Abstract
Purpose
To non-invasively assess the shear stiffness of the thyroid gland in vivo in order to determine whether Magnetic Resonance Elastography (MRE) might hold clinical utility in the diagnosis of thyroid disease.
Materials and Methods
Quantitative parametric images of thyroid stiffness in normal volunteers and patients were produced and quantitative stiffness values measured. Average gland stiffness was determined by region of interest analysis of the parametric images. This technique was used to assess stiffness of the thyroid in normal individuals (n=12), patients with Hashimoto's thyroiditis (HT; n=5), and patients with a solitary benign (n=8) or malignant (n=2) thyroid nodule.
Results
Mean shear modulus of normal thyroid glands was 1.9 ± 0.6 kPa at 100 Hz and 1.3 ± 0.5 kPa at 80 Hz, while that of HT glands was 2.8 ± 0.6 kPa and 1.8 ± 0.6 kPa at 80 Hz, respectively (p=0.004 at 100 Hz). Elastographic parameters could not differentiate benign from malignant thyroid nodules in these small sample sizes.
Conclusion
We have developed a method for the application of MRE to the study of thyroid gland pathology. Results show that the HT gland can be differentiated from normal thyroid. The clinical utility of this imaging modality in the diagnosis and management of thyroid disease awaits further study.
Keywords: Magnetic Resonance Elastography, Thyroid gland, Shear Stiffness, Hashimoto Thyroiditis, Chronic Lymphocytic Thyroiditis, Thyroid Nodule
INTRODUCTION
Manual palpation is commonly used in clinical medicine to assess tissues. Hard masses in the thyroid, breast or prostate are considered suspicious for malignancy. The palpatory finding of homogeneous firmness in a normally soft organ such as thyroid or liver points the clinician towards further testing for Hashimoto’s thyroiditis (HT; also termed chronic lymphocytic thyroiditis), or hepatic fibrosis. However, the utility of palpation as a means of gathering clinical information is limited; tissues or organs must be accessible to the examiner in order to be palpated and the degree of firmness is only roughly quantifiable by palpation. The stiffness of surrounding tissues might alter the examiner’s assessment of the stiffness of a nodule and subtle changes in the degree of firmness over time might not be easily appreciated, especially between multiple examiners.
Magnetic Resonance Elastography (MRE) is a recently developed technique that can directly visualize and quantitatively measure propagating waves in materials such as biological tissues (1). The feasibility of the MRE method has been demonstrated in many organs (muscle, lung, brain, hyalin cartilage, liver, vascular wall), (2–11). Stiffness imaging affords advantages over palpation, including quantitation and the ability to follow changes. Images can also demonstrate deep portions of the gland that might not be accessible to palpation.
There are three basic features of the MRE protocol: impart shear waves into the organ of interest, detect the displacement of tissues as the waves traverse the tissue, and analyze the detected data to produce parametric images of the shear modulus.
We undertook the current study to determine whether we might be able to quantify thyroid gland stiffness in normal and pathologic states using MRE. In particular, we wished to determine whether HT might be differentiated form normal thyroid gland using this methodology, whether MRE might be able to distinguish thyroid nodules from normal thyroid gland and whether MRE can distinguish benign nodules from malignant thyroid nodules.
MATERIALS AND METHODS
Patients with HT were recruited for participation in the study from the Thyroid Clinic (n=5, M:F=1:4, mean age = 47.6y). All had diffuse goiter, elevated serum antithyroperoxidase antibodies or cytologic confirmation of the condition, and were clinically and biochemically euthyroid. In addition, we recruited patients with solitary thyroid nodules found on fine needle aspiration biopsy either to be benign or suspicious for malignancy (n=10, M:F = 1:9, mean age = 53.2 years). Of these patients, 2 had cytology suspicious for malignancy and subsequently underwent thyroidectomy; one was found to have papillary carcinoma and the other follicular thyroid carcinoma. In patients with thyroid nodules, MRE of the thyroid gland was performed after the fine needle aspiration (FNA). Normal adult volunteers (n=12, M:F = 3:9, mean age = 39.6 years) were documented to be without history of thyroid disease or contraindication to MRI. This study was approved by the Institutional Review Board. Informed consent was obtained from all subjects after the nature of the procedure was fully explained to them.
