Abstract
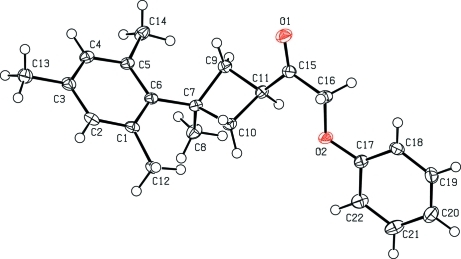
In the title compound, C22H26O2, the cyclobutane ring is puckered, with a dihedral angle of 24.97 (9)° between the two C3 planes. In the crystal, intermolecular non-classical C—H⋯O interactions between the methylcyclobutyl CH group and the O atom of the phenoxy group are found.
Related literature
For related cyclobutanes, see: Çukurovali et al. (2005 ▶); Dinçer et al. (2004 ▶); Kirilmiş et al. (2005a
▶,b
▶); Sari et al. (2002 ▶, 2004 ▶). For the anti-inflammatory and anti-depressant activity of three-substituted cyclobutane acid derivatives, see: Roger et al. (1977 ▶); Gerard (1979 ▶); Sawhney et al. (1978 ▶); Brown et al. (1974 ▶); for anti-microbial activity, see: Suziki et al. (1979 ▶); for anti-parasitic activity, see: Slip et al. (1974 ▶), for herbicidal activity, see: Foerster et al. (1979 ▶) and for their liquid-crystal properties, see: Dehmlow & Schmidt (1990 ▶).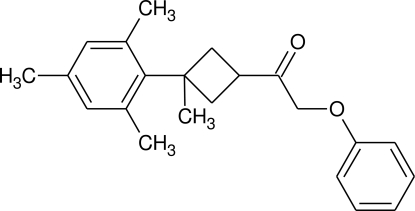
Experimental
Crystal data
C22H26O2
M r = 322.43
Triclinic,
a = 8.5884 (12) Å
b = 10.1725 (11) Å
c = 11.1018 (12) Å
α = 82.364 (4)°
β = 68.170 (3)°
γ = 86.235 (2)°
V = 892.24 (19) Å3
Z = 2
Mo Kα radiation
μ = 0.08 mm−1
T = 100 K
0.42 × 0.33 × 0.24 mm
Data collection
Nonius KappaCCD diffractometer
Absorption correction: multi-scan (SADABS; Bruker–Nonius, 2002 ▶) T min = 0.969, T max = 0.982
23641 measured reflections
3921 independent reflections
2968 reflections with I > 2σ(I)
R int = 0.054
Refinement
R[F 2 > 2σ(F 2)] = 0.047
wR(F 2) = 0.129
S = 1.07
3921 reflections
217 parameters
H-atom parameters constrained
Δρmax = 0.32 e Å−3
Δρmin = −0.25 e Å−3
Data collection: COLLECT (Bruker–Nonius, 2002 ▶); cell refinement: EVALCCD (Bruker–Nonius, 2002 ▶); data reduction: EVALCCD program(s) used to solve structure: SHELXS97 (Sheldrick, 2008 ▶); program(s) used to refine structure: SHELXL97 (Sheldrick, 2008 ▶); molecular graphics: PLATON (Spek, 2009 ▶); software used to prepare material for publication: SHELXL97.
Supplementary Material
Crystal structure: contains datablocks global, I. DOI: 10.1107/S1600536810003910/rk2189sup1.cif
Structure factors: contains datablocks I. DOI: 10.1107/S1600536810003910/rk2189Isup2.hkl
Additional supplementary materials: crystallographic information; 3D view; checkCIF report
Table 1. Hydrogen-bond geometry (Å, °).
| D—H⋯A | D—H | H⋯A | D⋯A | D—H⋯A |
|---|---|---|---|---|
| C10—H10A⋯O1i | 0.99 | 2.44 | 3.419 (3) | 172 (2) |
Symmetry code: (i) 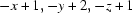 .
