Abstract
Epidermal growth factor receptor (EGFR) is a target in head and neck cancer. High EGFR expression and phosphorylated EGFR predicts poor survival in head and neck cancer patients, but does not correlate with advanced stage disease. The aim of this study is to determine if clinical biological correlates are more accurate when different aspects of EGFR are evaluated in combination. We analyzed the EGFR phosphorylation, expression, and mutations in 60 primary head and neck tumors. We not only found that head and neck tumors with either truncated or activated EGFR tend to have higher tumor and nodal stage but also discovered two novel EGFR truncations.
Keywords: Tyrosine kinase receptor, Truncated EGFR, Phosphorylated EGFR, EGFRvIII, Head and neck squamous cell carcinomas
INTRODUCTION
Aberrant expression and/or signaling of some tyrosine kinase receptors have been recognized to have important roles in carcinogenesis (1). To determine if a particular tyrosine kinase receptor carries prognostic value, many translational studies have used immunohistochemistry (IHC) to detect its expression in tumor specimens. While the conventional IHC approaches are relatively easy to perform on materials that are readily available, they evaluate only one aspect of the receptor. When IHC was used to examine the expression of the epidermal growth factor receptor (EGFR) in head and neck squamous cell carcinomas (HNSCC), a strong association was noted between high EGFR expression and poor survival (2, 3). However, the EGFR staining was not predictive of advanced stage disease. Subsequent studies used either fluorescence resonance energy transfer (FRET) or phospho-specific IHC to measure the EGFR phosphorylation in HNSCC. While there was a significant association between activated EGFR and disease-free survival, no correlation was found between phosphorylation and disease stage (4, 5). In spite of the critical role of EGFR in HNSCC, its expression and phosphorylation are not the only determinants of aggressive disease. A recent study reported that 42% of HNSCC expressed the EGFR truncation mutant, EGFRvIII, where it contributes to enhanced growth and resistance to EGFR targeting by Erbitux (6). Thus, the expression of EGFR variant(s) may be another important aspect related to the role of EGFR in HNSCC. The aim of this study was to determine if EGFR and tumor staging correlates when different aspects of the receptor are evaluated in combination. We used different molecular methods to examine the various aspects of EGFR in the primary tumors. These included immunoprecipitation followed by western blotting (IPW), IHC, and reverse transcriptase polymerase chain reaction (RT-PCR) coupled with DNA sequencing. The advantage of using multiple techniques to examine the status of an oncogenic receptor is most apparent from the correlation study linking HER-2 to survival in breast cancer (7). We analyzed EGFR in 60 primary HNSCC using a combination of IPW, IHC, and RT-PCR. We found that the combination of the phosphorylated and truncated EGFR correlates with advanced tumor and nodal stage in head and neck cancer. In addition, we discovered two novel EGFR truncations and two missense kinase mutations in these tumors.
MATERIALS AND METHODS
Collection of primary human tumors and HNSCC cell lines
Fresh, frozen primary human tumors were collected prospectively for this study through the National Cancer Institute (NCI)-sponsored Cooperative Human Tissue Network (CHTN). When head and neck cancer patients presented to the CHTN institutions for surgery, informed consent for participation in the research using excess tumor tissues was obtained by the CHTN staff at the local institutions. Once patients consented, our team was notified of the tumor arrival. At the time of procurement, the CHTN staff also reviewed patients’ medical charts to obtain relevant clinical information, such as age, sex, race, prior treatment, imaging reports, history of smoking, and alcohol use. Detailed pathology reports including information, such as tumor location, nodal involvement, size and number of involved nodes, histologic features, and staging, were sent to our team at the same time. These reports were standardized across institutions. In addition, the time from surgical excision of the tumor to storage in liquid nitrogen was recorded and provided by the CHTN for each tumor. All frozen tumors were shipped in dry ice, archived, and stored in our laboratory. The Institutional Review Board (IRB) approval for the study was obtained at our institution. One Hematoxylin and Eosin (H&E) stained and 10 unstained slides from each specimen were received at the same time. The following criteria were established for inclusion in our correlation analysis: Only histologically confirmed head and neck squamous cell carcinomas of the primary sites would be included and tumors with cancer to stroma ratio of ≤10% would be excluded to avoid false negative results.
The following HNSCC cell lines were purchased from American Type Culture Collection (ATCC): SCC9, SCC15, SCC25, and CAL27. MDA1386 was provided by Dr. Kepal Patel (New York University, New York, NY, USA).
Processing of primary tumors or cell lines for protein and RNA analyses
Primary tumors were homogenized in 1 ml of Triton X lysis buffer (50-mM Tris-Cl, pH = 8, 150-mM NaCl and 1% Triton X-100) with protease inhibitor and sodium orthovanadate using the POLYTRON system PT 10–35 GT (Kinematica AG). The homogenates were spun down, pellets were discarded, and the supernatants were saved for protein analyses. Separate pieces of the frozen tumor were homogenized in 1-ml Trizol Reagent using the POLYTRON system PT 1200 E (Kinematica AG). Each cell line was also lysed in Trizol reagent. Total RNA was isolated as per manufacturer’s recommendation (Life Technologies). Protein concentrations were determined by Bradford assay. RNA concentration and purity were determined using a NanoDrop spectrophotometer. Each tumor was homogenized with a disposable dispersing aggregate to avoid cross contamination between samples.
