Abstract
The title compound, C18H12N2O, comprises two aromatic fragments, viz., imidazo[2,1-a]isoquinoline and benzene, linked by oxygen and methylene bridges. Despite the absence of a common conjugative system within the molecule, it adopts an essentially planar conformation with an r.m.s. deviation of 0. 036 Å. In the crystal, due to this structure, molecules form stacks along the b axis by π⋯π stacking interactions, with shortest C⋯C distances in the range 3.340 (4)–3.510 (4) Å. The molecules are bound by intermolecular C—H⋯O interactions within the stacks and C—H⋯π interactions between the stacks.
Related literature
For background to cascade reactions, see: Bunce (1995 ▶); Tietze (1996 ▶); Parsons et al. (1996 ▶); Nicolaou et al. (2003 ▶, 2006 ▶); Wasilke et al. (2005 ▶); Pellissier (2006a
▶,b
▶); Parenty & Cronin (2008 ▶). For related compounds, see: Yadav et al. (2007 ▶); Kianmehr et al. (2009 ▶); Surpur et al. (2009 ▶).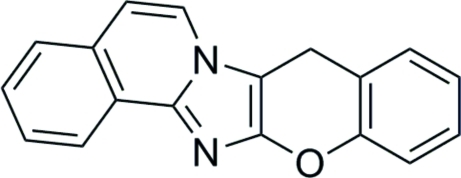
Experimental
Crystal data
C18H12N2O
M r = 272.30
Monoclinic,
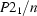
a = 11.9717 (15) Å
b = 6.0580 (8) Å
c = 17.948 (2) Å
β = 102.682 (3)°
V = 1269.9 (3) Å3
Z = 4
Mo Kα radiation
μ = 0.09 mm−1
T = 100 K
0.40 × 0.12 × 0.02 mm
Data collection
Bruker APEXII CCD diffractometer
Absorption correction: multi-scan (SADABS; Sheldrick, 2003 ▶) T min = 0.965, T max = 0.998
12413 measured reflections
2734 independent reflections
1821 reflections with I > 2σ(I)
R int = 0.056
Refinement
R[F 2 > 2σ(F 2)] = 0.066
wR(F 2) = 0.182
S = 1.00
2734 reflections
190 parameters
H-atom parameters constrained
Δρmax = 0.45 e Å−3
Δρmin = −0.23 e Å−3
Data collection: APEX2 (Bruker, 2005 ▶); cell refinement: SAINT-Plus (Bruker, 2001 ▶); data reduction: SAINT-Plus; program(s) used to solve structure: SHELXS97 (Sheldrick, 2008 ▶); program(s) used to refine structure: SHELXL97 (Sheldrick, 2008 ▶); molecular graphics: SHELXTL (Sheldrick, 2008 ▶); software used to prepare material for publication: SHELXTL.
Supplementary Material
Crystal structure: contains datablocks global, I. DOI: 10.1107/S1600536810006744/rk2193sup1.cif
Structure factors: contains datablocks I. DOI: 10.1107/S1600536810006744/rk2193Isup2.hkl
Additional supplementary materials: crystallographic information; 3D view; checkCIF report
Table 1. Hydrogen-bond geometry (Å, °).
Cg2 is the centroid of the O13,C12A,C8A,C8,C7A,C13A ring.
| D—H⋯A | D—H | H⋯A | D⋯A | D—H⋯A |
|---|---|---|---|---|
| C8—H8A⋯O13i | 0.99 | 2.71 | 3.637 (4) | 157 |
| C8—H8B⋯Cgii | 0.99 | 2.63 | 3.547 (3) | 154 |
Symmetry codes: (i) 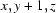 ; (ii)
; (ii) 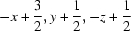 .
