Abstract
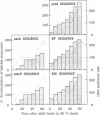
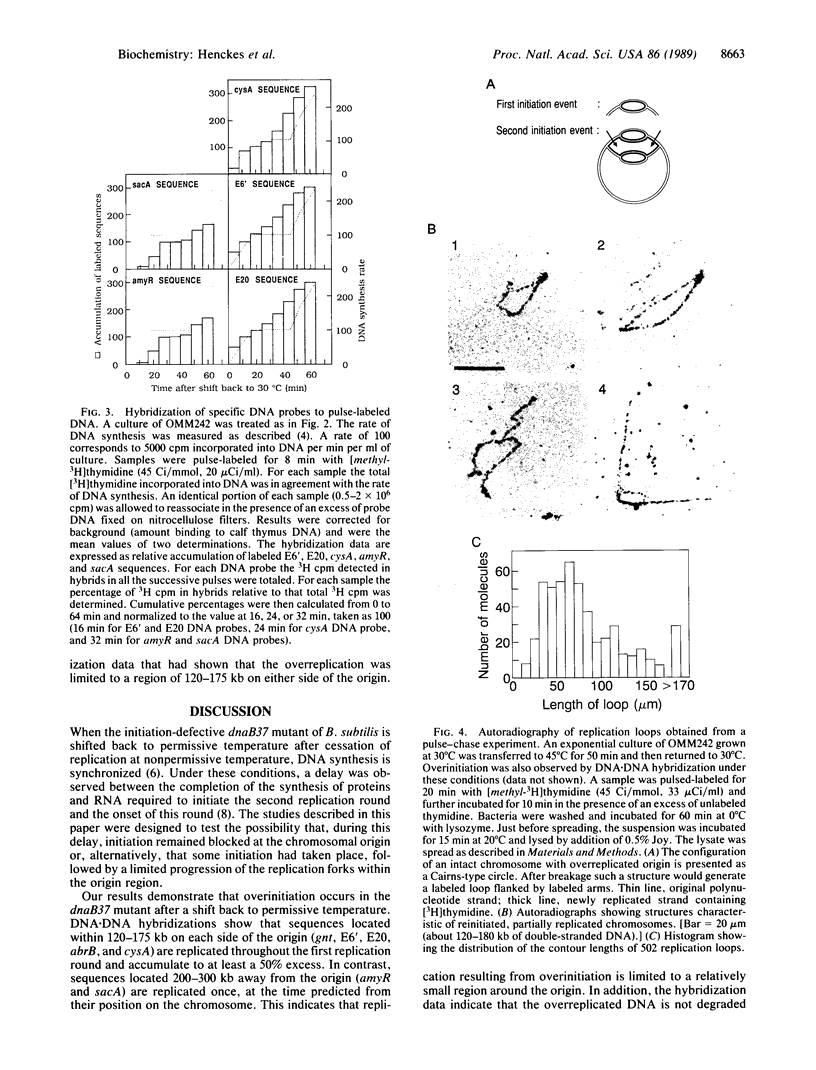
When the Bacillus subtilis dnaB37 mutant, defective in initiation, is returned to permissive temperature after accumulation of initiation proteins at 45 degrees C, we have shown, by extensive DNA.DNA hybridization analysis, that the origin region is replicated in excess (approximately 2-fold). However, this replication is limited to a region of about 120-175 kilobases on either side of the origin. This has been confirmed by autoradiographic analysis of the overreplicated region. During the second round of synchronized replication at 30 degrees C, replication in fact appears to resume from the stalled forks on either side of the origin. We propose that in B. subtilis, in addition to a first level of control at the origin, a second level of control exists downstream of the origin in order to limit overreplication of the chromosome. These two controls might normally be tightly coupled. We suggest that the second level of control is exerted through the reversible inhibition of replisome movement at specific regions on either side of the origin.