Protein analysis with IPW
Total tumor lysates, 1 mg, were incubated with the chimeric EGFR antibody, cetuximab (ImClone), which targets the extra-cellular domain of EGFR. Next day, the antibody conjugates were extracted using protein G-Sepharose beads and EGFR was eluted by incubation at 95°C in loading buffer. The proteins were subsequently resolved by 8% polyacrylamide gel electrophoresis under reducing conditions and transferred to Immobilon-P membrane (Millipore, Bedford, MA). Western blotting was performed first with the anti-phosphotyrosine 4G10 antibody (Upstate, Lake Placid, NY) to evaluate the phosphorylation status of EGFR. Next, the membrane is incubated with an anti-EGFR antibody (8) that recognizes the intracellular C-terminal domain of the receptor. Fluorescent secondary antibodies were used to develop the western blots so that the EGFR expression and phosphorylation can be quantified by the Odyssey Infrared Imaging System (LI-COR Biosciences, Lincoln, NE). The mean EGFR expression for all samples was calculated. Tumors with the EGFR expression above the mean were defined as high expressors.
RNA analysis with RT-PCR and DNA sequencing
Reverse transcription was carried out using a SuperScript Preamplification Kit (Life Technologies) on 1 μg of total RNA aliquots. PCR was performed using capillary LightCycler (Roche, Indianapolis, IN) with the following sets of EGFR primers. Set 1: 5′-GGCGAGTCGGGCTCTGGAGGAAAAG-3′ and 5′-GGCCCTTCGCACTTCTTACACTTG-3′. Set 2: 5′-CCTGGGGATCGGCCTCTTCAT-3′ and 5′-CACCCCGTAGCTCCAGACATCA-3′. Set 1 primer pair was designed to amplify the coding sequences from nucleotides 60 to 995 that cover the extracellular domain of EGFR (exon 1–8). Set 2 primer pair was designed to amplify the coding sequences from nucleotides 1983 to 2706 that cover the intra-cellular tyrosine kinase domain of EGFR (exon 17–22). PCR was performed under the following conditions: denaturating 10 s at 95°C, annealing 5 s at 60°C for set 1 primers and at 50°C for set 2 primers, extension 30 s at 72°C. A total of 45 and 37 cycles were performed, respectively, for set 1 and 2 primer pairs. The PCR end products were resolved by 2% agarose gel electrophoresis and isolated using Qiagen PCR Extraction Kit (QIAGEN Inc., Santa Clarita, CA). DNA sequencing was performed at the Sequencing Core Facility of the Stony Brook University Medical Center.
Immunohistochemistry and histology
Tumor sections were deparaffinized and rehydrated and antigen retrieval was performed by microwave heating in sodium citric solution. After washing with phosphate buffer solution (PBS), the sections were treated with blocking serum (Vectastain ABC-AP kit, Vector Laboratories, CA) and incubated with an anti-EGFR antibody (Cell Signaling, Beverly, MA) at 1:50 dilution or a phospho-EGFR specific to tyrosine 1068 antibody (Cell Signaling, Beverly, MA) at 1:400 dilution overnight at 4°C. The tumor sections were subsequently washed with PBS and stained using the ABC-AP kit and the Vector Red alkaline phosphatase substrate solution (Vector Red, Vector Laboratories, CA). All matched H&E-stained tumor sections were reviewed by a surgical pathologist (KRS) to confirm the diagnosis and determined the ratio of surface area occupied by the tumor to stroma. The histologic assessment was performed without knowledge of the molecular testing results and clinical data.
STATISTICAL ANALYSES
Statistical analyses were performed using SPSS Statistics 16.0 (SPSS Inc., Chicago, IL). Comparison between pEGFR+ and pEGFR− groups was performed using two-sample t-test. We performed log transformation to normalize the distribution of EGFR expression for comparison. Chi square or Fisher’s exact test was used to analyze the significance of the association between the EGFR status and tumor characteristics. Test of ΔEGFR and pEGFR interaction in their association with advanced stage tumor was performed under logistic regression model. No correction for multiple comparison was made in calculating p-values.
RESULTS
EGFR expression and activation analyses in HNSCC
The EGFR protein analysis was performed by IPW and IHC on 60 primary HNSCC. The IPW analysis surprisingly showed that only 40% (24/60) of HNSCC had tyrosine phosphorylated EGFR. As shown in Figure 1, despite having similar level of EGFR expression among the three tumors (D2232#, D2233#, and D223##), only D223## has detectable phosphorylated EGFR (Figure 1(a)). While EGFR phosphorylation strongly correlated with its expression level (i.e., the higher the EGFR expression, the more likely the receptor is phosphorylated), there were tumors with equivalent amounts of EGFR that differed in their activation status (Figure 1(b)). Nevertheless, it is clear from this data that tumors with low EGFR expression are unlikely to have activated EGFR. To evaluate the possibility that phosphorylation was lost during the processing of the tumors, we examined the difference in time taken from surgical excision of the tumor to liquid nitrogen storage between the phosphorylated and unphosphorylated tumors. There was no significant difference between their processing times. Mean processing time was 0.56 ± 0.24 hr and 0.59 ± 0.29 hr (p = .67) for pEGFR+ (n = 22) and pEGFR− (n = 34) tumors, respectively (Figure 1(c)). Processing time data was missing for four cases. Thus, the detection of EGFR phosphorylation is not directly related to the processing time. One shortcoming of the IPW method is its inability to localize protein expression to a specific cell type, due to the heterogeneity of the homogenized lysates. Therefore, IHC was performed to confirm the EGFR expression in the cancer cells. IHC also allows correlation of the EGFR expression with morphologic evidence of tumor viability. As shown in Figure 1(d), IHC detected EGFR expression only in the carcinomas and no staining was seen when the primary antibodies were omitted (data not shown). We also performed pEGFR IHC on the tumor sections. We have additional unstained slides for pEGFR IHC in 18 of the 24 IPW pEGFR+ tumors. Of these 18 samples, nine were positive for pEGFR by IHC. Therefore, the concordance rate between IPW and IHC for pEGFR+ tumor is 50%. Figure 1(e) showed a representative pEGFR+ HNSCC section. We performed pEGFR IHC on 18 corresponding IPW pEGFR− samples; five sections had some degree of nonspecific staining, the rest were all negative. Thus, the concordance rate between IPW and IHC for pEGFR− tumor is much better at 72%.