.
supplementary crystallographic information
Comment
Cascade reactions have emerged as powerful tools to allow rapidly increasing molecular complexity (Tietze, 1996; Parsons et al., 1996; Wasilke et al., 2005). These processes avoid the excessive handling and isolation of synthetic intermediates generating less waste and thus contribute towards "Green Chemistry". Cascade reactions, in which multiple reactions are combined into one synthetic operation, have been reported extensively in the literature and have already become "state–of–the–art" in synthetic organic chemistry (Bunce, 1995; Nicolaou et al., 2003, 2006; Pellissier, 2006a, 2006b; Parenty & Cronin, 2008).
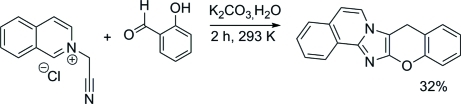
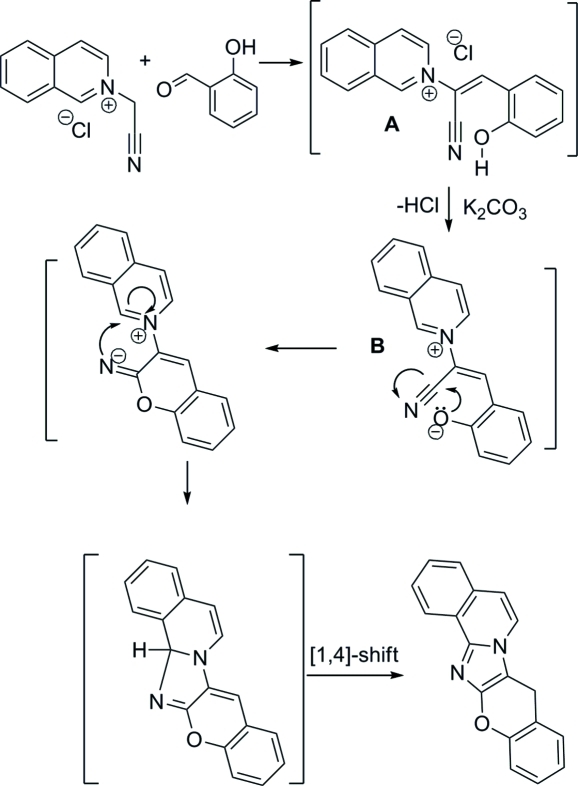
The title compound I, C18H12N2O, is the product of a novel cascade reaction (Fig. 1) (Yadav et al., 2007; Kianmehr et al., 2009; Surpur et al., 2009) starting with the Kroehnke condensation of salicylic aldehyde and isoquinolinium salt to afford the styryl derivative A, which forms zwitterion B upon thermally–induced cleavage of acetyl chloride. Then zwitterion B undergoes two consecutive nucleophilic cyclizations followed by [1,4]–proton shift to give the pentacycle I (Fig. 2). The single crystals of I suitable for X–ray diffraction analysis were obtained by slow crystallization from ethyl acetate solution.
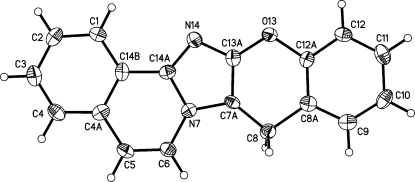
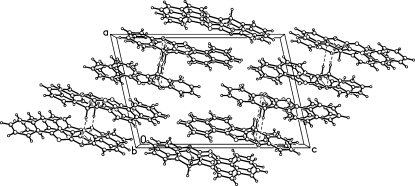
Compound I comprises two aromatic fragments - imidazo[2,1–a]isoquinoline and benzene linked by the oxygen and methylene bridges (Fig. 3). Despite the absence of common conjugative system within the molecule, it adopts practically planar conformation, with the r.m.s. deviation of 0.036Å. In the crystal, due to this structure, molecules form stacks along the b axis by the stacking interactions [C1···C7Ai = 3.340 (4)Å, C2···C8i = 3.510Å, C2···C8Ai = 3.451 (4)Å, C3···C12Ai = 3.394 (4)Å, C13A···C14Bi = 3.496 (4)Å and C14A···C14Ai = 3.426 (4)Å] (Fig. 4). The molecules are also bound by the C8—H8A···O13ii [H···O = 2.71Å, C—H···O 157°] interactions within the stacks and the C8—H8B···π (C12Aiii—O13iii—C13Aiii) [H···C12A = 2.94Å, H···O13 = 2.80Å and H···C13A 2.81Å, C—H···O 175°] interactions between the stacks. Symmetry codes: (i) 1-x, 1-y, z; (ii) x, 1+y, z; (iii) 1.5-x, 0.5+y, 0.5-z.