.
Acknowledgments
This study was supported financially by grant DPT/2003 K 120440-1 from the Scientific and Technical Research Council of Turkey (Project Manager Yıldırım Aydogdu). The authors extend special thanks to Frank W. Heinemann for the data collection.
supplementary crystallographic information
Comment
It is well known that three-substituted cyclobutane acid derivatives exhibit anti-inflammatory and anti-depressant activities (Roger et al., 1977; Gerard, 1979) and liquid-crystal properties (Dehmlow & Schmidt, 1990), moreover their of various thiazoles derivatives showed herbicidal (Foerster et al., 1979), anti-inflammatory (Sawhney et al., 1978; Brown et al., 1974), anti-microbial (Suziki et al., 1979) or anti-parasitic activity (Slip et al., 1974). Substituted cyclobutane contained similar structures, have been reported in the recent years (Kirilmiş et al., 2005a, 2005b; Sari et al., 2002, 2004;Çukurovali et al., 2005; Dinçer et al., 2004).
The four-atom bridge O2/C16/C15/C11 linking the cyclobutane and phenoxy rings is planar (Fig. 1). The torsion angle of O2—C16—C15—C11 is -1.0 (1)°. It corresponds to the (-) synclinical configuration. The cyclobutane ring is similar puckered as in a related compounds; the C9/C7/C10 plane forms a dihedaral angle of 24.97 (9)° with the C10/C11/C9 plane in the title compound. The same dihedral angles are presented in the literature: 25.74 (6)° - Çukurovali et al., (2005) and 19.8 (3)° - Dinçer et al., (2004). The mesityl and phenoxy rings are planar. The dihedral angle between these ring is 73.90 (5)°. The maximum deviation from mean plane of the atoms for mesityl and phenoxy rings are -0.046 (6)Å for C5 and 0.016 (3)Å for C17, respectively.
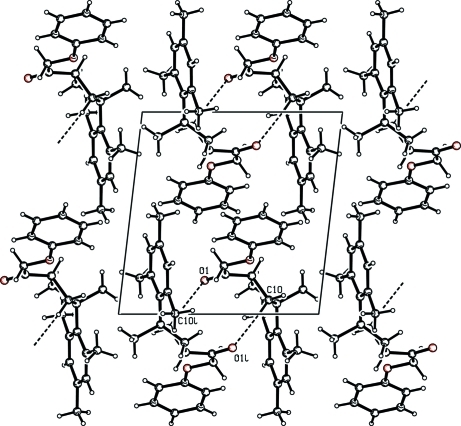
There is one intermolcular non-classical C—H···O hydrogen-bonding interactions in the crystal structure. This interactions lead to close packing between the neighbouring molecules. And the structure is stabilized by van der Waals interactions and symmetry-related molecules are linked to form dimerization chains via C—H···O intermolecular non-classical hydrogen bond. Intermolecular van der Waals interactions between the methylcyclobutyl CH group and the O atom of the phenoxy group (Fig. 2, Table 1).
In the IR spectra of 1-(3-mesityl-3-methylcyclobutyl)-2-phenoxy-1-ethanone very important bonds were observed as (ν, cm-1); 1711 (sharp C═O stretching) respectively and between 3063–2852 (broad aliphatic and aromatic C—H stretching) and 1070 (ether C–O–C stretching). The 1H-NMR spectra were reported in p.p.m. (δ) relative to tetramethylsilane (SiMe4) as the internal standard and referenced to deuterochloroform (CDCl3). In the 1H-NMR spectra were H signals obtained as (p.p.m.) 1.7 (s, 3H, cyclobutane-CH3), 2.2 (s, 9H, CH3-mesitylene), 2.5–2.9 (m, 4H, cyclobutane CH2), 3.9 (p,1H, cyclobutane CH), 4.2 (s, 2H, O—CH2) and aromatic protons observed at 6.7–7.3 (m, 7H, ArH). Elemental Analysis Calc. for C22H26O2 (322.44 g mol-1): C, 81.95; H, 8.13 Found: C, 81.88; H, 8.05.