Figure 1.
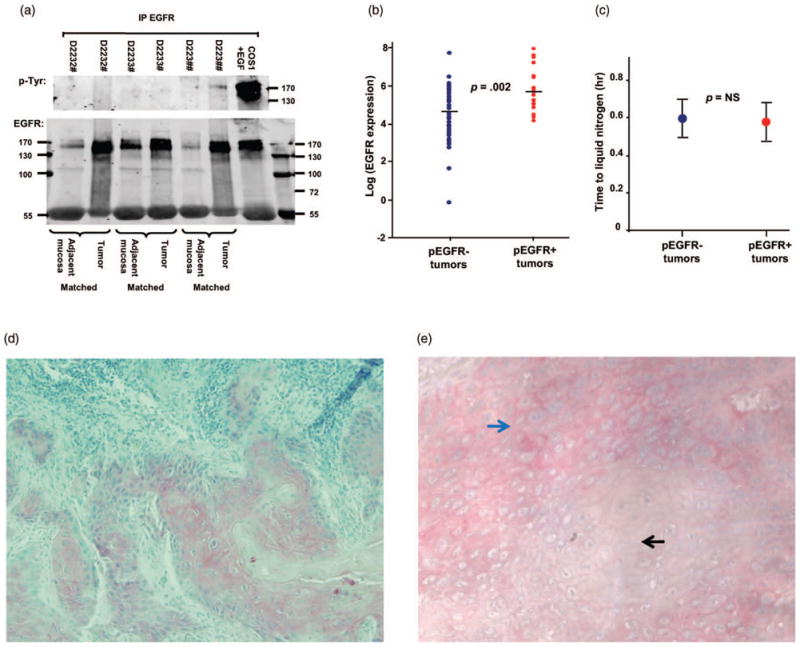
Protein analysis by IPW and IHC. (a) Representative EGFR IPW analysis of HNSCC with their matched adjacent noncancerous tissues. EGF-stimulated COS cells are positive controls. (b) Comparison of EGFR expression between pEGFR+ (n = 22) and pEGFR− (n = 34) HNSCC. EGFR expression is calculated on a logarithmic scale. Tumors with log (EGFR expression) <4 are never phosphorylated; tumors with log (EGFR expression) between 4 and 8 are variable in their receptor activity level. (c) Comparison of processing time between pEGFR+ and pEGFR− tumors. The circle represents the mean. The error bars show 95% confidence interval of the means. (d) EGFR immunohistochemistry of a representative HNSCC, D2232# (100X). Viable tumor comprised about 50% of the tissue section. Noted immunohistochemical staining is detected in tumor epithelial cells, with minimal expression in tumor stroma. (e) pEGFR immunohistochemistry of a representative HNSCC, 106169# (200X). The black arrow points to the center of a focal area with no staining, while the light blue arrow points to the center of an area with membraneous staining. Note that the pEGFR IHC stain tends to be membraneous, more focal, and less intense than the EGFR IHC.
Mutation analysis of EGFR in HNSCC by RT-PCR
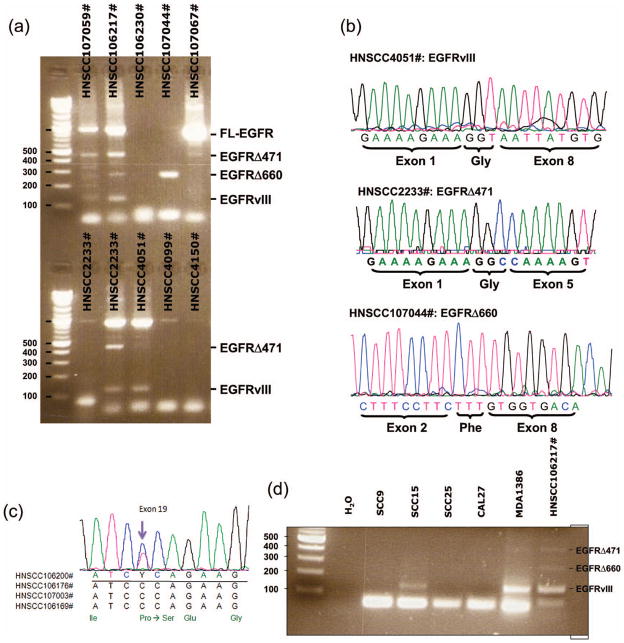
As we used an EGFR antibody that binds the extracellular domain to pull down EGFR in the IPW method, truncated variants of EGFR that lack portions of the extracellular domain, such as EGFRvIII, might not be recognized with this assay. In addition, IHC will not be able to distinguish between the truncated and full length EGFR because the EGFR antibody for IHC binds the C-terminal of the receptor, which is present in both forms. To overcome this limitation, we used RT-PCR to screen for truncated EGFR in 60 HNSCC. Like others (6), we found EGFRvIII in some HNSCC (Figure 2). DNA sequencing of the RT-PCR end products confirms the identity (upper panel, Figure 2(b)). Surprisingly, we also discovered two novel truncated variants of EGFR (Figure 2(a)). Both variants have not been previously reported. The first variant, EGFRΔ471, carries an in-frame deletion from nucleotide 89 of exon 1 to nucleotide 559 of exon 4 (Δ471). Most fascinating is the formation of a new codon (GGC) at the splice junction, which translates into a glycine residue (middle panel, Figure 2(b)); this is similar to that seen in EGFRvIII. The second truncated variant, EGFRΔ660, has an in-frame deletion from nucleotide 237 of exon 2 to nucleotide 896 of exon 8 (Δ660). Again, there is the formation of a new codon (TTT) at the splice junction, which translates into a phenylalanine residue (lower panel, Figure 2(b)). We also screened five HNSCC cell lines for the presence of these EGFR variants. While we did not find either EGFRΔ471 or Δ660 in these lines, we are surprised to see endogenous EGFRvIII expression in two of the cell lines (Figure 2(d)). To the best of our knowledge, no one has previously reported endogenous EGFRvIII expression in any head and neck squamous cell line. The two novel EGFR variants, Δ471 and Δ660, might possess oncogenic potential just like EGFRvIII.
