Abstract
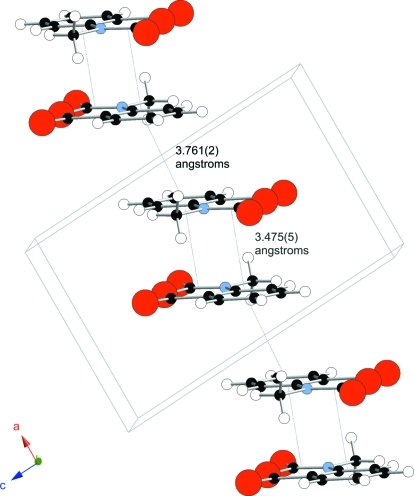
In its crystal structure, the title compound, C9H7NO3, forms π-stacked dimers, with a centroid–centroid distance of 3.475 (5) Å between the benzenoid and the 2,4 dicarbonyl oxazine rings. These dimers then form staircase-like linear chains through further π-stacking between the benzenoid rings [centroid–centroid distance of 3.761 (2) Å]. The methyl-H atoms are disordered due to rotation about the C—N bond and were modeled with equal occupancy.
Related literature
The title compound is a key intermediate for the synthesis of a variety of compounds, see: Coppola (1980 ▶); Kappe & Stadlbauer (1981 ▶); Shvekhgeimer (2001 ▶). Isatoaic anhydrides are important for the synthesis of a variety of commercial compounds. The crystal structures of two other isotoic anydrides have been reported: for the brominated 6-bromo-2H-3,1-benzoxazine-2,4(1H)-dione, see: Lubini & Wouters (1996 ▶) and for the unfunctionalized 2H-3,1-benzoxazine-2,4(1H)-dione, see: Kashino et al. (1978 ▶).
Experimental
Crystal data
C9H7NO3
M r = 177.16
Monoclinic,

a = 7.632 (2) Å
b = 8.818 (2) Å
c = 11.719 (3) Å
β = 93.599 (4)°
V = 787.1 (4) Å3
Z = 4
Mo Kα radiation
μ = 0.11 mm−1
T = 296 K
0.5 × 0.5 × 0.4 mm
Data collection
Bruker APEXII CCD diffractometer
Absorption correction: multi-scan (SADABS; Bruker, 2008 ▶) T min = 0.902, T max = 0.934
13548 measured reflections
2191 independent reflections
1532 reflections with I > 2σ(I)
R int = 0.029
Refinement
R[F 2 > 2σ(F 2)] = 0.052
wR(F 2) = 0.160
S = 1.07
2191 reflections
118 parameters
H-atom parameters constrained
Δρmax = 0.20 e Å−3
Δρmin = −0.21 e Å−3
Data collection: APEX2 (Bruker, 2008 ▶); cell refinement: SAINT-Plus (Bruker, 2008 ▶); data reduction: SAINT-Plus (Bruker, 2008 ▶); program(s) used to solve structure: SHELXS97 (Sheldrick, 2008 ▶); program(s) used to refine structure: SHELXL97 (Sheldrick, 2008 ▶); molecular graphics: CrystalMaker (CrystalMaker, 2009 ▶); software used to prepare material for publication: WinGX (Farrugia, 1999 ▶).
Supplementary Material
Crystal structure: contains datablocks I, global. DOI: 10.1107/S1600536810006094/fj2280sup1.cif
Structure factors: contains datablocks I. DOI: 10.1107/S1600536810006094/fj2280Isup2.hkl
Additional supplementary materials: crystallographic information; 3D view; checkCIF report
Acknowledgments
The authors thank Christopher Cahill for the use of his APEXII diffractometer and the Petroleum Research Fund (grant No. 48381-GB10) for travel funds.
supplementary crystallographic information
Comment
1-Methyl-2H-3,1- benzoxazine-2,4(1H)-dione (N-methylisatoic anhydride, 1) is a key intermediate for the synthesis of a variety of compounds, such as agricultural chemicals, dyes/pigments flavors, fragrances, pharmaceuticals, ultraviolet absorbers, as well as esters, thioesters and amides of N-methylanthranilic acid (Kappe and Stadlbauer, 1981; Coppola, 1980; Shvekhgeimer, 2001). During the investigation of a novel o-aminoaryl oxazoline synthesis from the reaction of N-substituted isatoic anhydrides and 2-chloroethylamine hydrochloride in DMSO using an equimolar quantity of base, o-amino thiomethyl esters were observed as side products. In some cases, an o-amino thiomethyl ester was the major product (Hunt and Cherney, unpublished results).