Experimental
A water solution of K2CO3 (0.4 g in 1 ml of H2O) was added to a solution of freshly distilled salicylic aldehyde (0.18 g, 1.47 mmol) and 2–(cyanomethyl)isoquinolinium chloride (0.30 g, 1.47 mmol) in H2O (5 ml). The resulting mixture was stirred for 3 hours at 293 K. The precipitate formed was filtered–off and recrystallized from ethyl acetate / hexane mixture to give product I as colourless needles. Yield is 32%. M.p. = 444 K. Found (%): C 79.13, H4.58, N 10.53. Calcd. for C18H12N2O (%): C79.39, H 4.44, N 10.29. 1H NMR (400 MHz, CDCl3): δ = 4.25 (s, 2H, CH2), 6.98 (dd, 1H, H12, J11,12 = 8.1, J10,12 = 1.2), 7.01 (d, 1H, H5, J5,6 = 7.5), 7.07–7.12 (m, 2H, H9+H10), 7.16 (dd, 1H, H11, J11,12 = 8.1, J9,11 = 1.2), 7.40–7.45 (m, 1H, H3), 7.48–7.53 (m, 1H, H2), 7.55 (d, 1H, H4, J3,4 = 7.5), 7.58 (d, 1H, H6, J5,6 = 7.5), 8.47(d, 1H, H1, J1,2 = 8.1). 13C NMR (100 MHz, CDCl3): δ = 23.2 (CH2), 112.9 (CH), 117.8 (Cq), 118.1 (Cq), 118.3 (CH), 120.3 (CH), 123.1 (CH), 123.2 (Cq), 123.5 (CH), 127.1 (CH), 127.8 (CH), 128.2 (2xCH), 129.1 (Cq), 130.3 (CH), 138.0 (Cq), 152.0 (Cq), 161.1 (Cq). Mass spectrum (EI MS), m/z (Ir, %): 272 (70) [M+.], 136 (11), 128 (10).
Refinement
The hydrogen atoms were placed in calculated positions with C—H = 0.95–0.99Å and refined in the riding model with fixed isotropic displacement parameters [Uiso(H) = 1.2Ueq(C)].
Figures
Fig. 1.
Synthesis of compound I.
Fig. 2.
The plausible formation mechanism of I.
Fig. 3.
Molecular structure of Iwith the atom numbering scheme. Displacement ellipsoids are shown atthe 50% probability level. H atoms are presented as a small spheres of arbitrary radius.
Fig. 4.
Crystal packing of I viewed down the b axis. Dashed lines indicate the C—H···O and C—H···π interactions.