Experimental
Elemental analysis were determined on a LECO CHNS–932 auto elemental analysis apparatus. Infrared spectra were obtained by using a Mattson 1000 F T–IR Spectrometer, from 4000–400 cm-1 in KBr pellet. 1H-NMR spectra were recorded on a Jeol FX–90Q Spectrometer 90 MHz.
Synthesis of 1-(3-mesityl-3-methylcyclobutyl)-2-phenoxy-1-ethanone: a mixture of 1-mesityl-1-methyl-3-(2-chloro-1-oxoethyl)cyclobutane (2.64 g, 10 mmol), phenol (1.035 g, 11 mmol) and K2CO3 (1.51 g, 11 mmol) in 200 ml dry acetone was refluxed for 8 h. The reaction mixture was poured into water (500 ml), the insoluble portion was filtered off, washed with water and crystallized from ethanol (yield 85%).
Refinement
All H atoms were placed geometrically (C—H = 0.95–0.99 Å) and refined in the riding model approximation, with Uiso(H) = 1.2Ueq(C) for CH and CH2, and Uiso(H) = 1.5Ueq(C) for CH3.
Figures
Fig. 1.
View of the title molecule with the atom numbering scheme. The displacement ellipsoids are drawn at the 50% probability level. H atoms are shown as a small circles of arbitrary radii.
Fig. 2.
A packing diagram of title compound with the intermolecular H bonds (dashed lines). Symmetry code: (i) -x+1, -y+2, -z+1.
Crystal data
| C22H26O2 | Z = 2 |
| Mr = 322.43 | F(000) = 348 |
| Triclinic, P1 | Dx = 1.200 Mg m−3 |
| Hall symbol: -P 1 | Mo Kα radiation, λ = 0.71073 Å |
| a = 8.5884 (12) Å | Cell parameters from 149 reflections |
| b = 10.1725 (11) Å | θ = 6.0–20.0° |
| c = 11.1018 (12) Å | µ = 0.08 mm−1 |
| α = 82.364 (4)° | T = 100 K |
| β = 68.170 (3)° | Block, colourless |
| γ = 86.235 (2)° | 0.42 × 0.33 × 0.24 mm |
| V = 892.24 (19) Å3 |
Data collection
| Nonius KappaCCD diffractometer | 3921 independent reflections |
| Radiation source: fine-focus sealed tube | 2968 reflections with I > 2σ(I) |
| graphite | Rint = 0.054 |
| Detector resolution: 9 pixels mm-1 | θmax = 27.1°, θmin = 3.0° |
| ω–scans with 2.00° and 40 sec per frame | h = −11→11 |
| Absorption correction: multi-scan (SADABS; Bruker–Nonius, 2002) | k = −13→12 |
| Tmin = 0.969, Tmax = 0.982 | l = −14→14 |
| 23641 measured reflections |
Refinement
| Refinement on F2 | Primary atom site location: structure-invariant direct methods |
| Least-squares matrix: full | Secondary atom site location: difference Fourier map |
| R[F2 > 2σ(F2)] = 0.047 | Hydrogen site location: inferred from neighbouring sites |
| wR(F2) = 0.129 | H-atom parameters constrained |