The title compound is a planar molecule (Fig. 1). The bond distances are consistent with an aromatic system. The packing diagram, (Fig. 2) shows the aromatic rings form pi stacked dimers between the benzenoid ring and the 2,4 dicarbonyl oxazine ring, 3.475 (5) Å for both centroid to centroid distances (Crystalmaker ver. 2.1.2). The dimer forms a staircase-like linear chain through additional pi stacking between the benzenoid rings (3.761 (2) Å centroid to centroid).
Experimental
During the course of the reaction of N-substituted isatoic anhydrides and 2-chloroethylamine hydrochloride, unreacted 1 was isolated from products via silica gel chromatography in a 98:2 dichloromethane/methanol mobile phase. The 98:2 eluent was slowly evaporated to produce colorless rods and blocks of 1. A block was chosen for X-ray analysis, the rods gave the same unit cell as the block.
Refinement
The structure was solved using direct methods. The hydrogen atoms were positioned geometrically. A small improvement in the refinement occurred when the methyl hydrogens were modeled as 50% disordered due to free rotation about the C—N bond.
Figures
Fig. 1.
Thermal ellipsoid plot at 50% probability, the disordered hydrogen atoms were removed for clarity.
Fig. 2.
The packing diagram viewed along the b axis shows π-stacked dimers that are separated by 3.475 (5) Å (centroid to centroid shown as a blue dashed line). A staircase-like linear chain forms from additional π-stacking through the bezenoid rings (3.761 (2) Å). Oxygen atoms are shown in red, nitrogen atoms in blue, carbon atoms in red and the hydrogen atoms in white.
Crystal data
| C9H7NO3 | F(000) = 368 |
| Mr = 177.16 | Dx = 1.495 Mg m−3 |
| Monoclinic, P21/n | Mo Kα radiation, λ = 0.71073 Å |
| Hall symbol: -P 2yn | Cell parameters from 5577 reflections |
| a = 7.632 (2) Å | θ = 5.8–59.2° |
| b = 8.818 (2) Å | µ = 0.11 mm−1 |
| c = 11.719 (3) Å | T = 296 K |
| β = 93.599 (4)° | Block, colorless |
| V = 787.1 (4) Å3 | 0.5 × 0.5 × 0.4 mm |
| Z = 4 |
Data collection
| Bruker APEXII CCD diffractometer | 2191 independent reflections |
| Radiation source: fine-focus sealed tube | 1532 reflections with I > 2σ(I) |
| graphite | Rint = 0.029 |
| ω and φ scans | θmax = 30.1°, θmin = 2.9° |
| Absorption correction: multi-scan (SADABS; Bruker, 2008) | h = −10→10 |
| Tmin = 0.902, Tmax = 0.934 | k = −9→12 |
| 13548 measured reflections | l = −16→16 |
Refinement
| Refinement on F2 | Primary atom site location: structure-invariant direct methods |
| Least-squares matrix: full | Secondary atom site location: difference Fourier map |
| R[F2 > 2σ(F2)] = 0.052 | Hydrogen site location: inferred from neighbouring sites |
| wR(F2) = 0.160 | H-atom parameters constrained |
| S = 1.07 | w = 1/[σ2(Fo2) + (0.0717P)2 + 0.1822P] where P = (Fo2 + 2Fc2)/3 |
| 2191 reflections | (Δ/σ)max < 0.001 |
| 118 parameters | Δρmax = 0.20 e Å−3 |
| 0 restraints | Δρmin = −0.21 e Å−3 |
Special details