Crystal data
| C18H12N2O | F(000) = 568 |
| Mr = 272.30 | Dx = 1.424 Mg m−3 |
| Monoclinic, P21/n | Mo Kα radiation, λ = 0.71073 Å |
| Hall symbol: -P 2yn | Cell parameters from 1700 reflections |
| a = 11.9717 (15) Å | θ = 2.3–26.3° |
| b = 6.0580 (8) Å | µ = 0.09 mm−1 |
| c = 17.948 (2) Å | T = 100 K |
| β = 102.682 (3)° | Needle, colourless |
| V = 1269.9 (3) Å3 | 0.40 × 0.12 × 0.02 mm |
| Z = 4 |
Data collection
| Bruker APEXII CCD diffractometer | 2734 independent reflections |
| Radiation source: fine–focus sealed tube | 1821 reflections with I > 2σ(I) |
| graphite | Rint = 0.056 |
| φ and ω scans | θmax = 27.0°, θmin = 1.9° |
| Absorption correction: multi-scan (SADABS; Sheldrick, 2003) | h = −15→15 |
| Tmin = 0.965, Tmax = 0.998 | k = −7→7 |
| 12413 measured reflections | l = −22→22 |
Refinement
| Refinement on F2 | Primary atom site location: structure-invariant direct methods |
| Least-squares matrix: full | Secondary atom site location: difference Fourier map |
| R[F2 > 2σ(F2)] = 0.066 | Hydrogen site location: inferred from neighbouring sites |
| wR(F2) = 0.182 | H-atom parameters constrained |
| S = 1.00 | w = 1/[σ2(Fo2) + (0.077P)2 + 1.7P] where P = (Fo2 + 2Fc2)/3 |
| 2734 reflections | (Δ/σ)max < 0.001 |
| 190 parameters | Δρmax = 0.45 e Å−3 |
| 0 restraints | Δρmin = −0.23 e Å−3 |
Special details
| Geometry. All s.u.'s (except the s.u. in the dihedral angle between two l.s. planes) are estimated using the full covariance matrix. The cell s.u.'s are taken into account individually in the estimation of s.u.'s in distances, angles and torsion angles; correlations between s.u.'s in cell parameters are only used when they are defined by crystal symmetry. An approximate (isotropic) treatment of cell s.u.'s is used for estimating s.u.'s involving l.s. planes. |
| Refinement. Refinement of F2 against ALL reflections. The weighted R–factor wR and goodness of fit S are based on F2, conventional R–factors R are based on F, with F set to zero for negative F2. The threshold expression of F2 > σ(F2) is used only for calculating R–factors(gt) etc. and is not relevant to the choice of reflections for refinement. R–factors based on F2 are statistically about twice as large as those based on F, and R–factors based on ALL data will be even larger. |
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2)
| x | y | z | Uiso*/Ueq | ||
| C1 | 0.6165 (2) | 0.1766 (5) | −0.07577 (16) | 0.0290 (6) | |
