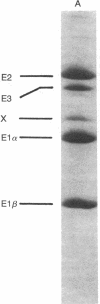
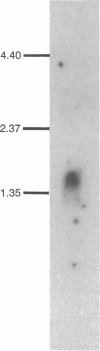
Abstract
The gene encoding the protein X component of the pyruvate dehydrogenase complex from Saccharomyces cerevisiae has been cloned and sequenced. A 487-base fragment of yeast genomic DNA encoding the amino-terminal region of protein X was amplified by the polymerase chain reaction using synthetic oligonucleotide primers based on amino-terminal and internal amino acid sequences. This DNA fragment was used as a probe to select two genomic DNA restriction fragments, which were cloned and sequenced. A 2.1-kilobase insert contains the complete sequence of the protein X gene. This insert has an open reading frame of 1230 nucleotides encoding a presequence of 30 amino acid residues and a mature protein of 380 amino acid residues (Mr, 42,052). Hybridization analysis showed that there is a single copy of the protein X gene and that the size of the mRNA is approximately 1.5 kilobases. Comparison of the deduced amino acid sequences of yeast protein X and dihydrolipoamide acetyltransferase indicates that the two proteins evolved from a common ancestor. The amino-terminal part of protein X (residues 1-195) resembles the acetyltransferase, but the remainder is quite different. There is strong homology between protein X and the acetyltransferase in the amino-terminal region (residues 1-84) that corresponds to the putative lipoyl domain. Protein X lacks the highly conserved sequence His-Xaa-Xaa-Xaa-Asp-Gly near the carboxyl terminus, which is thought to be part of the active site of all dihydrolipoamide acyltransferases.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cleveland D. W., Fischer S. G., Kirschner M. W., Laemmli U. K. Peptide mapping by limited proteolysis in sodium dodecyl sulfate and analysis by gel electrophoresis. J Biol Chem. 1977 Feb 10;252(3):1102–1106. [PubMed] [Google Scholar]
- Coppel R. L., McNeilage L. J., Surh C. D., Van de Water J., Spithill T. W., Whittingham S., Gershwin M. E. Primary structure of the human M2 mitochondrial autoantigen of primary biliary cirrhosis: dihydrolipoamide acetyltransferase. Proc Natl Acad Sci U S A. 1988 Oct;85(19):7317–7321. doi: 10.1073/pnas.85.19.7317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Marcucci O. G., Hodgson J. A., Lindsay J. G. The Mr-50 000 polypeptide of mammalian pyruvate dehydrogenase complex participates in the acetylation reactions. Eur J Biochem. 1986 Aug 1;158(3):587–594. doi: 10.1111/j.1432-1033.1986.tb09795.x. [DOI] [PubMed] [Google Scholar]
- De Marcucci O., Lindsay J. G. Component X. An immunologically distinct polypeptide associated with mammalian pyruvate dehydrogenase multi-enzyme complex. Eur J Biochem. 1985 Jun 18;149(3):641–648. doi: 10.1111/j.1432-1033.1985.tb08972.x. [DOI] [PubMed] [Google Scholar]
- Gopalakrishnan S., Rahmatullah M., Radke G. A., Powers-Greenwood S., Roche T. E. Role of protein X in the function of the mammalian pyruvate dehydrogenase complex. Biochem Biophys Res Commun. 1989 Apr 28;160(2):715–721. doi: 10.1016/0006-291x(89)92492-3. [DOI] [PubMed] [Google Scholar]
- Gulcher J. R., Stefansson K. Determination of contiguity of subclones using the polymerase chain reaction. Nucleic Acids Res. 1988 Nov 25;16(22):10931–10931. doi: 10.1093/nar/16.22.10931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodgson J. A., De Marcucci O. G., Lindsay J. G. Lipoic acid is the site of substrate-dependent acetylation of component X in ox heart pyruvate dehydrogenase multienzyme complex. Eur J Biochem. 1986 Aug 1;158(3):595–600. doi: 10.1111/j.1432-1033.1986.tb09796.x. [DOI] [PubMed] [Google Scholar]
- Jilka J. M., Rahmatullah M., Kazemi M., Roche T. E. Properties of a newly characterized protein of the bovine kidney pyruvate dehydrogenase complex. J Biol Chem. 1986 Feb 5;261(4):1858–1867. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Matsudaira P. Sequence from picomole quantities of proteins electroblotted onto polyvinylidene difluoride membranes. J Biol Chem. 1987 Jul 25;262(21):10035–10038. [PubMed] [Google Scholar]
- Miles J. S., Guest J. R. Molecular genetic aspects of the citric acid cycle of Escherichia coli. Biochem Soc Symp. 1987;54:45–65. [PubMed] [Google Scholar]
- Niu X. D., Browning K. S., Behal R. H., Reed L. J. Cloning and nucleotide sequence of the gene for dihydrolipoamide acetyltransferase from Saccharomyces cerevisiae. Proc Natl Acad Sci U S A. 1988 Oct;85(20):7546–7550. doi: 10.1073/pnas.85.20.7546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perham R. N., Packman L. C., Radford S. E. 2-Oxo acid dehydrogenase multi-enzyme complexes: in the beginning and halfway there. Biochem Soc Symp. 1987;54:67–81. [PubMed] [Google Scholar]
- Powers-Greenwood S. L., Rahmatullah M., Radke G. A., Roche T. E. Separation of protein X from the dihydrolipoyl transacetylase component of the mammalian pyruvate dehydrogenase complex and function of protein X. J Biol Chem. 1989 Mar 5;264(7):3655–3657. [PubMed] [Google Scholar]
- Rahmatullah M., Gopalakrishnan S., Andrews P. C., Chang C. L., Radke G. A., Roche T. E. Subunit associations in the mammalian pyruvate dehydrogenase complex. Structure and role of protein X and the pyruvate dehydrogenase component binding domain of the dihydrolipoyl transacetylase component. J Biol Chem. 1989 Feb 5;264(4):2221–2227. [PubMed] [Google Scholar]
- Rahmatullah M., Gopalakrishnan S., Radke G. A., Roche T. E. Domain structures of the dihydrolipoyl transacetylase and the protein X components of mammalian pyruvate dehydrogenase complex. Selective cleavage by protease Arg C. J Biol Chem. 1989 Jan 15;264(2):1245–1251. [PubMed] [Google Scholar]
- Rahmatullah M., Roche T. E. The catalytic requirements for reduction and acetylation of protein X and the related regulation of various forms of resolved pyruvate dehydrogenase kinase. J Biol Chem. 1987 Jul 25;262(21):10265–10271. [PubMed] [Google Scholar]
- Uhlinger D. J., Yang C. Y., Reed L. J. Phosphorylation-dephosphorylation of pyruvate dehydrogenase from bakers' yeast. Biochemistry. 1986 Sep 23;25(19):5673–5677. doi: 10.1021/bi00367a049. [DOI] [PubMed] [Google Scholar]
- von Heijne G. Mitochondrial targeting sequences may form amphiphilic helices. EMBO J. 1986 Jun;5(6):1335–1342. doi: 10.1002/j.1460-2075.1986.tb04364.x. [DOI] [PMC free article] [PubMed] [Google Scholar]