Figure 2.
RNA analysis by RT-PCR. (a) RT-PCR screening of EGFR variants in a representative panel of primary HNSCC. FL-EGFR: full length EGFR. (b) DNA sequences of the exon 1–8 RT-PCR end products from three representative HNSCC. Top panel is DNA sequence representing EGFRvIII; middle panel shows EGFRΔ471 sequence; and the bottom panel is EGFRΔ660 sequence. All EGFR truncations were confirmed in both directions. (c) DNA sequences of the exon 17–22 RT-PCR end products from four representative HNSCC. The black arrow points to the missense mutation (C → T) in nucleotide 2197 of EGFR exon 19 of HNSCC106200#. The change in amino acid of this P733S mutation is illustrated by the amino acid sequence at the bottom. DNA sequences of the remaining three HNSCC (106176#, 107003#, and 106169#) are wild type. EGFR kinase mutations were confirmed in both directions. (d) RT-PCR screening of EGFR variants in five HNSCC cell lines. HNSCC106217# is the known positive control that expresses all three EGFR truncation mutants (see panel A).
Next, to determine if any of the tumor samples had activating kinase mutations, we sequenced exon 17–22 of the EGFR tyrosine kinase domain. We found none of the known activating mutations in exon 18, 19, 20, and 21. Interestingly, in two of the samples, we identified two missense mutations. The first mutation is a change in nucleotide 2197 of exon 19, resulting in a change of the codon from CCA to TCA (Figure 2(c)). This changes the encoded amino acid from proline to serine (P733S). Using the PolyPhen program that predicts functional effect of human non-synonymous SNPs, the P733S mutation is predicted to have probable functional consequences. This mutation brings closest contact with the Tyr 922 phosphorylation site (3.918Å) and was identified in a T3N2 laryngeal tumor with high EGFR expression and truncated EGFR. The P733S mutation has been described only once previously in a synovial sarcoma (9). The second mutation is a change in nucleotide 2243 of exon 19, resulting in a change of the codon from AGA to ATA (data not shown). This changes the amino acid from arginine to isoleucine (R748I). However, the PolyPhen program predicts that the R748I mutation is likely benign. To our knowledge, this mutation has not been previously reported in any other tumors. Overall, the frequency of kinase mutations in our study population is low. This is consistent with the recent report that only 7% of HNSCC have kinase mutations (10). The mutations that we identified are different from the ones reported by these investigators. In addition, they did not find any extracellular mutations, while we found two novel truncations in addition to EGFRvIII.
Clinical biological correlates of EGFR expression, phosphorylation, and mutation in combination
Table 1 summarizes the clinical and pathological characteristics of our study population. The majority of the patients are Caucasian males, less than 65 years of age, who smoked and drank. The majority of the tumors are located in the oropharynx, moderately differentiated with equal distribution of low and high tumor or nodal stage. These characteristics are similar to other study populations in North America (2, 3). Of the 60 primary HNSCC, tumor stage was not addressed in the pathology report of one case and nodal staging was missing for four cases that did not include neck dissections. As shown in Table 2, there were no significant relations between phosphorylation or high expression of EGFR alone and advanced tumor, nodal, or overall stage. These results are consistent with what others have reported (2, 3, 5). On the other hand, when HNSCC with either phosphorylated or truncated EGFR were analyzed in combination, a statistically significant association was found between those that are positive for either phosphorylated or truncated EGFR and higher tumor or nodal stage (Table 2). In addition, there is a trend toward significant correlation between phosphorylated or truncated EGFR+ tumors and higher overall stage. We also tested the possibility of an interaction effect between truncated (Δ) and phosphorylated (p−) EGFR in their association with advanced stage disease using logistic regression model. Interestingly, we found that the effect of ΔEGFR in differentiating advanced tumor stage depends on the status of pEGFR (p = .001, test of interaction between ΔEGFR and pEGFR). In particular, among pEGFR− tumors, ΔEGFR expression is highly indicative of advanced T-stage. All ΔEGFR+ (11/11 or 100%) tumors were T3/4 stage, while only 37.5% (9/15) ΔEGFR− tumor were T3/4 (p= .0005, Fisher’s Exact test). On the other hand, ΔEGFR status was not a strong predictor for advanced T-stage among pEGFR+ tumors. This additional result further corroborates the synergistic effect of ΔEGFR and pEGFR in their correlation with HNSCC staging. To our surprise, we also noticed a significant association between truncated EGFR expression alone and advanced tumor as well as overall stage. However, the number of low-stage tumors with truncated EGFR is very small. While the small sample size might skew the chi square analysis toward significance, it is noted that results based on Fisher’s exact test remain similar. We also correlated the EGFR status to age, sex, risk factors, such as smoking and alcohol use, and histologic features, such as differentiation and invasion, but did not find any significant association (data not shown). Nevertheless, we found that patients with ΔEGFR+ HNSCC are significantly younger with mean age of 52.8 ± 9.0 years than ΔEGFR− patients (mean age = 59.6 ± 12.5 years, p= .02). Overall, these results suggest that HNSCC with either truncated or activated EGFR tend to have higher tumor and nodal stage.