| Geometry. All esds (except the esd in the dihedral angle between two l.s. planes) are estimated using the full covariance matrix. The cell esds are taken into account individually in the estimation of esds in distances, angles and torsion angles; correlations between esds in cell parameters are only used when they are defined by crystal symmetry. An approximate (isotropic) treatment of cell esds is used for estimating esds involving l.s. planes. |
| Refinement. Refinement of F2 against ALL reflections. The weighted R-factor wR and goodness of fit S are based on F2, conventional R-factors R are based on F, with F set to zero for negative F2. The threshold expression of F2 > σ(F2) is used only for calculating R-factors(gt) etc. and is not relevant to the choice of reflections for refinement. R-factors based on F2 are statistically about twice as large as those based on F, and R- factors based on ALL data will be even larger. |
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2)
| x | y | z | Uiso*/Ueq | Occ. (<1) | |
| O3 | 0.9373 (2) | 0.59606 (17) | 0.17004 (14) | 0.0830 (5) | |
| O2 | 0.9649 (2) | 1.0762 (2) | 0.27147 (12) | 0.0906 (6) | |
| O1 | 0.93997 (16) | 0.83752 (18) | 0.21847 (10) | 0.0668 (4) | |
| N1 | 0.80524 (17) | 0.76241 (15) | 0.04367 (11) | 0.0494 (3) | |
| C1 | 0.8953 (2) | 0.7229 (2) | 0.14315 (15) | 0.0576 (4) | |
| C2 | 0.9119 (2) | 0.9903 (2) | 0.19846 (14) | 0.0596 (5) | |
| C3 | 0.7855 (2) | 1.1789 (2) | 0.06087 (16) | 0.0607 (5) | |
| H3 | 0.8214 | 1.2561 | 0.1112 | 0.073* | |
| C4 | 0.6984 (3) | 1.2139 (2) | −0.04182 (18) | 0.0662 (5) | |
| H4 | 0.6748 | 1.3144 | −0.0613 | 0.079* | |
| C5 | 0.6463 (2) | 1.0989 (2) | −0.11575 (15) | 0.0591 (4) | |
| H5 | 0.5872 | 1.1228 | −0.1853 | 0.071* | |
| C6 | 0.6794 (2) | 0.95018 (19) | −0.08922 (13) | 0.0488 (4) | |
| H6 | 0.6430 | 0.8742 | −0.1404 | 0.059* | |
| C7 | 0.76789 (17) | 0.91246 (16) | 0.01464 (11) | 0.0402 (3) | |
| C8 | 0.82028 (18) | 1.02777 (18) | 0.08998 (12) | 0.0462 (4) | |
| C9 | 0.7552 (3) | 0.6393 (2) | −0.0348 (2) | 0.0780 (6) | |
| H9A | 0.6927 | 0.6799 | −0.1016 | 0.117* | 0.50 |
| H9B | 0.6811 | 0.5691 | 0.0022 | 0.117* | 0.50 |
| H9C | 0.8587 | 0.5878 | −0.0567 | 0.117* | 0.50 |
| H9D | 0.7956 | 0.5446 | −0.0025 | 0.117* | 0.50 |
| H9E | 0.8072 | 0.6554 | −0.1063 | 0.117* | 0.50 |
| H9F | 0.6297 | 0.6367 | −0.0474 | 0.117* | 0.50 |
Atomic displacement parameters (Å2)
| U11 | U22 | U33 | U12 | U13 | U23 | |
| O3 | 0.0874 (10) | 0.0758 (10) | 0.0840 (10) | 0.0213 (8) | −0.0077 (8) | 0.0243 (8) |
| O2 | 0.0825 (10) | 0.1184 (14) | 0.0673 (9) | −0.0039 (9) | −0.0231 (7) | −0.0386 (9) |
| O1 | 0.0639 (7) | 0.0889 (10) | 0.0455 (6) | 0.0043 (7) | −0.0147 (5) | 0.0028 (6) |
| N1 | 0.0527 (7) | 0.0442 (7) | 0.0499 (7) | 0.0013 (5) | −0.0079 (5) | −0.0009 (5) |
| C1 | 0.0515 (9) | 0.0646 (11) | 0.0558 (9) | 0.0072 (7) | −0.0025 (7) | 0.0104 (8) |
| C2 | 0.0470 (8) | 0.0819 (13) | 0.0489 (8) | −0.0014 (8) | −0.0060 (6) | −0.0148 (8) |
| C3 | 0.0554 (9) | 0.0509 (10) | 0.0758 (11) | −0.0090 (7) | 0.0049 (8) | −0.0169 (8) |
| C4 | 0.0670 (11) | 0.0474 (10) | 0.0839 (13) | −0.0007 (8) | 0.0030 (9) | 0.0084 (8) |
| C5 | 0.0597 (10) | 0.0622 (11) | 0.0549 (9) | 0.0018 (8) | −0.0015 (7) | 0.0128 (8) |
| C6 | 0.0505 (8) | 0.0531 (9) | 0.0418 (7) | −0.0028 (7) | −0.0048 (6) | −0.0009 (6) |