MRE data were acquired in a single 2D axial plane through the thyroid gland in patients lying supine in a 1.5T MRI scanner. If thyroid nodules were present was chosen to include the largest portion of the nodule. A custom-made receive-only surface coil was placed on the neck overlying the thyroid gland (Fig. 1). There was no evidence on the MRI images of artifact or signal abnormality caused by the surface coil. The entire examination took approximately 10 minutes of set-up time and 20 minutes of scanning time. A single axial 5mm thick scan plane was imaged with a 2D gradient echo acquisition with a flip angle of 60 degrees and a TR of 150ms and the minimal TE. The field of view was 14cm with an in-plane resolution of 256x160 pixels. The MR image acquisition was synchronized to the imparted harmonic shear waves. Six acquisitions were acquired, each at a different phase offset from the shear wave. Data sets were acquired using both 80 Hz shear waves and 100 Hz shear waves. The data sets were used to measure the displacement pattern as the shear waves traversed the tissue. Six phase offsets, separated by one third Pi, were used for both the 80 Hz and 100 Hz acquisition.
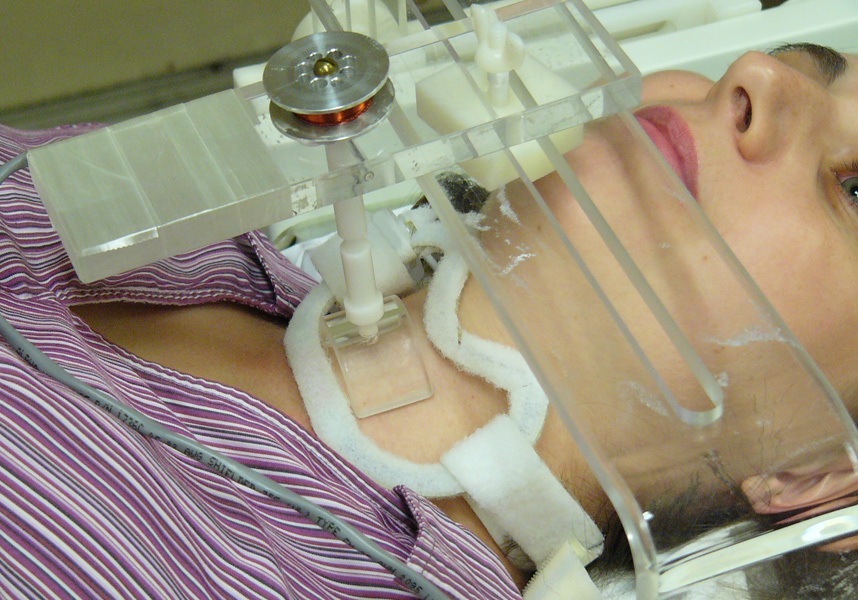
Figure 1.
Demonstration of the placement of a custom-made 8 cm receive-only MR surface coil over the thyroid gland. Contacts of a custom-made electromechanical transducer gently rest on the skin overlying the thyroid lobes.
Anatomic landmarks were obtained for region of interest (ROI) drawing with coregistered T1 weighted gradient echo (flip angle 30 degrees, TR/TE 200/7, field of view 14cm, matrix size 256x160). The shear modulus was calculated from the image data by solving the partial differential wave equation that describes the relationship of stiffness to wavelength (12,13).
The margins of the right and left thyroid lobes were identified and outlined by a neuroradiologist on the T1 weighted MRI. These ROI were used for the analysis of the MRE data. The mean stiffness of the right and left glands was used in the analysis of the normal volunteers and HT patients.
Similar images were obtained from thyroids containing a solitary nodule. The margins of the nodules were not well demarcated from the surrounding thyroid tissue on the T1 weighted anatomic images. Because the margins of the nodules were not visible on the anatomic images, the right and left lobes of the thyroid gland were outlined as above. The entire lobe containing the nodule was used as the ROI. This was done because the margins of the nodules were not well demarcated from the surrounding thyroid tissue on anatomic images and therefore there was no indicator for the placement of the ROI margins. The average stiffness values from each lobe were determined. The thyroid lobes containing a nodule were compared to the paired contralateral lobes. The lobar MRE data from each subject was then sorted according to the whether the solitary nodule was present or absent in a particular thyroid lobe, based on clinical palpation by a thyroidologist.
