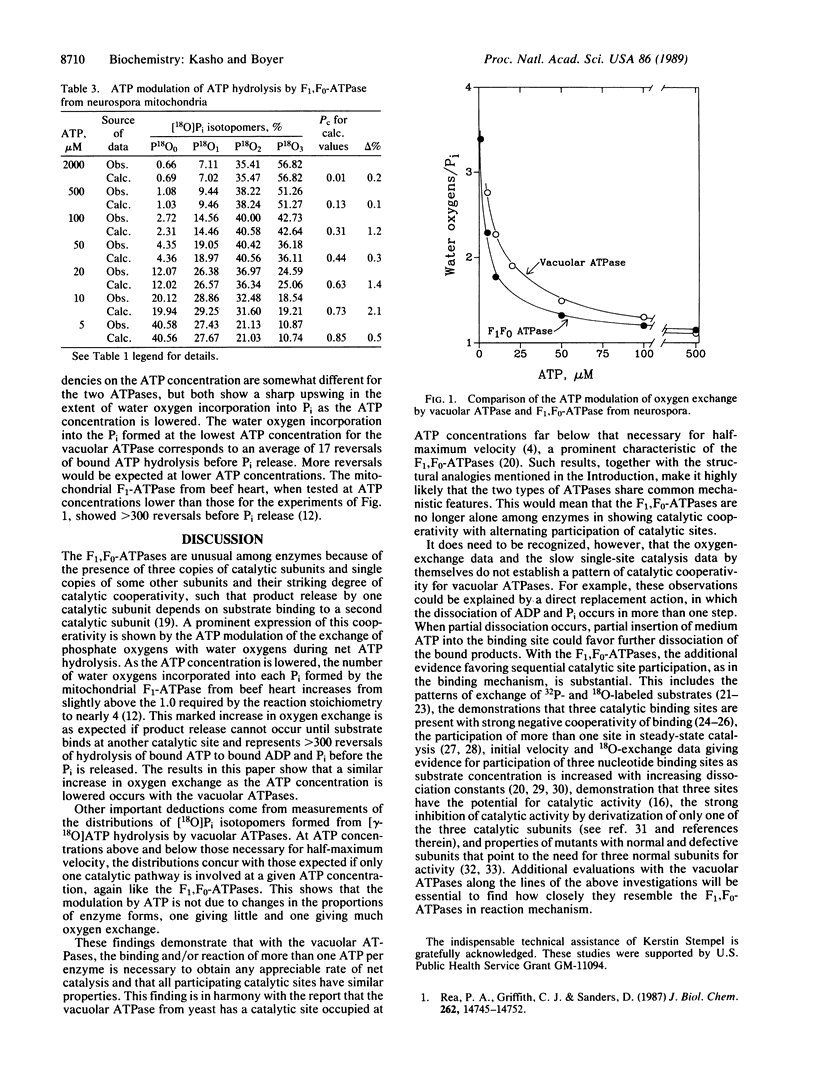
Abstract
Recent studies with vacuolar ATPases have shown that multiple copies catalytic subunits are present and that these have definite sequence homology with catalytic subunits of the F1,F0-ATPases. Experiments are reported that assess whether the vacuolar ATPases may have the unusual catalytic cooperativity with sequential catalytic site participation as in the binding change mechanism for the F1,F0-ATPases. The extent of reversal of bound ATP hydrolysis to bound ADP and Pi as medium ATP concentration was lowered was determined by 18O-exchange measurements for yeast and neurospora vacuolar ATPases. The results show a pronounced increase in the extent of water oxygen incorporation into the Pi formed as ATP concentration is decreased to the micromolar range. The F1,F0-ATPase from neurospora mitochondria showed an even more pronounced modulation, similar to that of other F1-type ATPases. The vacuolar ATPases thus appear to have a catalytic mechanism quite analogous to that of the F1,F0-ATPases.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arai H., Terres G., Pink S., Forgac M. Topography and subunit stoichiometry of the coated vesicle proton pump. J Biol Chem. 1988 Jun 25;263(18):8796–8802. [PubMed] [Google Scholar]
- Aris J. P., Blobel G. Identification and characterization of a yeast nucleolar protein that is similar to a rat liver nucleolar protein. J Cell Biol. 1988 Jul;107(1):17–31. doi: 10.1083/jcb.107.1.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowman B. J., Allen R., Wechser M. A., Bowman E. J. Isolation of genes encoding the Neurospora vacuolar ATPase. Analysis of vma-2 encoding the 57-kDa polypeptide and comparison to vma-1. J Biol Chem. 1988 Oct 5;263(28):14002–14007. [PubMed] [Google Scholar]
- Bowman E. J., Bowman B. J. Purification of vacuolar membranes, mitochondria, and plasma membranes from Neurospora crassa and modes of discriminating among the different H+-ATPases. Methods Enzymol. 1988;157:562–573. doi: 10.1016/0076-6879(88)57104-5. [DOI] [PubMed] [Google Scholar]
- Bowman E. J. Comparison of the vacuolar membrane ATPase of Neurospora crassa with the mitochondrial and plasma membrane ATPases. J Biol Chem. 1983 Dec 25;258(24):15238–15244. [PubMed] [Google Scholar]
- Bowman E. J., Mandala S., Taiz L., Bowman B. J. Structural studies of the vacuolar membrane ATPase from Neurospora crassa and comparison with the tonoplast membrane ATPase from Zea mays. Proc Natl Acad Sci U S A. 1986 Jan;83(1):48–52. doi: 10.1073/pnas.83.1.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowman E. J., Tenney K., Bowman B. J. Isolation of genes encoding the Neurospora vacuolar ATPase. Analysis of vma-1 encoding the 67-kDa subunit reveals homology to other ATPases. J Biol Chem. 1988 Oct 5;263(28):13994–14001. [PubMed] [Google Scholar]
- Boyer P. D. The unusual enzymology of ATP synthase. Biochemistry. 1987 Dec 29;26(26):8503–8507. doi: 10.1021/bi00400a001. [DOI] [PubMed] [Google Scholar]