Table 1.
Clinical and Pathological Characteristics of Patient Population
| Characteristics | No. of Patients (%) |
|---|---|
| Sex | |
| Male | 45 (75) |
| Female | 15 (25) |
| Race | |
| White | 53 (88.3) |
| Black | 2 (3.3) |
| Asian | 1 (1.7) |
| Unknown | 4 (6.7) |
| Age (years) | |
| <65 | 43 (71.7) |
| ≥65 | 17 (28.3) |
| Smoking | |
| Yes | 37 (61.7) |
| No | 11 (18.3) |
| Unknown | 12 (20) |
| Alcohol | |
| Yes | 34 (56.7) |
| No | 12 (20) |
| Unknown | 14 (23.3) |
| Tumorsite | |
| Qropharynx | 41 (68.3) |
| Larynx | 19 (31.7) |
| T stage | |
| Tis/T1/T2 | 26 (43.3) |
| T3/T4 | 33 (55) |
| Tx | 1 (17) |
| N stage | |
| NO/N1 | 27 (45) |
| N2 | 27 (45) |
| nx | 6 (10) |
| Differentiation | |
| Well | 10 (16.7) |
| Moderate | 42 (70) |
| Poor | 5 (8.3) |
| Unknown | 3 (5) |
| Perineural Invasion | |
| Yes | 25 (41.7) |
| No | 15 (25) |
| Unknown | 20 (33) |
| Lymphovascular Invasion | |
| Yes | 18 (30) |
| No | 35 (58.3) |
| Unknown | 7 (11.7) |
Table 2.
Correlation of Disease Stage and Aspect of EGFR Alone or in Combination
| pEGFR |
p | ΔEGIFR |
p | EGIFR Expression |
p | p− or Δ EG FR |
p | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| + | − | + | − | High | Low | + | − | |||||
| T-stage | ||||||||||||
| Tis/1/2 | 11 | 15 | 3 | 23 | 14 | 12 | 11 | 15 | ||||
| T3/4 | 13 | 20 | NS | 13 | 20 | .02 | 22 | 11 | NS | 24 | 9 | .02 |
| N-stage | ||||||||||||
| NO/1 | 8 | 19 | 6 | 21 | 16 | 11 | 13 | 14 | ||||
| N2 | 13 | 14 | .16 | 11 | 16 | .14 | 18 | 9 | NS | 20 | 7 | .05 |
| Staging | ||||||||||||
| 1/2 | 4 | 6 | 0 | 10 | 5 | 5 | 4 | 6 | ||||
| 3/4 | 17 | 27 | NS | 27 | 17 | .0007 | 29 | 15 | NS | 29 | 15 | 0.13 |
pEGFR: phosphorylated EGFR; ΔEGFR: truncated EGFR (i.e., EGFRvIII, EGFR Δ471, or EGFR Δ660); p− or ΔEGFR: phosphorylated or truncated EGFR; NS: not significant, p > .3.
DISCUSSION
Ample data over the last 20 years strongly support an important role of EGFR and its ligands in the development and progression of HNSCC. Overexpression of EGFR has been reported in up to 80% of HNSCC (11); EGFR mRNA and protein levels are also increased in dysplastic lesions and histologically normal mucosa from HNSCC patients (12). Studies further exploring the prognostic value of EGFR in HNSCC began to emerge in the late 1990s. Quantitative differences in EGFR protein levels are reliable predictors of adverse outcome in head and neck cancer patients (2, 3, 13). With these data, strategies to block the EGFR activity were developed. EGFR targeting agents are now in clinical trials for HNSCC. Despite the mountain of evidence suggesting the importance of EGFR in HNSCC, the results of various clinical trials testing different EGFR inhibitors are not as dramatic as one would expect. Phase II studies testing the efficacy of EGFR inhibitors, such as gefitinib (Iressa), erlotinib (Tarceva), and cetuximab (Erbitux), in treating recurrent or metastatic HNSCC yield a response rate of 5–10% (14–16). While the phase III randomized clinical trial showed that Erbitux improved the local regional control and significantly prolonged the progression-free survival of patients with advanced stage HNSCC, the overall survival at 3 years only improved by 10% (17). Apart from this limited therapeutic benefit of EGFR inhibitors, another puzzling observation noted in these studies was the lack of correlation between EGFR expression and disease stage (2, 3); this suggests that other factors may contribute to disease aggressiveness. A recent study found that 42% of HNSCC expresses the EGFR truncation mutant, EGFRvIII (6). EGFRvIII is a deletion mutant of the EGFR gene that was first discovered in glioblastomas (18). This mutant contains an in-frame deletion from exon 2–7 (Δ801) and encodes a truncated receptor that is constitutively active, but lacks the majority of the extracellular domain. Evidence that cancer cells with EGFRvIII expression were not sensitive to Erbitux or Tarceva is now emerging in different reports (6, 19). Thus, truncated forms of EGFR may be another important aspect of EGFR in HNSCC.