Parametric images of the shear modulus were created from the MRE data using the local frequency estimation inversion method (1,12). The equations on which this method is based assume a purely elastic model for a simple isotropic Hookean material (1). The thyroid gland stiffness was measured by ROI analysis of the parametric shear modulus images.
Data were analyzed using a paired t-test to compare average stiffness over each lobe between the two groups being studied (i.e. HT versus Normal or thyroid lobe containing solitary nodule versus the paired contralateral lobe).
RESULTS
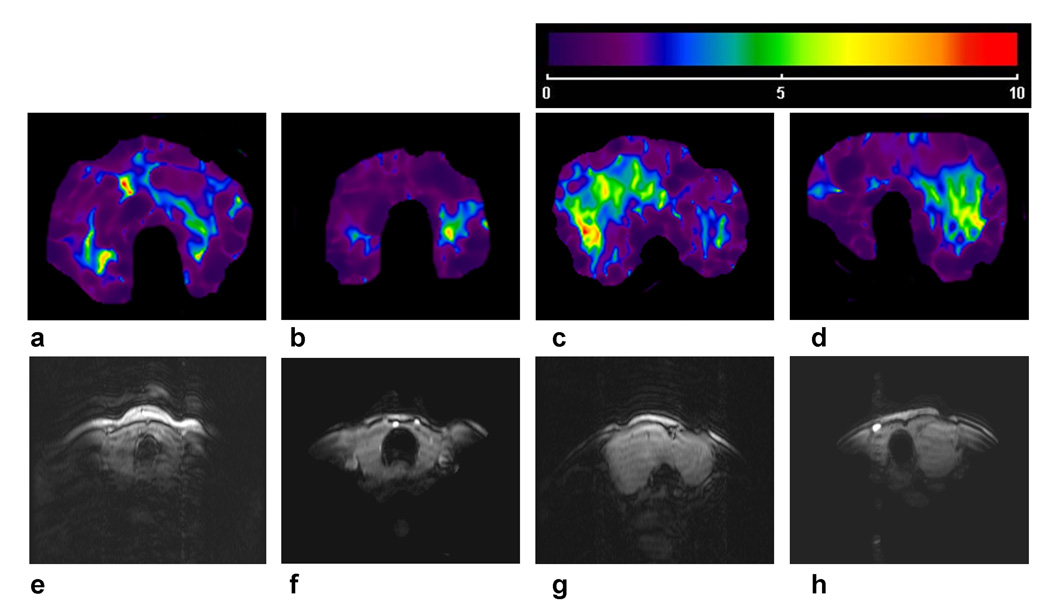
We imaged normal and HT glands using MRE and found that the elastic properties of the glands were visually quite dissimilar as were the axial T1 weighted anatomic MR images obtained coregistered with the MRE (Fig. 2). The color scale ranged linearly from purple-blue-green-yellow-orange-red as the local stiffness of the glands ranged from 0 to 10 kilopascal (kPa) with yellow-green indicating a stiffness of 5 kPa.
Figure 2.
Figures 2a and 2b show parametric shear modulus images from Normal volunteers and the Figures 2c and 2d are from patients with HT. Figures 2e-h are axial T1 weighted anatomic MR images of the thyroid gland that were obtained coregistered with the MRE and correspond to Figures 2a-d, respectively.