| C7 | 0.0368 (6) | 0.0443 (8) | 0.0393 (6) | −0.0016 (5) | 0.0001 (5) | −0.0022 (5) |
| C8 | 0.0390 (7) | 0.0530 (9) | 0.0463 (7) | −0.0044 (6) | 0.0003 (5) | −0.0095 (6) |
| C9 | 0.0984 (15) | 0.0469 (10) | 0.0854 (14) | 0.0068 (10) | −0.0219 (11) | −0.0154 (9) |
Geometric parameters (Å, °)
| O3—C1 | 1.201 (2) | C4—H4 | 0.9300 |
| O2—C2 | 1.194 (2) | C5—C6 | 1.368 (2) |
| O1—C1 | 1.371 (2) | C5—H5 | 0.9300 |
| O1—C2 | 1.382 (3) | C6—C7 | 1.3944 (19) |
| N1—C1 | 1.361 (2) | C6—H6 | 0.9300 |
| N1—C7 | 1.3912 (19) | C7—C8 | 1.3888 (19) |
| N1—C9 | 1.459 (2) | C9—H9A | 0.9600 |
| C2—C8 | 1.450 (2) | C9—H9B | 0.9600 |
| C3—C4 | 1.373 (3) | C9—H9C | 0.9600 |
| C3—C8 | 1.397 (3) | C9—H9D | 0.9600 |
| C3—H3 | 0.9300 | C9—H9E | 0.9600 |
| C4—C5 | 1.376 (3) | C9—H9F | 0.9600 |
| C1—O1—C2 | 125.43 (13) | C7—C8—C3 | 120.04 (14) |
| C1—N1—C7 | 122.51 (14) | C7—C8—C2 | 119.62 (15) |
| C1—N1—C9 | 116.62 (15) | C3—C8—C2 | 120.34 (15) |
| C7—N1—C9 | 120.81 (13) | N1—C9—H9A | 109.5 |
| O3—C1—N1 | 125.14 (18) | N1—C9—H9B | 109.5 |
| O3—C1—O1 | 117.80 (16) | H9A—C9—H9B | 109.5 |
| N1—C1—O1 | 117.06 (15) | N1—C9—H9C | 109.5 |
| O2—C2—O1 | 117.05 (18) | H9A—C9—H9C | 109.5 |
| O2—C2—C8 | 127.4 (2) | H9B—C9—H9C | 109.5 |
| O1—C2—C8 | 115.59 (14) | N1—C9—H9D | 109.5 |
| C4—C3—C8 | 120.15 (16) | H9A—C9—H9D | 141.1 |
| C4—C3—H3 | 119.9 | H9B—C9—H9D | 56.3 |
| C8—C3—H3 | 119.9 | H9C—C9—H9D | 56.3 |
| C3—C4—C5 | 119.44 (17) | N1—C9—H9E | 109.5 |
| C3—C4—H4 | 120.3 | H9A—C9—H9E | 56.3 |
| C5—C4—H4 | 120.3 | H9B—C9—H9E | 141.1 |
| C6—C5—C4 | 121.41 (16) | H9C—C9—H9E | 56.3 |
| C6—C5—H5 | 119.3 | H9D—C9—H9E | 109.5 |
| C4—C5—H5 | 119.3 | N1—C9—H9F | 109.5 |
| C5—C6—C7 | 119.98 (15) | H9A—C9—H9F | 56.3 |
| C5—C6—H6 | 120.0 | H9B—C9—H9F | 56.3 |
| C7—C6—H6 | 120.0 | H9C—C9—H9F | 141.1 |
| C8—C7—N1 | 119.63 (13) | H9D—C9—H9F | 109.5 |
| C8—C7—C6 | 118.98 (14) | H9E—C9—H9F | 109.5 |
| N1—C7—C6 | 121.39 (13) |
Footnotes
Supplementary data and figures for this paper are available from the IUCr electronic archives (Reference: FJ2280).
References
- Bruker (2008). APEX2, SAINT and SADABS Bruker AXS Inc., Madison, Wisconsin, USA.
- Coppola, G. M. (1980). Synthesis, pp. 505–536.
- CrystalMaker (2009). CrystalMaker CrystalMaker Software, Bicester, England (www.CrystalMaker.com).
- Farrugia, L. J. (1999). J. Appl. Cryst.32, 837–838.
- Kappe, T. & Stadlbauer, W. (1981). Advances in Heterocyclic Chemistry, Vol. 28, Isatoic Anhydrides and their Use in Heterocyclic Chemistry, pp. 231–361. London: Academic Press.
- Kashino, S., Nakashima, S. & Haisa, M. (1978). Acta Cryst. B34, 2191–2195.
- Lubini, P. & Wouters, J. (1996). Acta Cryst. C52, 3108–3110.
- Sheldrick, G. M. (2008). Acta Cryst. A64, 112–122. [DOI] [PubMed]
- Shvekhgeimer, M.-G. A. (2001). Chem. Heterocycl. Compd, 37, 285–443.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Crystal structure: contains datablocks I, global. DOI: 10.1107/S1600536810006094/fj2280sup1.cif
Structure factors: contains datablocks I. DOI: 10.1107/S1600536810006094/fj2280Isup2.hkl
Additional supplementary materials: crystallographic information; 3D view; checkCIF report