In the current study, we investigate whether EGFR status and disease staging correlate when the different aspects of the receptor are evaluated in combination. Several interesting yet unexpected results surfaced from our analyses. The first is that HN-SCC with equivalent amount of EGFR do not necessarily possess similar receptor activity level. Of the 60 HNSCC, only 40% have phosphorylated EGFR. While not all EGFR-expressing tumors have phosphorylated EGFR, the higher the expression, the more likely the EGFR is active. Our data are consistent with previous reports that both high EGFR expression and phosphorylated EGFR correlated with worse disease-free survival (2, 3). However, phosphorylated EGFR alone does not predict advanced stage disease. Thus, we examined other aspects of the receptor and searched for EGFR mutations. Like others, we found EGFRvIII in 23% HNSCC. Surprisingly, we also discovered two novel EGFR truncations, EGFRΔ471 and Δ660. To our knowledge, these mutants have not been previously described in primary tumors; both contain in-frame deletions of the EGFR extracellular domain, similar to EGFRvIII. When analyzed in combination, truncated EGFR synergizes with phosphorylated EGFR in correlation with advanced stage disease. HNSCC with either truncated forms of EGFR or activated full-length EGFR tend to be of higher tumor and nodal stage. In addition, we found a highly significant interaction effect between truncated and phosphorylated EGFR in their correlation with advanced tumor stage. We excluded the possibility of kinase mutants contributing to the correlation, as only two samples carry kinase mutations. Therefore, activated and truncated EGFR together might represent biomarkers for aggressive HNSCC. We acknowledged that multiple statistical tests were performed in our analysis and this could increase the chance of seeing significant results. However, our investigation is exploratory in nature and the current findings are suggestive of an association between combined truncated or phosphorylated EGFR and advanced stage disease. Another interesting finding from this study is the discovery of three potentially significant EGFR mutations: two novel extracellular mutants and the P733S missense kinase mutation. While other EGFR deletion mutants have been identified in glioblastomas (20), the ones that we discovered in HNSCC were distinctly different. They closely resemble EGFRvIII and therefore might possess oncogenic potential. A recent report has suggested that genomic deletions occur at breakpoints around Alu repeat elements in EGFR introns 1 and 7, which could be the potential mechanism of EGFRvIII synthesis (21). Given this finding, we postulate that EGFRΔ471 and 660 production may be the result of genomic alterations. While EGFR kinase mutations are rare in HNSCC, they do exist in a low percentage of tumors as demonstrated in this and other studies (10, 22, 23). Analyses of multiple sequence alignments and protein 3D-structures by the PolyPhen program predict with high confidence that the P733S mutation affects the protein structure and function. Further biochemical characterization of these mutations is needed to define their functional significance.
It has become increasingly clear from this and other studies that even tumors with the same histologic diagnoses are not the same in terms of their molecular profile. Some of these differences may dictate patients’ response to molecular target therapy. For instance, only about 10% of patients with non-small cell lung cancer have activating mutations in the EGFR tyrosine kinase domain; these patients demonstrated dramatic response to gefitinib (24). As shown in our study, not all EGFR-expressing HNSCC are the same. Thus, there has been increasing emphasis on the application of molecular biomarkers to further classify cancer of the same histologic diagnosis. To better understand the target, we used various methods to analyze EGFR in HN-SCC. It is apparent that each technique offers an advantage on examining a certain aspect of the receptor. The discrepancy between IPW and IHC in detecting pEGFR is a good example of why different assays are needed to examine a molecular target. The 4G10 antibody used detects all the phosphorylated tyrosine residues of EGFR in IPW and thus is likely more sensitive than the Tyr-1068 specific pEGFR antibody used in IHC. In addition, pEGFR IHC stain tend to be more focal and less intense, which makes interpretation subjective and difficult. On the other hand, the IPW data is less prone to interpretation bias. Thus, the IPW method offers an added dimension to the analysis of EGFR status in this case. As most translational studies still rely on pEGFR-1068 IHC to perform correlation analysis, our results support the need to consider additional technique(s) when evaluating EGFR in primary tumors. Overall, the combination of results from various assays provided a more in-depth molecular characterization of EGFR in the tumor. The value of this approach can be seen when more accurate clinical biological correlation was observed with different EGFR status evaluated in combination. Although many translational studies used IHC to analyze EGFR expression, protein expression is only one aspect of the receptor status; by itself, expression may not always tell the whole story.
CONCLUSION
While many HNSCC express high level of EGFR, not all high EGFR expressors have the activated receptor. In addition, some HNSCC express truncated forms of EGFR. The combination of truncated and activated EGFR appears to be associated with advanced tumor and nodal stage. We also discovered three potentially significant EGFR mutations in HNSCC: two novel extracellular mutants and the P733S missense kinase mutation. Efforts are ongoing to further characterize the oncogenic potential of these novel EGFR mutants.