| H1 | 0.5719 | 0.0665 | −0.0583 | 0.035* | |
| C2 | 0.6436 (2) | 0.1563 (5) | −0.14599 (17) | 0.0349 (7) | |
| H2 | 0.6185 | 0.0302 | −0.1766 | 0.042* | |
| C3 | 0.7068 (2) | 0.3167 (6) | −0.17271 (17) | 0.0372 (7) | |
| H3 | 0.7237 | 0.3008 | −0.2217 | 0.045* | |
| C4 | 0.7447 (2) | 0.4957 (5) | −0.12988 (17) | 0.0349 (7) | |
| H4 | 0.7885 | 0.6033 | −0.1494 | 0.042* | |
| C4A | 0.7213 (2) | 0.5276 (5) | −0.05755 (16) | 0.0309 (6) | |
| C5 | 0.7591 (2) | 0.7182 (5) | −0.01180 (16) | 0.0332 (7) | |
| H5 | 0.8052 | 0.8253 | −0.0295 | 0.040* | |
| C6 | 0.7306 (2) | 0.7478 (5) | 0.05559 (16) | 0.0310 (6) | |
| H6 | 0.7538 | 0.8777 | 0.0845 | 0.037* | |
| N7 | 0.66701 (19) | 0.5884 (4) | 0.08314 (13) | 0.0269 (5) | |
| C7A | 0.6332 (2) | 0.5823 (4) | 0.15238 (15) | 0.0251 (6) | |
| C8 | 0.6498 (2) | 0.7432 (5) | 0.21381 (16) | 0.0326 (7) | |
| H8A | 0.6141 | 0.8858 | 0.1950 | 0.039* | |
| H8B | 0.7325 | 0.7676 | 0.2349 | 0.039* | |
| C8A | 0.5921 (2) | 0.6458 (5) | 0.27520 (16) | 0.0301 (6) | |
| C9 | 0.5902 (2) | 0.7644 (5) | 0.34032 (18) | 0.0347 (7) | |
| H9 | 0.6261 | 0.9050 | 0.3466 | 0.042* | |
| C10 | 0.5385 (3) | 0.6886 (5) | 0.39654 (18) | 0.0382 (7) | |
| H10 | 0.5390 | 0.7752 | 0.4407 | 0.046* | |
| C11 | 0.4851 (2) | 0.4809 (6) | 0.38774 (18) | 0.0387 (8) | |
| H11 | 0.4483 | 0.4261 | 0.4258 | 0.046* | |
| C12 | 0.4864 (2) | 0.3558 (5) | 0.32275 (16) | 0.0304 (6) | |
| H12 | 0.4516 | 0.2141 | 0.3163 | 0.037* | |
| C12A | 0.5393 (2) | 0.4417 (5) | 0.26757 (15) | 0.0264 (6) | |
| O13 | 0.53167 (16) | 0.2981 (3) | 0.20489 (11) | 0.0319 (5) | |
| C13A | 0.5786 (2) | 0.3795 (5) | 0.14856 (15) | 0.0297 (6) | |
| N14 | 0.57698 (19) | 0.2676 (4) | 0.08385 (13) | 0.0297 (5) | |
| C14A | 0.6311 (2) | 0.3960 (5) | 0.04379 (16) | 0.0291 (6) | |
| C14B | 0.6554 (2) | 0.3618 (5) | −0.02983 (14) | 0.0278 (6) |
Atomic displacement parameters (Å2)
| U11 | U22 | U33 | U12 | U13 | U23 | |
| C1 | 0.0230 (13) | 0.0287 (15) | 0.0340 (15) | −0.0002 (11) | 0.0032 (11) | 0.0065 (12) |
| C2 | 0.0304 (15) | 0.0366 (16) | 0.0339 (16) | 0.0086 (13) | −0.0012 (12) | −0.0084 (13) |
| C3 | 0.0285 (15) | 0.056 (2) | 0.0270 (15) | 0.0063 (14) | 0.0051 (12) | 0.0020 (14) |
| C4 | 0.0300 (15) | 0.0386 (17) | 0.0360 (17) | 0.0009 (13) | 0.0072 (12) | 0.0117 (13) |
| C4A | 0.0256 (13) | 0.0281 (15) | 0.0354 (16) | 0.0053 (11) | −0.0012 (12) | −0.0019 (12) |
| C5 | 0.0312 (15) | 0.0325 (15) | 0.0360 (16) | −0.0054 (12) | 0.0080 (12) | 0.0073 (13) |
