Abstract
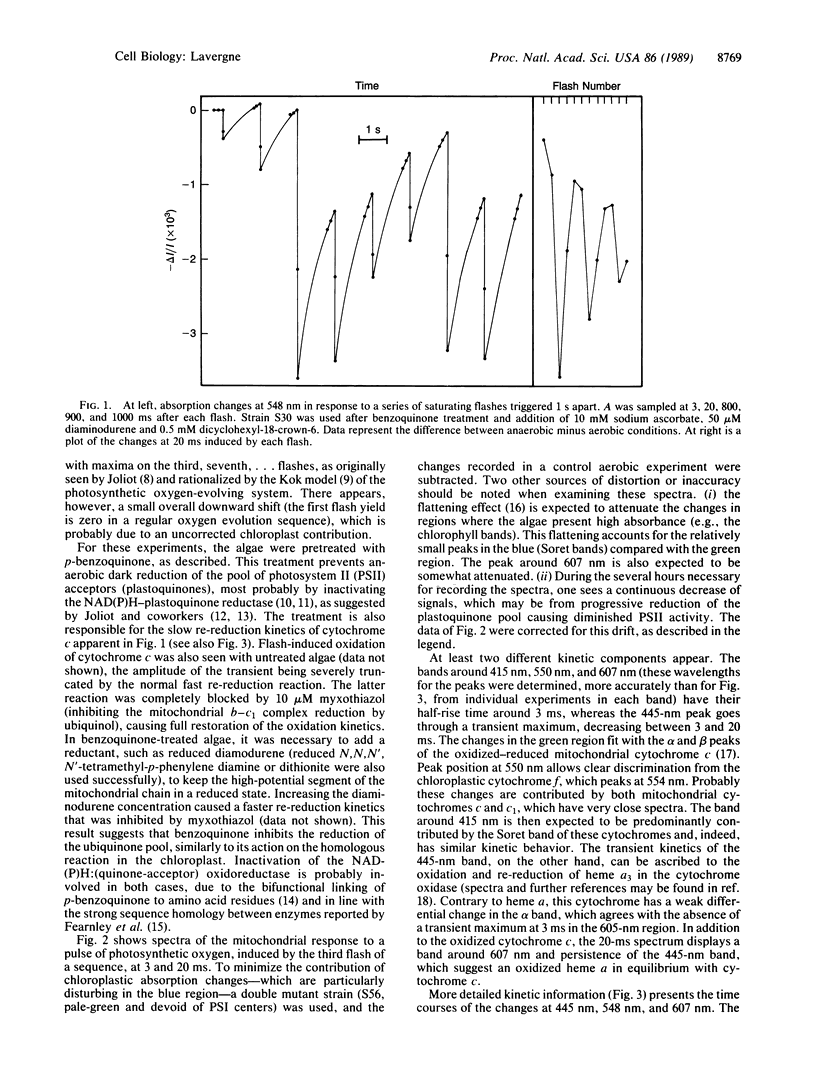
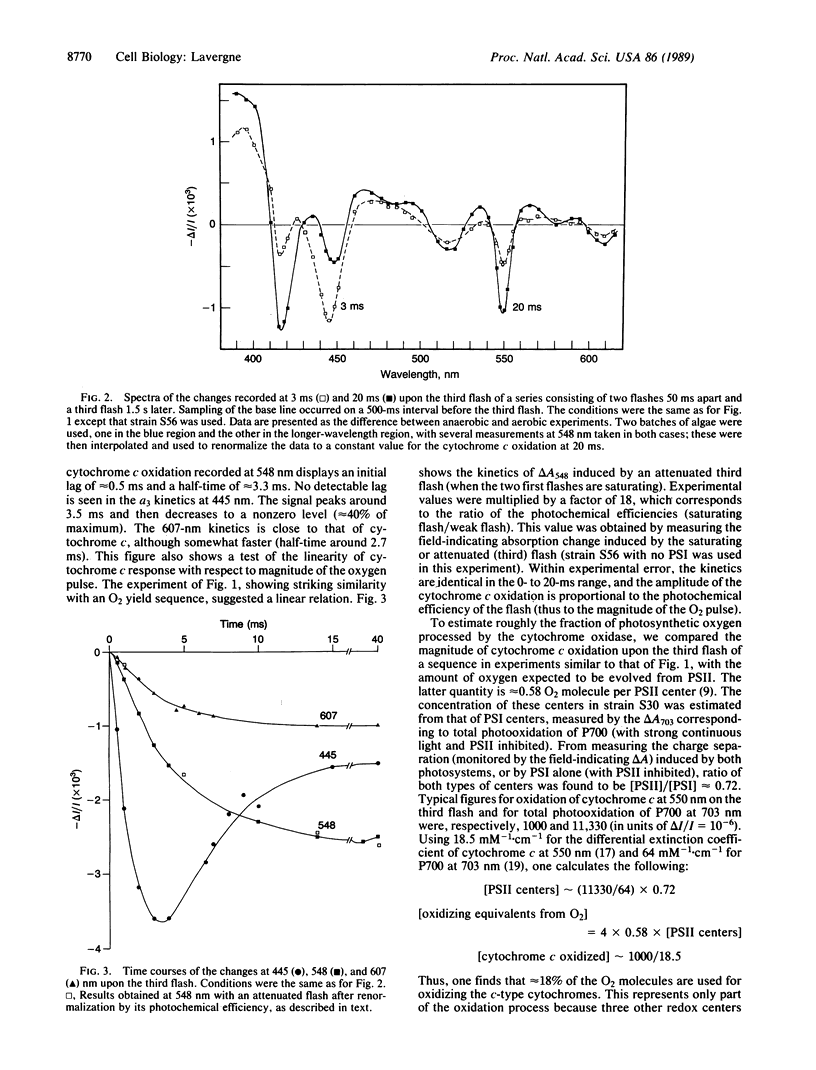
When submitting anaerobic algal cells to a series of saturating flashes, transient absorption changes of mitochondrial origin were detected, showing the characteristic flash-number dependence of photosynthetic oxygen evolution. The faster kinetic event is the oxidation of heme a3 of the cytochrome-c oxidase, which reaches a maximum at [unk]3.5 ms before again being reduced within 20 ms. The oxidation of cytochrome c involves an initial submillisecond lag, and its half-time is [unk]3.3 ms. Another component, probably indicating oxidation of heme a, is seen around 607 nm, with a kinetic behavior similar to that of cytochrome c. The fast time scale of these reactions excludes long-range diffusion and supports a direct intracellular trapping of O2. It is estimated that, under appropriate conditions, the yield of this process is >30%. The linearity of these responses with respect to the amplitude of the oxygen pulse implies that a single turnover of the cytochrome oxidase is involved. These results suggest that the intracellular oxygen pathway may be of physiological importance in green algae. On the other hand, this technique seems promising both as an alternative to polarographic detection of photosynthetic oxygen and as a means of studying the cytochrome oxidase response in vivo to single-turnover oxygen pulses.
Keywords: oxygen evolution, green algae, mitochondrion, cytochrome c, cytochrome c oxidase
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bennoun P. Evidence for a respiratory chain in the chloroplast. Proc Natl Acad Sci U S A. 1982 Jul;79(14):4352–4356. doi: 10.1073/pnas.79.14.4352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DUYSENS L. N. The flattening of the absorption spectrum of suspensions, as compared to that of solutions. Biochim Biophys Acta. 1956 Jan;19(1):1–12. doi: 10.1016/0006-3002(56)90380-8. [DOI] [PubMed] [Google Scholar]