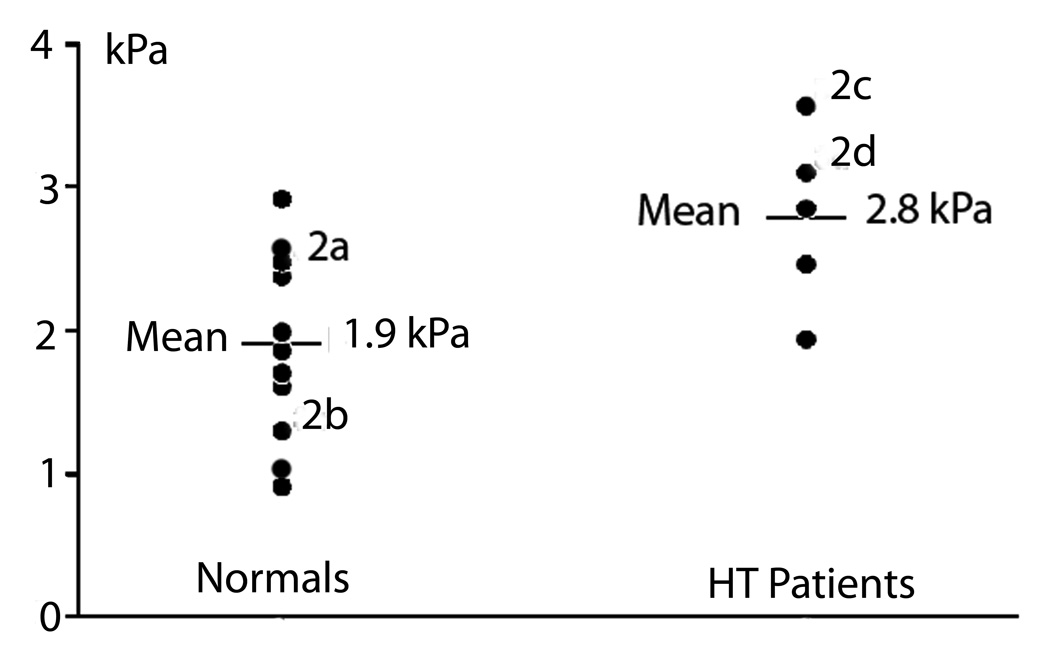
We then quantitatively analyzed the MRE data using both 80 Hz and 100 Hz sheer waves. The difference between the mean stiffness of the Normal and the HT groups was significant at 100 Hz (p=0.004), and of borderline significance at 80 Hz {p=0.046) (Table 1, Fig. 3).
Table 1.
Mean Stiffness of the Thyroid Gland
| Normal | Hashimoto’s Thyroiditis | p | |
|---|---|---|---|
| 80 Hz shear waves | 1.3 ± 0.5 kPa | 1.8 ± 0.6 kPa | 0.046 |
| 100 Hz shear waves | 1.9 ± 0.6 kPa | 2.8 ± 0.6 kPa | 0.004 |
Figure 3.
The shear modulus values using 100 Hz shear waves for all Normal and HT volunteers in the study. The difference between the mean stiffness of the Normal and the HT group was significant at 100 Hz (p=0.004). The superscripts 2a-2d correspond to the thyroid gland images in Figure 2.
In contrast, quantitative analysis did not demonstrate a significant difference in stiffness between lobes containing a nodule and the contralateral lobes that did not contain a nodule, or between the lobes with and without nodules and the Normal glands (data not shown). Further, the lobes containing a malignant nodule (n=2) did not significantly differ from the lobes containing a benign nodule (n=8).
DISCUSSION
Our data suggest that MRE offers a quantitative method for assessing the thyroid gland for the presence of HT. It is probable that the inflammatory lymphocytic infiltration and fibrosis of the thyroid gland characteristic of HT accounts for the differences in the elastographic parameters measured between HT and normal thyroid gland. This modality does not appear to be useful in the evaluation of thyroid nodules as the thyroid lobes containing a nodule could not be distinguished from the paired contralateral lobes, and malignant nodules could not be distinguished from benign. This is likely due to the similar compressible qualities of these tissues and perhaps to the small number of malignant nodules studied. The sensitivity of the results to stiffness differences between nodules and surrounding tissue was decreased because the entire lobe containing the nodule was used as a region of interest instead of the isolated nodule. This was done because the margins of the nodules were not well demarcated from the surrounding thyroid tissue on anatomic images and therefore there was no indicator for the placement of the ROI margins. The primary nodule or the most suspicious nodules based on US findings, palpation and clinical findings was chosen for biopsy, as is the standard of practice (14). The FNA needle was very fine compared to the size of the nodule and there was no MRI evidence of hemorrhage or architectural distortion in the region of the biopsy. Therefore, we do not believe that the small FNA aspiration could alter the MRE results. The results of the studies of nodules are limited due to the small sample size and difficulty in identifying the margins of the nodules for ROI placement.