| C6 | 0.0333 (15) | 0.0264 (14) | 0.0335 (15) | 0.0002 (12) | 0.0078 (12) | 0.0053 (12) |
| N7 | 0.0261 (11) | 0.0246 (12) | 0.0282 (12) | 0.0025 (9) | 0.0021 (9) | 0.0000 (10) |
| C7A | 0.0187 (12) | 0.0265 (14) | 0.0297 (14) | −0.0019 (10) | 0.0045 (10) | 0.0056 (11) |
| C8 | 0.0258 (14) | 0.0372 (16) | 0.0350 (16) | −0.0040 (12) | 0.0072 (12) | −0.0058 (13) |
| C8A | 0.0209 (13) | 0.0325 (15) | 0.0350 (15) | 0.0021 (11) | 0.0020 (11) | 0.0023 (13) |
| C9 | 0.0278 (14) | 0.0313 (15) | 0.0440 (17) | 0.0019 (12) | 0.0055 (12) | 0.0008 (13) |
| C10 | 0.0365 (16) | 0.0407 (18) | 0.0362 (16) | 0.0091 (14) | 0.0054 (13) | −0.0132 (14) |
| C11 | 0.0304 (15) | 0.051 (2) | 0.0388 (17) | 0.0089 (14) | 0.0175 (13) | 0.0082 (15) |
| C12 | 0.0239 (13) | 0.0275 (14) | 0.0397 (16) | 0.0001 (11) | 0.0067 (12) | 0.0026 (13) |
| C12A | 0.0214 (12) | 0.0294 (14) | 0.0283 (14) | 0.0068 (11) | 0.0055 (11) | −0.0016 (11) |
| O13 | 0.0344 (11) | 0.0285 (10) | 0.0352 (11) | −0.0058 (8) | 0.0130 (9) | −0.0022 (9) |
| C13A | 0.0256 (14) | 0.0345 (15) | 0.0290 (14) | 0.0048 (12) | 0.0059 (11) | 0.0012 (12) |
| N14 | 0.0254 (11) | 0.0327 (13) | 0.0314 (13) | 0.0016 (10) | 0.0070 (9) | 0.0015 (10) |
| C14A | 0.0252 (13) | 0.0257 (14) | 0.0347 (15) | 0.0004 (11) | 0.0029 (11) | −0.0018 (12) |
| C14B | 0.0227 (13) | 0.0376 (16) | 0.0214 (13) | 0.0105 (11) | 0.0014 (10) | 0.0021 (12) |
Geometric parameters (Å, °)
| C1—C2 | 1.374 (4) | C8—C8A | 1.540 (4) |
| C1—C14B | 1.410 (4) | C8—H8A | 0.9900 |
| C1—H1 | 0.9500 | C8—H8B | 0.9900 |
| C2—C3 | 1.380 (5) | C8A—C9 | 1.377 (4) |
| C2—H2 | 0.9500 | C8A—C12A | 1.382 (4) |
| C3—C4 | 1.349 (5) | C9—C10 | 1.373 (4) |
| C3—H3 | 0.9500 | C9—H9 | 0.9500 |
| C4—C4A | 1.400 (4) | C10—C11 | 1.405 (5) |
| C4—H4 | 0.9500 | C10—H10 | 0.9500 |
| C4A—C5 | 1.432 (4) | C11—C12 | 1.394 (4) |
| C4A—C14B | 1.432 (4) | C11—H11 | 0.9500 |
| C5—C6 | 1.339 (4) | C12—C12A | 1.389 (4) |
| C5—H5 | 0.9500 | C12—H12 | 0.9500 |
| C6—N7 | 1.386 (4) | C12A—O13 | 1.409 (3) |
| C6—H6 | 0.9500 | O13—C13A | 1.353 (3) |
| N7—C14A | 1.382 (4) | C13A—N14 | 1.341 (4) |
| N7—C7A | 1.390 (4) | N14—C14A | 1.321 (4) |
| C7A—C13A | 1.386 (4) | C14A—C14B | 1.429 (4) |
| C7A—C8 | 1.452 (4) | ||
| C2—C1—C14B | 119.6 (3) | C8A—C8—H8B | 110.5 |
| C2—C1—H1 | 120.2 | H8A—C8—H8B | 108.7 |
| C14B—C1—H1 | 120.2 | C9—C8A—C12A | 117.3 (3) |
| C1—C2—C3 | 120.9 (3) | C9—C8A—C8 | 120.1 (3) |
| C1—C2—H2 | 119.6 | C12A—C8A—C8 | 122.6 (3) |
| C3—C2—H2 | 119.6 | C10—C9—C8A | 122.9 (3) |