Acknowledgments
Tissue samples were provided by the Cooperative Human Tissue Network, which is funded by the National Cancer Institute. This work was supported in part by research grants from the Long Island League to Abolish Cancer (E.L.C. and M.J.H.), the National Cancer Institute R21CA133642–01A2 (M.J.H.), the Carol Baldwin Breast Cancer Research Foundation, the Stony Brook University Medical Center GCRC M01 RR10710, and the Sunrise Fund (E.L.C.). Dr. Chan is the recipient of NIH Pediatric Research Loan Repayment Program Award, the clinical scholar award from the Stony Brook Clinical Research Training Program K30 RR022285–01 and the mentored research scholar grant from the American Cancer Society 117718-MRSG-09–172-01-CCE (E.L.C.). We also want to thank Dr. Kepal Patel for providing the MDA1386 cell line.
Footnotes
DECLARATION OF INTEREST
Dr. Chan and Mr. James Keller, in conjunction with the Office of Technology Licensing at Stony Brook University are filing a provisional patent application on EGFRΔ471 and Δ660. The authors alone are responsible for the content and writing of this paper.
References
- 1.Kolibaba KS, Druker BJ. Protein tyrosine kinases and cancer. Biochim Biophys Acta. 1997 Dec 9;1333(3):F217–F248. doi: 10.1016/s0304-419x(97)00022-x. [DOI] [PubMed] [Google Scholar]
- 2.Rubin Grandis J, Melhem MF, Gooding WE, Day R, Holst VA, Wagener MM, Drenning SD, Tweardy DJ. Levels of TGF-alpha and EGFR protein in head and neck squamous cell carcinoma and patient survival. J Natl Cancer Inst. 1998 Jun 3;90(11):824–832. doi: 10.1093/jnci/90.11.824. [DOI] [PubMed] [Google Scholar]
- 3.Ang KK, Berkey BA, Tu X, Zhang HZ, Katz R, Hammond EH, Fu KK, Milas L. Impact of epidermal growth factor receptor expression on survival and pattern of relapse in patients with advanced head and neck carcinoma. Cancer Res. 2002 Dec 15;62( 24):7350–7356. [PubMed] [Google Scholar]
- 4.Kong A, Leboucher P, Leek R, Calleja V, Winter S, Harris A, Parker PJ, Larijani B. Prognostic value of an activation state marker for epidermal growth factor receptor in tissue microarrays of head and neck cancer. Cancer Res. 2006 Mar 1;66(5):2834–2843. doi: 10.1158/0008-5472.CAN-05-2994. [DOI] [PubMed] [Google Scholar]
- 5.Hiraishi Y, Wada T, Nakatani K, Negoro K, Fujita S. Immuno-histochemical expression of EGFR and p-EGFR in oral squamous cell carcinomas. Pathol Oncol Res. 2006;12(2):87–91. doi: 10.1007/BF02893450. [DOI] [PubMed] [Google Scholar]
- 6.Sok JC, Coppelli FM, Thomas SM, Lango MN, Xi S, Hunt JL, Freilino ML, Graner MW, Wikstrand CJ, Bigner DD, Gooding WE, Furnari FB, Grandis JR. Mutant epidermal growth factor receptor (EGFRvIII) contributes to head and neck cancer growth and resistance to EGFR targeting. Clin Cancer Res. 2006 Sep 1;12(17):5064–5073. doi: 10.1158/1078-0432.CCR-06-0913. [DOI] [PubMed] [Google Scholar]
- 7.Slamon DJ, Godolphin W, Jones LA, Holt JA, Wong SG, Keith DE, Levin WJ, Stuart SG, Udove J, Ullrich A, Press MF. Studies of the HER-2/neu proto-oncogene in human breast and ovarian cancer. Science. 1989 May 12;244(4905):707–712. doi: 10.1126/science.2470152. [DOI] [PubMed] [Google Scholar]
- 8.Agazie YM, Hayman MJ. Molecular mechanism for a role of SHP2 in epidermal growth factor receptor signaling. Mol Cell Biol. 2003 Nov;23(21):7875–7886. doi: 10.1128/MCB.23.21.7875-7886.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bode B, Frigerio S, Behnke S, Senn B, Odermatt B, Zimmermann DR, Moch H. Mutations in the tyrosine kinase domain of the EGFR gene are rare in synovial sarcoma. Mod Pathol. 2006 Apr;19(4):541–547. doi: 10.1038/modpathol.3800560. [DOI] [PubMed] [Google Scholar]
- 10.Hama T, Yuza Y, Saito Y, Ou J, Kondo S, Okabe M, Yamada H, Kato T, Moriyama H, Kurihara S, Urashima M. Prognostic significance of epidermal growth factor receptor phosphorylation and mutation in head and neck squamous cell carcinoma. Oncologist. 2009 Sep;14(9):900–908. doi: 10.1634/theoncologist.2009-0058. [DOI] [PubMed] [Google Scholar]
- 11.Quon H, Liu FF, Cummings BJ. Potential molecular prognostic markers in head and neck squamous cell carcinomas. Head Neck. 2001 Feb;23(2):147–159. doi: 10.1002/1097-0347(200102)23:2<147::aid-hed1010>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- 12.Ford AC, Grandis JR. Targeting epidermal growth factor receptor in head and neck cancer. Head Neck. 2003 Jan;25(1):67–73. doi: 10.1002/hed.10224. [DOI] [PubMed] [Google Scholar]