| S = 1.07 | w = 1/[σ2(Fo2) + (0.0644P)2 + 0.2801P] where P = (Fo2 + 2Fc2)/3 |
| 3921 reflections | (Δ/σ)max = 0.001 |
| 217 parameters | Δρmax = 0.32 e Å−3 |
| 0 restraints | Δρmin = −0.25 e Å−3 |
Special details
| Geometry. All s.u.'s (except the s.u. in the dihedral angle between two l.s. planes) are estimated using the full covariance matrix. The cell s.u.'s are taken into account individually in the estimation of s.u.'s in distances, angles and torsion angles; correlations between s.u.'s in cell parameters are only used when they are defined by crystal symmetry. An approximate (isotropic) treatment of cell s.u.'s is used for estimating s.u.'s involving l.s. planes. |
| Refinement. Refinement of F2 against ALL reflections. The weighted R-factor wR and goodness of fit S are based on F2, conventional R-factors R are based on F, with F set to zero for negative F2. The threshold expression of F2 > σ(F2) is used only for calculating R-factors(gt) etc. and is not relevant to the choice of reflections for refinement. R-factors based on F2 are statistically about twice as large as those based on F, and R-factors based on ALL data will be even larger. |
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2)
| x | y | z | Uiso*/Ueq | ||
| C1 | 0.66157 (19) | 0.63782 (15) | 0.69324 (15) | 0.0191 (3) | |
| C2 | 0.6658 (2) | 0.62422 (15) | 0.81884 (15) | 0.0212 (3) | |
| H2 | 0.5724 | 0.5864 | 0.8899 | 0.025* | |
| C3 | 0.8017 (2) | 0.66400 (15) | 0.84382 (15) | 0.0216 (3) | |
| C4 | 0.9374 (2) | 0.71600 (15) | 0.73790 (15) | 0.0202 (3) | |
| H4 | 1.0328 | 0.7416 | 0.7525 | 0.024* | |
| C5 | 0.93938 (19) | 0.73229 (15) | 0.61020 (15) | 0.0181 (3) | |
| C6 | 0.79712 (19) | 0.69657 (14) | 0.58680 (15) | 0.0168 (3) | |
| C7 | 0.79622 (18) | 0.71572 (15) | 0.44807 (15) | 0.0175 (3) | |
| C8 | 0.8730 (2) | 0.59338 (16) | 0.37804 (16) | 0.0238 (4) | |
| H8A | 0.8710 | 0.6069 | 0.2895 | 0.036* | |
| H8B | 0.8079 | 0.5150 | 0.4267 | 0.036* | |
| H8C | 0.9892 | 0.5802 | 0.3731 | 0.036* | |
| C9 | 0.86927 (19) | 0.84742 (16) | 0.36088 (15) | 0.0190 (3) | |
| H9A | 0.8701 | 0.9204 | 0.4114 | 0.023* | |
| H9B | 0.9806 | 0.8360 | 0.2918 | 0.023* | |
| C10 | 0.62618 (19) | 0.75748 (15) | 0.42989 (15) | 0.0189 (3) | |
| H10B | 0.5741 | 0.6854 | 0.4069 | 0.023* | |
| H10A | 0.5440 | 0.7998 | 0.5038 | 0.023* | |
| C11 | 0.72371 (19) | 0.85766 (15) | 0.31115 (15) | 0.0181 (3) | |
| H11 | 0.7558 | 0.8179 | 0.2273 | 0.022* | |
| C12 | 0.5091 (2) | 0.58448 (17) | 0.67897 (17) | 0.0255 (4) | |