- Fearnley I. M., Runswick M. J., Walker J. E. A homologue of the nuclear coded 49 kd subunit of bovine mitochondrial NADH-ubiquinone reductase is coded in chloroplast DNA. EMBO J. 1989 Mar;8(3):665–672. doi: 10.1002/j.1460-2075.1989.tb03424.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill B. C., Greenwood C., Nicholls P. Intermediate steps in the reaction of cytochrome oxidase with molecular oxygen. Biochim Biophys Acta. 1986;853(2):91–113. doi: 10.1016/0304-4173(86)90006-6. [DOI] [PubMed] [Google Scholar]
- Ke B. The rise time of photoreduction, difference spectrum, and oxidation-reduction potential of P430. Arch Biochem Biophys. 1972 Sep;152(1):70–77. doi: 10.1016/0003-9861(72)90194-4. [DOI] [PubMed] [Google Scholar]
- Kok B., Forbush B., McGloin M. Cooperation of charges in photosynthetic O2 evolution-I. A linear four step mechanism. Photochem Photobiol. 1970 Jun;11(6):457–475. doi: 10.1111/j.1751-1097.1970.tb06017.x. [DOI] [PubMed] [Google Scholar]
- MARGOLIASH E., FROHWIRT N. Spectrum of horse-heart cytochrome c. Biochem J. 1959 Mar;71(3):570–572. doi: 10.1042/bj0710570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rebeille F., Gans P. Interaction between Chloroplasts and Mitochondria in Microalgae: Role of Glycolysis. Plant Physiol. 1988 Dec;88(4):973–975. doi: 10.1104/pp.88.4.973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sternberg R., Bindra D. S., Wilson G. S., Thévenot D. R. Covalent enzyme coupling on cellulose acetate membranes for glucose sensor development. Anal Chem. 1988 Dec 15;60(24):2781–2786. doi: 10.1021/ac00175a028. [DOI] [PubMed] [Google Scholar]


