |
| N2···C7iii | 3.387 (2) | H7A···N2 | 2.7700 |
| N2···H7A | 2.7700 | H7A···C6 | 2.6600 |
| N2···H7B | 2.4800 | H7A···H6 | 2.3000 |
| N2···H7Biii | 2.7000 | H7B···N2 | 2.4800 |
| C1···Cl1viii | 3.5263 (14) | H7B···N2iii | 2.7000 |
| C1···O2 | 3.1942 (19) | H9···Cl1iii | 3.0200 |
| C1···C6i | 3.592 (2) | H10···Cl2ix | 3.1400 |
| C2···Cl1viii | 3.5736 (14) | H11···O2 | 2.7700 |
| C2···C5i | 3.469 (2) | H11···O2vii | 2.4200 |
| C1—O1—C7 | 118.85 (12) | N2—C9—C10 | 112.42 (17) |
| N2—N1—C8 | 120.45 (13) | C9—C10—C11 | 105.58 (16) |
| N2—N1—C11 | 111.72 (13) | N1—C11—C10 | 106.55 (16) |
| C8—N1—C11 | 127.80 (13) | C2—C3—H3 | 121.00 |
| N1—N2—C9 | 103.74 (14) | C4—C3—H3 | 121.00 |
| O1—C1—C2 | 116.28 (13) | C4—C5—H5 | 120.00 |
| O1—C1—C6 | 125.34 (14) | C6—C5—H5 | 120.00 |
| C2—C1—C6 | 118.38 (14) | C1—C6—H6 | 120.00 |
| Cl1—C2—C1 | 118.80 (12) | C5—C6—H6 | 120.00 |
| Cl1—C2—C3 | 119.68 (12) | O1—C7—H7A | 109.00 |
| C1—C2—C3 | 121.49 (14) | O1—C7—H7B | 109.00 |
| C2—C3—C4 | 118.88 (15) | C8—C7—H7A | 109.00 |
| Cl2—C4—C3 | 119.31 (13) | C8—C7—H7B | 109.00 |
| Cl2—C4—C5 | 119.64 (14) | H7A—C7—H7B | 108.00 |
| C3—C4—C5 | 121.04 (16) | N2—C9—H9 | 124.00 |
| C4—C5—C6 | 119.73 (16) | C10—C9—H9 | 124.00 |
| C1—C6—C5 | 120.47 (16) | C9—C10—H10 | 127.00 |
| O1—C7—C8 | 111.44 (13) | C11—C10—H10 | 127.00 |
| O2—C8—N1 | 121.06 (14) | N1—C11—H11 | 127.00 |
| O2—C8—C7 | 124.89 (14) | C10—C11—H11 | 127.00 |
| N1—C8—C7 | 114.05 (13) | ||
| C7—O1—C1—C2 | 160.01 (13) | C6—C1—C2—C3 | −0.8 (2) |
| C7—O1—C1—C6 | −20.0 (2) | O1—C1—C6—C5 | −179.24 (15) |
| C1—O1—C7—C8 | 85.03 (17) | C2—C1—C6—C5 | 0.8 (2) |
| C8—N1—N2—C9 | −177.90 (15) | Cl1—C2—C3—C4 | −178.20 (13) |
| C11—N1—N2—C9 | 0.21 (18) | C1—C2—C3—C4 | 0.0 (2) |
| N2—N1—C8—O2 | 174.44 (14) | C2—C3—C4—Cl2 | 179.37 (12) |
| N2—N1—C8—C7 | −6.0 (2) | C2—C3—C4—C5 | 0.8 (3) |
| C11—N1—C8—O2 | −3.3 (3) | Cl2—C4—C5—C6 | −179.41 (14) |
| C11—N1—C8—C7 | 176.21 (15) | C3—C4—C5—C6 | −0.9 (3) |
| N2—N1—C11—C10 | −0.18 (19) | C4—C5—C6—C1 | 0.0 (3) |
| C8—N1—C11—C10 | 177.76 (16) | O1—C7—C8—O2 | −9.1 (2) |
| N1—N2—C9—C10 | −0.2 (2) | O1—C7—C8—N1 | 171.34 (13) |
| O1—C1—C2—Cl1 | −2.56 (18) | N2—C9—C10—C11 | 0.1 (2) |
| O1—C1—C2—C3 | 179.19 (14) | C9—C10—C11—N1 | 0.1 (2) |
| C6—C1—C2—Cl1 | 177.44 (12) |
Symmetry codes: (i) x−1, y, z; (ii) −x+1, −y+2, −z+1; (iii) −x+2, −y+2, −z; (iv) −x, −y+2, −z+1; (v) x−1, y, z+1; (vi) −x+2, −y+1, −z+1; (vii) −x+1, −y+1, −z; (viii) x+1, y, z; (ix) x+1, y, z−1.
Hydrogen-bond geometry (Å, °)
| D—H···A | D—H | H···A | D···A | D—H···A |
| C11—H11···O2vii | 0.93 | 2.42 | 3.339 (2) | 170 |
Symmetry codes: (vii) −x+1, −y+1, −z.
Footnotes
Supplementary data and figures for this paper are available from the IUCr electronic archives (Reference: SI2292).
References
- Bernstein, J., Davis, R. E., Shimoni, L. & Chang, N.-L. (1995). Angew. Chem. Int. Ed. Engl.34, 1555–1573.
- Bruker (2005). SADABS Bruker AXS Inc., Madison, Wisconsin, USA.
- Bruker (2009). APEX2 and SAINT Bruker AXS Inc., Madison, Wisconsin, USA.
- Crafts, A. S. (1957). Adv. Pest Control Res.1, 39–79.
- Farrugia, L. J. (1997). J. Appl. Cryst.30, 565.
- Farrugia, L. J. (1999). J. Appl. Cryst.32, 837–838.
- Khan, M. A., Tahir, M. N., Ather, A. Q., Shaheen, M. & Khan, R. A. (2009). Acta Cryst. E65, o1615. [DOI] [PMC free article] [PubMed]
- Sheldrick, G. M. (2008). Acta Cryst. A64, 112–122. [DOI] [PubMed]
- Spek, A. L. (2009). Acta Cryst. D65, 148–155. [DOI] [PMC free article] [PubMed]
- Wang, Y., Wan, R., Han, F. & Wang, P. (2009). Acta Cryst. E65, o1761. [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Crystal structure: contains datablocks global, I. DOI: 10.1107/S1600536810035087/si2292sup1.cif
Structure factors: contains datablocks I. DOI: 10.1107/S1600536810035087/si2292Isup2.hkl
Additional supplementary materials: crystallographic information; 3D view; checkCIF report