| H12A | 0.5442 | 0.5430 | 0.5980 | 0.038* | |
| H12B | 0.4298 | 0.6575 | 0.6761 | 0.038* | |
| H12C | 0.4549 | 0.5185 | 0.7536 | 0.038* | |
| C13 | 0.8009 (2) | 0.64947 (18) | 0.98136 (16) | 0.0283 (4) | |
| H13A | 0.7400 | 0.5695 | 1.0308 | 0.042* | |
| H13B | 0.7456 | 0.7273 | 1.0240 | 0.042* | |
| H13C | 0.9166 | 0.6421 | 0.9784 | 0.042* | |
| C14 | 1.09987 (19) | 0.78228 (18) | 0.50263 (16) | 0.0233 (4) | |
| H14A | 1.1277 | 0.7295 | 0.4294 | 0.035* | |
| H14B | 1.1912 | 0.7741 | 0.5361 | 0.035* | |
| H14C | 1.0846 | 0.8756 | 0.4727 | 0.035* | |
| C15 | 0.63834 (19) | 0.98988 (16) | 0.30363 (15) | 0.0196 (3) | |
| C16 | 0.4891 (2) | 1.00148 (15) | 0.26088 (16) | 0.0217 (3) | |
| H16A | 0.3888 | 1.0322 | 0.3314 | 0.026* | |
| H16B | 0.5121 | 1.0673 | 0.1824 | 0.026* | |
| C17 | 0.35862 (19) | 0.87125 (16) | 0.16126 (15) | 0.0193 (3) | |
| C18 | 0.2842 (2) | 0.98135 (16) | 0.11452 (16) | 0.0231 (4) | |
| H18 | 0.2968 | 1.0674 | 0.1337 | 0.028* | |
| C19 | 0.1910 (2) | 0.96324 (19) | 0.03910 (18) | 0.0302 (4) | |
| H19 | 0.1404 | 1.0381 | 0.0062 | 0.036* | |
| C20 | 0.1703 (2) | 0.8385 (2) | 0.01108 (17) | 0.0304 (4) | |
| H20 | 0.1088 | 0.8280 | −0.0425 | 0.036* | |
| C21 | 0.2405 (2) | 0.72904 (18) | 0.06224 (18) | 0.0290 (4) | |
| H21 | 0.2240 | 0.6427 | 0.0459 | 0.035* | |
| C22 | 0.3340 (2) | 0.74507 (17) | 0.13673 (17) | 0.0253 (4) | |
| H22 | 0.3819 | 0.6697 | 0.1714 | 0.030* | |
| O1 | 0.68046 (15) | 1.08915 (12) | 0.33319 (12) | 0.0270 (3) | |
| O2 | 0.45853 (14) | 0.87559 (11) | 0.23207 (11) | 0.0235 (3) |
Atomic displacement parameters (Å2)
| U11 | U22 | U33 | U12 | U13 | U23 | |
| C1 | 0.0195 (8) | 0.0117 (7) | 0.0253 (8) | −0.0002 (6) | −0.0076 (6) | −0.0015 (6) |
| C2 | 0.0221 (8) | 0.0149 (8) | 0.0224 (8) | −0.0030 (6) | −0.0038 (7) | 0.0004 (6) |
| C3 | 0.0289 (9) | 0.0143 (7) | 0.0217 (8) | −0.0001 (6) | −0.0088 (7) | −0.0040 (6) |
| C4 | 0.0214 (8) | 0.0183 (8) | 0.0239 (8) | −0.0011 (6) | −0.0104 (7) | −0.0058 (6) |
| C5 | 0.0179 (7) | 0.0156 (7) | 0.0211 (8) | 0.0010 (6) | −0.0066 (6) | −0.0056 (6) |
| C6 | 0.0179 (7) | 0.0129 (7) | 0.0203 (7) | 0.0013 (6) | −0.0069 (6) | −0.0047 (6) |
| C7 | 0.0159 (7) | 0.0175 (8) | 0.0198 (7) | −0.0008 (6) | −0.0066 (6) | −0.0043 (6) |
| C8 | 0.0258 (8) | 0.0229 (8) | 0.0263 (8) | 0.0048 (7) | −0.0122 (7) | −0.0099 (7) |
| C9 | 0.0168 (7) | 0.0216 (8) | 0.0177 (7) | −0.0018 (6) | −0.0047 (6) | −0.0035 (6) |