The current approach to diagnosis and management of HT includes obtaining the clinical history, palpating of the gland, performing an ultrasound examination and measuring serum antithyroperoxidase antibodies and thyroid hormone levels (15). Typically, HT appears on ultrasound as a diffusely hypoechoic gland. Grey scale ultrasonography to further characterize tissue echogenicity has been shown to correlate with functional status of the gland and highly elevated levels of antithyroperoxidase antibodies (16). Occasionally fine-needle aspiration biopsy is performed if the diagnosis remains in doubt or if nodules are present. In general, HT is treated with thyroid hormone when the immunologic process has progressed to the point that hormone production is significantly impaired. Figure 3 demonstrates that despite a significant difference in the mean elasticity of the HT glands and normal controls, there is some overlap of the individual values of elasticity between these two groups. This suggests that MRE findings alone may not be sufficient to confirm the diagnosis of HT. However, the clinician uses a constellation of clinical, laboratory and imaging findings when evaluating patients with an enlarged thyroid gland. Similarly, MRI might therefore be considered as another useful modality to aid in clinical decision making.
Another thyroid disorder that is characterized by a diffusely firm goiter on palpation is Reidel’s thyroiditis. No quantitative results of MRE in Reidel’s are known, but it is likely that that the firm gland would also appear to be more firm on MRE. However, HT and Reidels generally can be easily distinguished by the remarkably firm “woody” nature of a Reidel’s gland on palpation and its extension beyond the thyroid gland with extensive lymphadenopathy on ultrasound examination (17). A diffusely enlarged colloid (sporadic)goiter can also be differentiated from HT by being soft on palpation, lacking antiperoxidase antibodies and having a diffusely hyperechoic structure on ultrasound examination (18).
The practical utility of this modality in the setting of HT at present may be limited as currently available tools generally supply the clinician with adequate information to address the currently important diagnostic and management issues. However, this modality might be considered as an adjunct to palpation, ultrasound examination and laboratory evaluation. Stiffness imaging using MRE does afford advantages over palpation, including quantitation of the gland consistency (“firmness”) and potentially the ability to accurately follow changes over time. Images can also demonstrate deep portions of the gland that might not be accessible to palpation. Future studies could address whether MRE can play a role in identifying lymphoma developing in a Hashimoto’s gland. Also, quantitative monitoring of HT patients with MRE may be used in future to evaluate changes in elasticity of the gland over time following the administration of novel immunologic therapies.
In conclusion, we have developed a method for the application of MRE to the study of thyroid gland pathology that can clearly distinguish the HT gland from normal thyroid. At present, the clinical utility of this modality in the setting of HT is limited. However, as novel therapeutics aimed at forestalling the immunologic insult responsible for HT are developed, quantitative monitoring of HT progression using MRE might offer advantages in the management of this condition.
Acknowledgments
This study was funded by NIH Grant EB001981.
REFERENCES
- 1.Kruse SA, Smith JA, Lawrence AJ, et al. Tissue characterization using magnetic resonance elastography: preliminary results. Physics in Medicine & Biology. 2000;45(6):1579–1590. doi: 10.1088/0031-9155/45/6/313. [DOI] [PubMed] [Google Scholar]