- Cross R. L., Grubmeyer C., Penefsky H. S. Mechanism of ATP hydrolysis by beef heart mitochondrial ATPase. Rate enhancements resulting from cooperative interactions between multiple catalytic sites. J Biol Chem. 1982 Oct 25;257(20):12101–12105. [PubMed] [Google Scholar]
- Cross R. L., Nalin C. M. Adenine nucleotide binding sites on beef heart F1-ATPase. Evidence for three exchangeable sites that are distinct from three noncatalytic sites. J Biol Chem. 1982 Mar 25;257(6):2874–2881. [PubMed] [Google Scholar]
- Gresser M. J., Myers J. A., Boyer P. D. Catalytic site cooperativity of beef heart mitochondrial F1 adenosine triphosphatase. Correlations of initial velocity, bound intermediate, and oxygen exchange measurements with an alternating three-site model. J Biol Chem. 1982 Oct 25;257(20):12030–12038. [PubMed] [Google Scholar]
- Grubmeyer C., Penefsky H. S. The presence of two hydrolytic sites on beef heart mitochondrial adenosine triphosphatase. J Biol Chem. 1981 Apr 25;256(8):3718–3727. [PubMed] [Google Scholar]
- Hackney D. D., Boyer P. D. Evaluation of the partitioning of bound inorganic phosphate during medium and intermediate phosphate in equilibrium water oxygen exchange reactions of yeast inorganic pyrophosphatase. Proc Natl Acad Sci U S A. 1978 Jul;75(7):3133–3137. doi: 10.1073/pnas.75.7.3133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutton R. L., Boyer P. D. Subunit interaction during catalysis. Alternating site cooperativity of mitochondrial adenosine triphosphatase. J Biol Chem. 1979 Oct 25;254(20):9990–9993. [PubMed] [Google Scholar]
- Kaestner K. H., Randall S. K., Sze H. N,N'-dicyclohexylcarbodiimide-binding proteolipid of the vacuolar H+-ATPase from oat roots. J Biol Chem. 1988 Jan 25;263(3):1282–1287. [PubMed] [Google Scholar]
- Kasho V. N., Yoshida M., Boyer P. D. F1 ATPase from the thermophilic bacterium PS3 (TF1) shows ATP modulation of oxygen exchange. Biochemistry. 1989 Aug 22;28(17):6949–6954. doi: 10.1021/bi00443a026. [DOI] [PubMed] [Google Scholar]
- Kayalar C., Rosing J., Boyer P. D. An alternating site sequence for oxidative phosphorylation suggested by measurement of substrate binding patterns and exchange reaction inhibitions. J Biol Chem. 1977 Apr 25;252(8):2486–2491. [PubMed] [Google Scholar]
- Mandala S., Taiz L. Partial purification of a tonoplast ATPase from corn coleoptiles. Plant Physiol. 1985 Jun;78(2):327–333. doi: 10.1104/pp.78.2.327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuno-Yagi A., Hatefi Y. Kinetic modalities of ATP synthesis. Regulation by the mitochondrial respiratory chain. J Biol Chem. 1986 Oct 25;261(30):14031–14038. [PubMed] [Google Scholar]
- Melese T., Xue Z. X., Stempel K. E., Boyer P. D. Catalytic properties of chloroplast F1-ATPase modified at catalytic or noncatalytic sites by 2-azido adenine nucleotides. J Biol Chem. 1988 Apr 25;263(12):5833–5840. [PubMed] [Google Scholar]
- Noumi T., Taniai M., Kanazawa H., Futai M. Replacement of arginine 246 by histidine in the beta subunit of Escherichia coli H+-ATPase resulted in loss of multi-site ATPase activity. J Biol Chem. 1986 Jul 15;261(20):9196–9201. [PubMed] [Google Scholar]