| C10 | 0.0174 (7) | 0.0177 (8) | 0.0237 (8) | −0.0006 (6) | −0.0094 (6) | −0.0039 (6) |
| C11 | 0.0187 (7) | 0.0190 (8) | 0.0182 (7) | −0.0002 (6) | −0.0078 (6) | −0.0048 (6) |
| C12 | 0.0228 (8) | 0.0225 (9) | 0.0302 (9) | −0.0085 (7) | −0.0104 (7) | 0.0061 (7) |
| C13 | 0.0371 (10) | 0.0267 (9) | 0.0210 (8) | −0.0066 (8) | −0.0103 (7) | −0.0013 (7) |
| C14 | 0.0171 (8) | 0.0321 (9) | 0.0218 (8) | −0.0037 (7) | −0.0072 (7) | −0.0049 (7) |
| C15 | 0.0203 (8) | 0.0197 (8) | 0.0174 (7) | −0.0029 (6) | −0.0048 (6) | −0.0026 (6) |
| C16 | 0.0219 (8) | 0.0167 (8) | 0.0282 (8) | 0.0012 (6) | −0.0099 (7) | −0.0070 (7) |
| C17 | 0.0148 (7) | 0.0211 (8) | 0.0218 (8) | 0.0001 (6) | −0.0062 (6) | −0.0036 (6) |
| C18 | 0.0218 (8) | 0.0190 (8) | 0.0277 (8) | −0.0038 (6) | −0.0093 (7) | 0.0018 (7) |
| C19 | 0.0283 (9) | 0.0314 (10) | 0.0328 (9) | −0.0060 (7) | −0.0169 (8) | 0.0095 (8) |
| C20 | 0.0268 (9) | 0.0434 (11) | 0.0240 (9) | −0.0084 (8) | −0.0121 (7) | −0.0023 (8) |
| C21 | 0.0248 (9) | 0.0301 (9) | 0.0351 (10) | 0.0000 (7) | −0.0106 (8) | −0.0154 (8) |
| C22 | 0.0215 (8) | 0.0204 (8) | 0.0365 (9) | 0.0040 (7) | −0.0124 (7) | −0.0081 (7) |
| O1 | 0.0317 (7) | 0.0214 (6) | 0.0334 (7) | −0.0009 (5) | −0.0166 (6) | −0.0082 (5) |
| O2 | 0.0276 (6) | 0.0151 (6) | 0.0350 (6) | 0.0000 (5) | −0.0196 (5) | −0.0036 (5) |
Geometric parameters (Å, °)
| C1—C2 | 1.397 (2) | C12—H12A | 0.9800 |
| C1—C6 | 1.410 (2) | C12—H12B | 0.9800 |
| C1—C12 | 1.518 (2) | C12—H12C | 0.9800 |
| C2—C3 | 1.392 (2) | C13—H13A | 0.9800 |
| C2—H2 | 0.9500 | C13—H13B | 0.9800 |
| C3—C4 | 1.385 (2) | C13—H13C | 0.9800 |
| C3—C13 | 1.512 (2) | C14—H14A | 0.9800 |
| C4—C5 | 1.399 (2) | C14—H14B | 0.9800 |
| C4—H4 | 0.9500 | C14—H14C | 0.9800 |
| C5—C6 | 1.418 (2) | C15—O1 | 1.2177 (19) |
| C5—C14 | 1.514 (2) | C15—C16 | 1.516 (2) |
| C6—C7 | 1.529 (2) | C16—O2 | 1.4237 (18) |
| C7—C8 | 1.535 (2) | C16—H16A | 0.9900 |
| C7—C9 | 1.562 (2) | C16—H16B | 0.9900 |
| C7—C10 | 1.571 (2) | C17—O2 | 1.3682 (18) |
| C8—H8A | 0.9800 | C17—C18 | 1.389 (2) |
| C8—H8B | 0.9800 | C17—C22 | 1.392 (2) |
| C8—H8C | 0.9800 | C18—C19 | 1.391 (2) |
| C9—C11 | 1.536 (2) | C18—H18 | 0.9500 |
| C9—H9A | 0.9900 | C19—C20 | 1.383 (3) |
| C9—H9B | 0.9900 | C19—H19 | 0.9500 |
| C10—C11 | 1.553 (2) | C20—C21 | 1.387 (3) |
| C10—H10B | 0.9900 | C20—H20 | 0.9500 |
| C10—H10A | 0.9900 | C21—C22 | 1.380 (2) |
| C11—C15 | 1.497 (2) | C21—H21 | 0.9500 |
| C11—H11 | 1.0000 | C22—H22 | 0.9500 |
| C2—C1—C6 | 119.74 (14) | C10—C11—H11 | 111.2 |