- 2.Bensamoun SF, Ringleb SI, Chen Q, Ehman RL, An K-N, Brennan M. Thigh muscle stiffness assessed with magnetic resonance elastography in hyperthyroid patients before and after medical treatment. Journal of Magnetic Resonance Imaging. 2007;26(3):708–713. doi: 10.1002/jmri.21073. [DOI] [PubMed] [Google Scholar]
- 3.Bensamoun SF, Ringleb SI, Littrell L, et al. Determination of thigh muscle stiffness using magnetic resonance elastography. Journal of Magnetic Resonance Imaging. 2006;23(2):242–247. doi: 10.1002/jmri.20487. [DOI] [PubMed] [Google Scholar]
- 4.Goss BC, McGee KP, Ehman EC, Mandua A, Ehman RL. Magnetic resonance elastography of the lung: Technical feasibility. Magnetic Resonance in Medicine. 2006;56(5):1060–1066. doi: 10.1002/mrm.21053. [DOI] [PubMed] [Google Scholar]
- 5.Kruse SA, Rose GH, Glaser KJ, et al. Magnetic resonance elastography of the brain. NeuroImage. 2008;39(1):231–237. doi: 10.1016/j.neuroimage.2007.08.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lopez O, Amrami KK, Manduca A, Ehman RL. Characterization of the dynamic shear properties of hyaline cartilage using high-frequency dynamic MR elastography. Magnetic Resonance in Medicine. 2008;59(2):356–364. doi: 10.1002/mrm.21474. [DOI] [PubMed] [Google Scholar]
- 7.McGee KP, Hubmayr RD, Ehman RL. MR elastography of the lung with hyperpolarized 3He. Magnetic Resonance in Medicine. 2008;59(1):14–18. doi: 10.1002/mrm.21465. [DOI] [PubMed] [Google Scholar]
- 8.Ringleb SI, Bensamoun SF, Chen Q, Manduca A, An K-N, Ehman RL. Applications of magnetic resonance elastography to healthy and pathologic skeletal muscle. Journal of Magnetic Resonance Imaging. 2007;25(2):301–309. doi: 10.1002/jmri.20817. [DOI] [PubMed] [Google Scholar]
- 9.Rouviere O, Yin M, Dresner MA, et al. MR Elastography of the Liver: Preliminary Results. Radiology. 2006;240(2):440–448. doi: 10.1148/radiol.2402050606. [DOI] [PubMed] [Google Scholar]
- 10.Talwalkar JA, Yin M, Fidler JL, Sanderson SO, Kamath PS, Ehman RL. Magnetic resonance imaging of hepatic fibrosis: Emerging clinical applications. Hepatology. 2008;47(1):332–342. doi: 10.1002/hep.21972. [DOI] [PubMed] [Google Scholar]
- 11.Woodrum DA, Romano AJ, Lerman A, et al. Vascular wall elasticity measurement by magnetic resonance imaging. Magnetic Resonance in Medicine. 2006;56(3):593–600. doi: 10.1002/mrm.20991. [DOI] [PubMed] [Google Scholar]
- 12.Manduca A, Muthupillai R, Rossman PJ, Greenleaf JF, Ehman RL. In: Visualization of tissue elasticity by magnetic resonance elastography. Hone KH, Kikinis R, editors. Hamburg, Germany: Springer; 1996. pp. 63–68. [Google Scholar]
- 13.Manduca A, Oliphant TE, Dresner MA, et al. Magnetic resonance elastography: Non-invasive mapping of tissue elasticity. Medical Image Analysis. 2001;5(4):237–254. doi: 10.1016/s1361-8415(00)00039-6. [DOI] [PubMed] [Google Scholar]
- 14.Mandel SJ. What are the ultrasound criteria for fine-needle aspiration cytology of nonpalpable thyroid nodules? Nature Clinical Practice Endocrinology & Metabolism. 2006;2(7):372–373. [Google Scholar]
- 15.Pearce E, Farwell A, Braverman L. Thyroiditis. N Engl J Med. 2003;348:2646–2655. doi: 10.1056/NEJMra021194. [DOI] [PubMed] [Google Scholar]
- 16.Schiemann U, Avenhaus W, Konturek JW, Gellner R, Hengst K, Gross M. Relationship of clinical features and laboratory parameters to thyroid echogenicity measured by standardized grey scale ultrasonography in patients with Hashimoto's thyroiditis. Med Sci Monit. 2003;9(4):MT13–MT17. [PubMed] [Google Scholar]
- 17.Ozbayrak M, Kantarci F, Olgun D, Akman C, Mihmanli I, Kadioglu P. Riedel thyroiditis associated with massive neck fibrosis. J Ultrasound Med. 2009;28(2):267–271. doi: 10.7863/jum.2009.28.2.267. [DOI] [PubMed] [Google Scholar]
- 18.Berghout A, WM W, NJ S, JL T. The value of thyroid volume measured by ultrasonography in the diagnosis of goitre. Clin Endocrinol. 1988;28:409–414. doi: 10.1111/j.1365-2265.1988.tb03672.x. [DOI] [PubMed] [Google Scholar]