| C4—C3—C2 | 120.6 (3) | C10—C9—H9 | 118.5 |
| C4—C3—H3 | 119.7 | C8A—C9—H9 | 118.5 |
| C2—C3—H3 | 119.7 | C9—C10—C11 | 119.0 (3) |
| C3—C4—C4A | 121.9 (3) | C9—C10—H10 | 120.5 |
| C3—C4—H4 | 119.0 | C11—C10—H10 | 120.5 |
| C4A—C4—H4 | 119.0 | C12—C11—C10 | 119.5 (3) |
| C4—C4A—C5 | 122.7 (3) | C12—C11—H11 | 120.2 |
| C4—C4A—C14B | 117.6 (3) | C10—C11—H11 | 120.2 |
| C5—C4A—C14B | 119.7 (3) | C12A—C12—C11 | 118.9 (3) |
| C6—C5—C4A | 121.0 (3) | C12A—C12—H12 | 120.5 |
| C6—C5—H5 | 119.5 | C11—C12—H12 | 120.5 |
| C4A—C5—H5 | 119.5 | C8A—C12A—C12 | 122.4 (3) |
| C5—C6—N7 | 119.9 (3) | C8A—C12A—O13 | 125.4 (2) |
| C5—C6—H6 | 120.1 | C12—C12A—O13 | 112.2 (2) |
| N7—C6—H6 | 120.1 | C13A—O13—C12A | 114.0 (2) |
| C14A—N7—C6 | 122.6 (2) | N14—C13A—O13 | 122.2 (3) |
| C14A—N7—C7A | 108.4 (2) | N14—C13A—C7A | 114.1 (2) |
| C6—N7—C7A | 129.0 (2) | O13—C13A—C7A | 123.7 (2) |
| C13A—C7A—N7 | 101.9 (2) | C14A—N14—C13A | 104.9 (2) |
| C13A—C7A—C8 | 128.1 (2) | N14—C14A—N7 | 110.7 (2) |
| N7—C7A—C8 | 130.0 (2) | N14—C14A—C14B | 129.9 (3) |
| C7A—C8—C8A | 106.1 (2) | N7—C14A—C14B | 119.4 (3) |
| C7A—C8—H8A | 110.5 | C1—C14B—C14A | 123.3 (3) |
| C8A—C8—H8A | 110.5 | C1—C14B—C4A | 119.4 (2) |
| C7A—C8—H8B | 110.5 | C14A—C14B—C4A | 117.3 (3) |
| C14B—C1—C2—C3 | −1.1 (4) | C11—C12—C12A—O13 | −178.9 (2) |
| C1—C2—C3—C4 | 0.9 (4) | C8A—C12A—O13—C13A | −2.1 (4) |
| C2—C3—C4—C4A | −0.5 (4) | C12—C12A—O13—C13A | 177.7 (2) |
| C3—C4—C4A—C5 | −178.9 (3) | C12A—O13—C13A—N14 | −178.5 (2) |
| C3—C4—C4A—C14B | 0.2 (4) | C12A—O13—C13A—C7A | 2.1 (4) |
| C4—C4A—C5—C6 | 176.9 (3) | N7—C7A—C13A—N14 | −0.3 (3) |
| C14B—C4A—C5—C6 | −2.3 (4) | C8—C7A—C13A—N14 | 180.0 (3) |
| C4A—C5—C6—N7 | 2.4 (4) | N7—C7A—C13A—O13 | 179.1 (2) |
| C5—C6—N7—C14A | 0.2 (4) | C8—C7A—C13A—O13 | −0.6 (4) |
| C5—C6—N7—C7A | 176.8 (3) | O13—C13A—N14—C14A | −179.3 (2) |
| C14A—N7—C7A—C13A | 0.3 (3) | C7A—C13A—N14—C14A | 0.1 (3) |
| C6—N7—C7A—C13A | −176.7 (3) | C13A—N14—C14A—N7 | 0.1 (3) |
| C14A—N7—C7A—C8 | −179.9 (3) | C13A—N14—C14A—C14B | 179.9 (3) |
| C6—N7—C7A—C8 | 3.1 (5) | C6—N7—C14A—N14 | 177.0 (2) |
| C13A—C7A—C8—C8A | −1.0 (4) | C7A—N7—C14A—N14 | −0.3 (3) |
| N7—C7A—C8—C8A | 179.3 (2) | C6—N7—C14A—C14B | −2.8 (4) |
| C7A—C8—C8A—C9 | −177.9 (2) | C7A—N7—C14A—C14B | 179.9 (2) |
| C7A—C8—C8A—C12A | 1.0 (4) | C2—C1—C14B—C14A | 179.9 (3) |
| C12A—C8A—C9—C10 | −0.1 (4) | C2—C1—C14B—C4A | 0.8 (4) |
| C8—C8A—C9—C10 | 178.9 (3) | N14—C14A—C14B—C1 | 3.9 (4) |