Full text
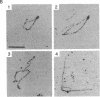
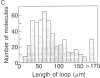
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Atlung T., Løbner-Olesen A., Hansen F. G. Overproduction of DnaA protein stimulates initiation of chromosome and minichromosome replication in Escherichia coli. Mol Gen Genet. 1987 Jan;206(1):51–59. doi: 10.1007/BF00326535. [DOI] [PubMed] [Google Scholar]
- BURTON K. A study of the conditions and mechanism of the diphenylamine reaction for the colorimetric estimation of deoxyribonucleic acid. Biochem J. 1956 Feb;62(2):315–323. doi: 10.1042/bj0620315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fouet A., Klier A., Rapoport G. Cloning and expression in Escherichia coli of the sucrase gene from Bacillus subtilis. Mol Gen Genet. 1982;186(3):399–404. doi: 10.1007/BF00729460. [DOI] [PubMed] [Google Scholar]
- Fujita Y., Nihashi J., Fujita T. The characterization and cloning of a gluconate (gnt) operon of Bacillus subtilis. J Gen Microbiol. 1986 Jan;132(1):161–169. doi: 10.1099/00221287-132-1-161. [DOI] [PubMed] [Google Scholar]
- Helmstetter C. E., Krajewski C. A., Leonard A. C., Weinberger M. Discontinuity in DNA replication during expression of accumulated initiation potential in dnaA mutants of Escherichia coli. J Bacteriol. 1986 Feb;165(2):631–637. doi: 10.1128/jb.165.2.631-637.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill T. M., Pelletier A. J., Tecklenburg M. L., Kuempel P. L. Identification of the DNA sequence from the E. coli terminus region that halts replication forks. Cell. 1988 Nov 4;55(3):459–466. doi: 10.1016/0092-8674(88)90032-3. [DOI] [PubMed] [Google Scholar]
- Lampe M. F., Bott K. F. Cloning the gyrA gene of Bacillus subtilis. Nucleic Acids Res. 1984 Aug 10;12(15):6307–6323. doi: 10.1093/nar/12.15.6307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laurent S. J. Control of deoxyribonucleic acid synthesis in a Bacillus subtilis mutant temperature sensitive for initiation of chromosome replication. J Bacteriol. 1974 Feb;117(2):329–336. doi: 10.1128/jb.117.2.329-336.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laurent S. J. Initiation of deoxyribonucleic acid replication in a temperature-sensitive mutant of B. subtilis: evidence for a transcriptional step. J Bacteriol. 1973 Oct;116(1):141–145. doi: 10.1128/jb.116.1.141-145.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laurent S. J., Vannier F. S. Temperature-sensitive initiation of chromosome replication in a mutant of Bacillus subtilis. J Bacteriol. 1973 May;114(2):474–484. doi: 10.1128/jb.114.2.474-484.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine A., Henckes G., Vannier F., Séror S. J. Chromosomal initiation in Bacillus subtilis may involve two closely linked origins. Mol Gen Genet. 1987 Jun;208(1-2):37–44. doi: 10.1007/BF00330419. [DOI] [PubMed] [Google Scholar]
- Løbner-Olesen A., Skarstad K., Hansen F. G., von Meyenburg K., Boye E. The DnaA protein determines the initiation mass of Escherichia coli K-12. Cell. 1989 Jun 2;57(5):881–889. doi: 10.1016/0092-8674(89)90802-7. [DOI] [PubMed] [Google Scholar]
- McDonell M. W., Simon M. N., Studier F. W. Analysis of restriction fragments of T7 DNA and determination of molecular weights by electrophoresis in neutral and alkaline gels. J Mol Biol. 1977 Feb 15;110(1):119–146. doi: 10.1016/s0022-2836(77)80102-2. [DOI] [PubMed] [Google Scholar]
- Moriya S., Ogasawara N., Yoshikawa H. Structure and function of the region of the replication origin of the Bacillus subtilis chromosome. III. Nucleotide sequence of some 10,000 base pairs in the origin region. Nucleic Acids Res. 1985 Apr 11;13(7):2251–2265. doi: 10.1093/nar/13.7.2251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- OISHI M., YOSHIKAWA H., SUEOKA N. SYNCHRONOUS AND DICHOTOMOUS REPLICATIONS OF THE BACILLUS SUBTILIS CHROMOSOME DURING SPORE GERMINATION. Nature. 1964 Dec 12;204:1069–1073. doi: 10.1038/2041069a0. [DOI] [PubMed] [Google Scholar]
- Ogasawara N., Mizumoto S., Yoshikawa H. Replication origin of the Bacillus subtilis chromosome determined by hybridization of the first-replicating DNA with cloned fragments from the replication origin region of the chromosome. Gene. 1984 Oct;30(1-3):173–182. doi: 10.1016/0378-1119(84)90118-5. [DOI] [PubMed] [Google Scholar]
- Ogasawara N., Seiki M., Yoshikawa H. Effect of novobiocin on initiation of DNA replication in Bacillus subtilis. Nature. 1979 Oct 25;281(5733):702–704. doi: 10.1038/281702a0. [DOI] [PubMed] [Google Scholar]
- Pierucci O., Helmstetter C. E., Rickert M., Weinberger M., Leonard A. C. Overexpression of the dnaA gene in Escherichia coli B/r: chromosome and minichromosome replication in the presence of rifampin. J Bacteriol. 1987 May;169(5):1871–1877. doi: 10.1128/jb.169.5.1871-1877.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piggot P. J., Hoch J. A. Revised genetic linkage map of Bacillus subtilis. Microbiol Rev. 1985 Jun;49(2):158–179. doi: 10.1128/mr.49.2.158-179.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rigby P. W., Dieckmann M., Rhodes C., Berg P. Labeling deoxyribonucleic acid to high specific activity in vitro by nick translation with DNA polymerase I. J Mol Biol. 1977 Jun 15;113(1):237–251. doi: 10.1016/0022-2836(77)90052-3. [DOI] [PubMed] [Google Scholar]
- Wake R. G. Visualization of reinitiated chromosomes in Bacillus subtilis. J Mol Biol. 1972 Jul 28;68(3):501–509. doi: 10.1016/0022-2836(72)90102-7. [DOI] [PubMed] [Google Scholar]
- Xu Y. C., Bremer H. Chromosome replication in Escherichia coli induced by oversupply of DnaA. Mol Gen Genet. 1988 Jan;211(1):138–142. doi: 10.1007/BF00338404. [DOI] [PubMed] [Google Scholar]
- de Massy B., Patte J., Louarn J. M., Bouché J. P. oriX: a new replication origin in E. coli. Cell. 1984 Jan;36(1):221–227. doi: 10.1016/0092-8674(84)90092-8. [DOI] [PubMed] [Google Scholar]