- 13.Etienne MC, Pivot X, Formento JL, Bensadoun RJ, Formento P, Dassonville O, Francoual M, Poissonnet G, Fontana X, Schneider M, Demard F, Milano G. A multifactorial approach including tumoural epidermal growth factor receptor, p53, thymidylate synthase and dihydropyrimidine dehydrogenase to predict treatment outcome in head and neck cancer patients receiving 5-fluorouracil. Br J Cancer. 1999 Apr;79(11–12):1864–1869. doi: 10.1038/sj.bjc.6690297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cohen EE, Rosen F, Stadler WM, Recant W, Stenson K, Huo D, Vokes DE. Phase-II trial of ZD1839 in recurrent or metastatic squamous cell carcinoma of the head and neck. J Clin Oncol. 2003 May 15;21(10):1980–1987. doi: 10.1200/JCO.2003.10.051. [DOI] [PubMed] [Google Scholar]
- 15.Soulieres D, Senzer NN, Vokes EE, Hidalgo M, Agarwala SS, Siu LL. Multicenter phase II study of erlotinib, an oral epidermal growth factor receptor tyrosine kinase inhibitor, in patients with recurrent or metastatic squamous cell cancer of the head and neck. J Clin Oncol. 2004 Jan 1;22(1):77–85. doi: 10.1200/JCO.2004.06.075. [DOI] [PubMed] [Google Scholar]
- 16.Baselga J, Trigo JM, Bourhis J, Tortochaux J, Cortes-Funes H, Hitt R, Gascon P, Amellal N, Harstrick A, Eckhardt A. Phase II multicenter study of the antiepidermal growth factor receptor monoclonal antibody cetuximab in combination with platinum-based chemotherapy in patients with platinum-refractory metastatic and/or recurrent squamous cell carcinoma of the head and neck. J Clin Oncol. 2005 Aug 20;23(24):5568–5577. doi: 10.1200/JCO.2005.07.119. [DOI] [PubMed] [Google Scholar]
- 17.Bonner JA, Harari PM, Giralt J, Azarnia N, Shin DM, Cohen RB, Jones CU, Sur R, Raben D, Jassem J, Ove R, Kies MS, Baselga J, Youssoufian H, Amellal N, Rowinsky EK, Ang KK. Radiotherapy plus cetuximab for squamous-cell carcinoma of the head and neck. N Engl J Med. 2006 Feb 9;354(6):567–578. doi: 10.1056/NEJMoa053422. [DOI] [PubMed] [Google Scholar]
- 18.Pedersen MW, Meltorn M, Damstrup L, Poulsen HS. The type-III epidermal growth factor receptor mutation. Biological significance and potential target for anti-cancer therapy. Ann Oncol. 2001 Jun;12(6):745–760. doi: 10.1023/a:1011177318162. [DOI] [PubMed] [Google Scholar]
- 19.Ji H, Zhao X, Yuza Y, Shimamura T, Li D, Protopopov A, Jung BL, McNamara K, Xia H, Glatt KA, Thomas RK, Sasaki H, Horner JW, Eck M, Mitchell A, Sun Y, Al-Hashem R, Bronson RT, Rabindran SK, Discafani CM, Maher E, Shapiro GI, Meyerson M, Wong KK. Epidermal growth factor receptor variant III mutations in lung tumorigenesis and sensitivity to tyrosine kinase inhibitors. Proc Natl Acad Sci USA. 2006 May 16;103(20):7817–7822. doi: 10.1073/pnas.0510284103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Frederick L, Wang XY, Eley G, James CD. Diversity and frequency of epidermal growth factor receptor mutations in human glioblastomas. Cancer Res. 2000 Mar 1;60(5):1383–1387. [PubMed] [Google Scholar]
- 21.Frederick L, Eley G, Wang XY, James CD. Analysis of genomic rearrangements associated with EGRFvIII expression suggests involvement of Alu repeat elements. Neuro Oncol. 2000 Jul;2( 3):159–163. doi: 10.1093/neuonc/2.3.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Loeffler-Ragg J, Witsch-Baumgartner M, Tzankov A, Hilbe W, Schwentner I, Sprinzl GM, Utermann G, Zwierzina H. Low incidence of mutations in EGFR kinase domain in Caucasian patients with head and neck squamous cell carcinoma. Eur J Cancer. 2006 Jan;42(1):109–111. doi: 10.1016/j.ejca.2005.08.034. [DOI] [PubMed] [Google Scholar]
- 23.Lee JW, Soung YH, Kim SY, Nam HK, Park WS, Nam SW, Kim MS, Sun DI, Lee YS, Jang JJ, Lee JY, Yoo NJ, Lee SH. Somatic mutations of EGFR gene in squamous cell carcinoma of the head and neck. Clin Cancer Res. 2005 Apr 15;11( 8):2879–2882. doi: 10.1158/1078-0432.CCR-04-2029. [DOI] [PubMed] [Google Scholar]
- 24.Lynch TJ, Bell DW, Sordella R, Gurubhagavatula S, Okimoto RA, Brannigan BW, Harris PL, Haserlat SM, Supko JG, Haluska FG, Louis DN, Christiani DC, Settleman J, Haber DA. Activating mutations in the epidermal growth factor receptor underlying responsiveness of non-small-cell lung cancer to gefitinib. N Engl J Med. 2004 May 20;350(21):2129–2139. doi: 10.1056/NEJMoa040938. [DOI] [PubMed] [Google Scholar]