- O'Neal C. C., Boyer P. D. Assessment of the rate of bound substrate interconversion and of ATP acceleration of product release during catalysis by mitochondrial adenosine triphosphatase. J Biol Chem. 1984 May 10;259(9):5761–5767. [PubMed] [Google Scholar]
- Rao R., Senior A. E. The properties of hybrid F1-ATPase enzymes suggest that a cyclical catalytic mechanism involving three catalytic sites occurs. J Biol Chem. 1987 Dec 25;262(36):17450–17454. [PubMed] [Google Scholar]
- Rea P. A., Griffith C. J., Sanders D. Purification of the N,N'-dicyclohexylcarbodiimide-binding proteolipid of a higher plant tonoplast H+-ATPase. J Biol Chem. 1987 Oct 25;262(30):14745–14752. [PubMed] [Google Scholar]
- Rosen G., Gresser M., Vinkler C., Boyer P. D. Assessment of total catalytic sites and the nature of bound nucleotide participation in photophosphorylation. J Biol Chem. 1979 Nov 10;254(21):10654–10661. [PubMed] [Google Scholar]
- Stempel K. E., Boyer P. D. Refinements in oxygen-18 methodology for the study of phosphorylation mechanisms. Methods Enzymol. 1986;126:618–639. doi: 10.1016/s0076-6879(86)26065-6. [DOI] [PubMed] [Google Scholar]
- Stroop S. D., Boyer P. D. Catalytic and regulatory effects of light intensity on chloroplast ATP synthase. Biochemistry. 1987 Mar 10;26(5):1479–1484. doi: 10.1021/bi00379a040. [DOI] [PubMed] [Google Scholar]
- Sun S. Z., Xie X. S., Stone D. K. Isolation and reconstitution of the dicyclohexylcarbodiimide-sensitive proton pore of the clathrin-coated vesicle proton translocating complex. J Biol Chem. 1987 Oct 25;262(30):14790–14794. [PubMed] [Google Scholar]
- Uchida E., Ohsumi Y., Anraku Y. Characterization and function of catalytic subunit alpha of H+-translocating adenosine triphosphatase from vacuolar membranes of Saccharomyces cerevisiae. A study with 7-chloro-4-nitrobenzo-2-oxa-1,3-diazole. J Biol Chem. 1988 Jan 5;263(1):45–51. [PubMed] [Google Scholar]
- Wu D., Boyer P. D. Bound adenosine 5'-triphosphate formation, bound adenosine 5'-diphosphate and inorganic phosphate retention, and inorganic phosphate oxygen exchange by chloroplast adenosinetriphosphatase in the presence of Ca2+ or Mg2+. Biochemistry. 1986 Jun 3;25(11):3390–3396. doi: 10.1021/bi00359a044. [DOI] [PubMed] [Google Scholar]
- Xue Z. X., Melese T., Stempel K. E., Reedy T. J., Boyer P. D. Properties of chloroplast F1-ATPase partially modified by 2-azido adenine nucleotides, including demonstration of three catalytic pathways. J Biol Chem. 1988 Nov 15;263(32):16880–16885. [PubMed] [Google Scholar]
- Xue Z. X., Zhou J. M., Melese T., Cross R. L., Boyer P. D. Chloroplast F1 ATPase has more than three nucleotide binding sites, and 2-azido-ADP or 2-azido-ATP at both catalytic and noncatalytic sites labels the beta subunit. Biochemistry. 1987 Jun 30;26(13):3749–3753. doi: 10.1021/bi00387a001. [DOI] [PubMed] [Google Scholar]
- Zimniak L., Dittrich P., Gogarten J. P., Kibak H., Taiz L. The cDNA sequence of the 69-kDa subunit of the carrot vacuolar H+-ATPase. Homology to the beta-chain of F0F1-ATPases. J Biol Chem. 1988 Jul 5;263(19):9102–9112. [PubMed] [Google Scholar]