| C2—C1—C12 | 116.96 (14) | C1—C12—H12A | 109.5 |
| C6—C1—C12 | 123.28 (14) | C1—C12—H12B | 109.5 |
| C3—C2—C1 | 122.43 (15) | H12A—C12—H12B | 109.5 |
| C3—C2—H2 | 118.8 | C1—C12—H12C | 109.5 |
| C1—C2—H2 | 118.8 | H12A—C12—H12C | 109.5 |
| C4—C3—C2 | 117.38 (14) | H12B—C12—H12C | 109.5 |
| C4—C3—C13 | 121.65 (15) | C3—C13—H13A | 109.5 |
| C2—C3—C13 | 120.97 (15) | C3—C13—H13B | 109.5 |
| C3—C4—C5 | 122.47 (15) | H13A—C13—H13B | 109.5 |
| C3—C4—H4 | 118.8 | C3—C13—H13C | 109.5 |
| C5—C4—H4 | 118.8 | H13A—C13—H13C | 109.5 |
| C4—C5—C6 | 119.57 (14) | H13B—C13—H13C | 109.5 |
| C4—C5—C14 | 116.94 (14) | C5—C14—H14A | 109.5 |
| C6—C5—C14 | 123.43 (13) | C5—C14—H14B | 109.5 |
| C1—C6—C5 | 118.27 (14) | H14A—C14—H14B | 109.5 |
| C1—C6—C7 | 121.45 (13) | C5—C14—H14C | 109.5 |
| C5—C6—C7 | 120.21 (13) | H14A—C14—H14C | 109.5 |
| C6—C7—C8 | 110.39 (12) | H14B—C14—H14C | 109.5 |
| C6—C7—C9 | 117.38 (12) | O1—C15—C11 | 123.12 (14) |
| C8—C7—C9 | 111.83 (13) | O1—C15—C16 | 117.80 (14) |
| C6—C7—C10 | 118.07 (12) | C11—C15—C16 | 119.06 (13) |
| C8—C7—C10 | 110.26 (12) | O2—C16—C15 | 109.41 (13) |
| C9—C7—C10 | 87.08 (11) | O2—C16—H16A | 109.8 |
| C7—C8—H8A | 109.5 | C15—C16—H16A | 109.8 |
| C7—C8—H8B | 109.5 | O2—C16—H16B | 109.8 |
| H8A—C8—H8B | 109.5 | C15—C16—H16B | 109.8 |
| C7—C8—H8C | 109.5 | H16A—C16—H16B | 108.2 |
| H8A—C8—H8C | 109.5 | O2—C17—C18 | 124.76 (14) |
| H8B—C8—H8C | 109.5 | O2—C17—C22 | 115.14 (14) |
| C11—C9—C7 | 89.84 (11) | C18—C17—C22 | 120.10 (15) |
| C11—C9—H9A | 113.7 | C17—C18—C19 | 118.77 (16) |
| C7—C9—H9A | 113.7 | C17—C18—H18 | 120.6 |
| C11—C9—H9B | 113.7 | C19—C18—H18 | 120.6 |
| C7—C9—H9B | 113.7 | C20—C19—C18 | 121.37 (17) |
| H9A—C9—H9B | 110.9 | C20—C19—H19 | 119.3 |
| C11—C10—C7 | 88.89 (11) | C18—C19—H19 | 119.3 |
| C11—C10—H10B | 113.8 | C19—C20—C21 | 119.17 (16) |
| C7—C10—H10B | 113.8 | C19—C20—H20 | 120.4 |
| C11—C10—H10A | 113.8 | C21—C20—H20 | 120.4 |
| C7—C10—H10A | 113.8 | C22—C21—C20 | 120.27 (16) |
| H10B—C10—H10A | 111.1 | C22—C21—H21 | 119.9 |
| C15—C11—C9 | 117.93 (13) | C20—C21—H21 | 119.9 |
| C15—C11—C10 | 114.89 (13) | C21—C22—C17 | 120.25 (16) |
| C9—C11—C10 | 88.64 (11) | C21—C22—H22 | 119.9 |
| C15—C11—H11 | 111.2 | C17—C22—H22 | 119.9 |
| C9—C11—H11 | 111.2 | C17—O2—C16 | 118.20 (12) |
Hydrogen-bond geometry (Å, °)
| D—H···A | D—H | H···A | D···A | D—H···A |
| C10—H10A···O1i | 0.99 | 2.44 | 3.419 (3) | 172 (2) |
Symmetry codes: (i) −x+1, −y+2, −z+1.