| C8A—C9—C10—C11 | 0.0 (4) | N7—C14A—C14B—C1 | −176.3 (2) |
| C9—C10—C11—C12 | 0.6 (4) | N14—C14A—C14B—C4A | −176.9 (3) |
| C10—C11—C12—C12A | −1.0 (4) | N7—C14A—C14B—C4A | 2.8 (4) |
| C9—C8A—C12A—C12 | −0.4 (4) | C4—C4A—C14B—C1 | −0.4 (4) |
| C8—C8A—C12A—C12 | −179.3 (2) | C5—C4A—C14B—C1 | 178.8 (2) |
| C9—C8A—C12A—O13 | 179.4 (2) | C4—C4A—C14B—C14A | −179.5 (2) |
| C8—C8A—C12A—O13 | 0.5 (4) | C5—C4A—C14B—C14A | −0.4 (4) |
| C11—C12—C12A—C8A | 0.9 (4) |
Hydrogen-bond geometry (Å, °)
| Cg2 is the centroid of the O13,C12A,C8A,C8,C7A,C13A ring. |
| D—H···A | D—H | H···A | D···A | D—H···A |
| C8—H8A···O13i | 0.99 | 2.71 | 3.637 (4) | 157 |
| C8—H8B···Cgii | 0.99 | 2.63 | 3.547 (3) | 154 |
Symmetry codes: (i) x, y+1, z; (ii) −x+3/2, y+1/2, −z+1/2.
Footnotes
Supplementary data and figures for this paper are available from the IUCr electronic archives (Reference: RK2193).
References
- Bruker (2001). SAINT-Plus Bruker AXS Inc., Madison, Wisconsin, USA.
- Bruker (2005). APEX2 Bruker AXS Inc., Madison, Wisconsin, USA.
- Bunce, R. A. (1995). Tetrahedron, 51, 13103–13159.
- Kianmehr, E., Faramarzi, R. & Estiri, H. (2009). Heterocycles, 78, 415–423.
- Nicolaou, K. C., Edmonds, D. J. & Bulger, P. G. (2006). Angew. Chem. Int. Ed.45, 7134–7186. [DOI] [PubMed]
- Nicolaou, K. C., Montagnon, T. & Snyder, S. A. (2003). Chem. Commun. pp. 551–564. [DOI] [PubMed]
- Parenty, A. D. C. & Cronin, L. (2008). Synthesis, pp. 1479–1485.
- Parsons, P. J., Penkett, C. S. & Shell, A. J. (1996). Chem. Rev.96, 195–206. [DOI] [PubMed]
- Pellissier, H. (2006a). Tetrahedron, 62, 1619–1665.
- Pellissier, H. (2006b). Tetrahedron, 62, 2143–2173.
- Sheldrick, G. M. (2003). SADABS University of Göttingen, Germany.
- Sheldrick, G. M. (2008). Acta Cryst. A64, 112–122. [DOI] [PubMed]
- Surpur, M. P., Kshirsagar, S. & Samant, S. D. (2009). Tetrahedron Lett.50, 719–722.
- Tietze, L. F. (1996). Chem. Rev.96, 115–136. [DOI] [PubMed]
- Wasilke, J.–C., Obrey, S. J., Baker, R. T. & Bazan, G. C. (2005). Chem. Rev.105, 1001–1020. [DOI] [PubMed]
- Yadav, J. S., Subba Reddy, B. V., Gupta, M. K., Prathap, I. & Pandey, S. K. (2007). Catal. Commun.8, 2208–2211.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Crystal structure: contains datablocks global, I. DOI: 10.1107/S1600536810006744/rk2193sup1.cif
Structure factors: contains datablocks I. DOI: 10.1107/S1600536810006744/rk2193Isup2.hkl
Additional supplementary materials: crystallographic information; 3D view; checkCIF report