Footnotes
Supplementary data and figures for this paper are available from the IUCr electronic archives (Reference: RK2189).
References
- Brown, K., Cater, D. P., Cavalla, J. F., Green, D., Newberry, R. A. & Wilson, A. B. (1974). J. Med. Chem.14, 1177–1181. [DOI] [PubMed]
- Bruker–Nonius (2002). COLLECT, EVALCCD and SADABS Bruker AXS Inc., Madison, Wisconsin, USA.
- Çukurovalı, A., Özdemir, N., Yılmaz, I. & Dinçer, M. (2005). Acta Cryst. E61, o1754–o1756.
- Dehmlow, E. V. & Schmidt, S. (1990). Liebigs Ann. Chem.5, 411–414.
- Dinçer, M., Özdemir, N., Çukurovalı, A., Yılmaz, İ. & Büyükgüngör, O. (2004). Acta Cryst. E60, o1523–o1524. [DOI] [PubMed]
- Foerster, H., Hofer, W., Mues, V., Eue, L. & Schmidt, R. R. (1979). German Patent 2, 822 155.
- Gerard, G. (1979). Eur. J. Med. Chem.14, 493–497.
- Kırılmış, C., Koca, M., Arıcı, C., Heinemann, F. W. & Ahmedzade, M. (2005a). Acta Cryst. E61, o555–o556.
- Kırılmış, C., Koca, M., Arıcı, C., Heinemann, F. W. & Ahmedzade, M. (2005b). Acta Cryst. E61, o1176–o1177.
- Roger, E., Pier, C. J., Paulet, V., Gerard, G., Chepat, J. P. & Robert, G. (1977). Eur. J. Med. Chem. Chem. Ther. pp. 12501–?????
- Sari, U., Güven, K., Yılmaz, I., Çukurovali, A. & Aksoy, I. (2002). Anal. Sci.18, 725–726. [DOI] [PubMed]
- Sari, U., Yılmaz, I., Güven, K., Çukurovali, A. & Aksoy, I. (2004). J. Chem. Crystallogr.34, 571–575.
- Sawhney, S. N., Arora, S. K. & Sing, J. V. (1978). Indian J. Chem. Sect. B, 16, 605–609.
- Sheldrick, G. M. (2008). Acta Cryst. A64, 112–122. [DOI] [PubMed]
- Slip, P. I., Closier, M. & Neville, M. (1974). J. Med. Chem.17, 207–209. [DOI] [PubMed]
- Spek, A. L. (2009). Acta Cryst. D65, 148–155. [DOI] [PMC free article] [PubMed]
- Suziki, N., Tanaka, Y. & Dohmori, R. (1979). Chem. Pharm. Bull.27, 1–11. [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Crystal structure: contains datablocks global, I. DOI: 10.1107/S1600536810003910/rk2189sup1.cif
Structure factors: contains datablocks I. DOI: 10.1107/S1600536810003910/rk2189Isup2.hkl
Additional supplementary materials: crystallographic information; 3D view; checkCIF report