Abstract
In the asymmetric unit of the title compound, C17H14F2N2O, there are three independent molecules (A, B and C) which differ slightly in the relative orientations of the two fluorophenyl rings. In molecules A and C one of the fluorophenyl rings is disordered over two positions, with occupancy ratios of 0.72 (2):0.28 (2) for molecule A and 0.67 (2):0.33 (2) for molecule C. The dihedral angle between the two fluorophenyl rings in the independent molecules lie in the range 70.3 (3)–84.0 (3)°. In the crystal structure, the molecules are linked via intermolecular C—H⋯O and C—H⋯F hydrogen bonds and π⋯π stacking interactions [centroid–centroid distance = 3.7508 (13) Å], forming a three-dimensional network.
Related literature
For the biological activity of pyrazoline derivatives, see: Amir et al. (2008 ▶); Garge et al. (1971 ▶); Hes et al. (1978 ▶); Manna et al. (2005 ▶); Regaila et al. (1979 ▶). For the role of pyrazolines in organic synthesis, see: Bhaskarreddy et al. (1997 ▶); Klimova et al. (1999 ▶). For related structures, see: Anuradha et al. (2008 ▶); Jian et al. (2006 ▶); Jian & Wang (2006 ▶); Lu et al. (2008 ▶); Wang et al. (2005 ▶). For the stability of the temperature controller used in the data collection, see: Cosier & Glazer (1986 ▶).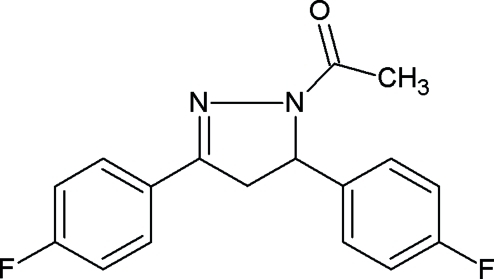
Experimental
Crystal data
C17H14F2N2O
M r = 300.30
Triclinic,
a = 7.1447 (1) Å
b = 17.2332 (3) Å
c = 18.4871 (4) Å
α = 102.880 (1)°
β = 97.941 (1)°
γ = 96.373 (1)°
V = 2173.86 (7) Å3
Z = 6
Mo Kα radiation
μ = 0.11 mm−1
T = 100 K
0.40 × 0.27 × 0.21 mm
Data collection
Bruker SMART APEXII CCD area-detector diffractometer
Absorption correction: multi-scan (SADABS; Bruker, 2009 ▶) T min = 0.960, T max = 0.978
40717 measured reflections
12544 independent reflections
6078 reflections with I > 2σ(I)
R int = 0.039
Refinement
R[F 2 > 2σ(F 2)] = 0.078
wR(F 2) = 0.242
S = 1.03
12544 reflections
726 parameters
510 restraints
H-atom parameters constrained
Δρmax = 0.32 e Å−3
Δρmin = −0.22 e Å−3
Data collection: APEX2 (Bruker, 2009 ▶); cell refinement: SAINT (Bruker, 2009 ▶); data reduction: SAINT; program(s) used to solve structure: SHELXTL (Sheldrick, 2008 ▶); program(s) used to refine structure: SHELXTL; molecular graphics: SHELXTL; software used to prepare material for publication: SHELXTL and PLATON (Spek, 2009 ▶).
Supplementary Material
Crystal structure: contains datablocks global, I. DOI: 10.1107/S1600536810004435/ci5026sup1.cif
Structure factors: contains datablocks I. DOI: 10.1107/S1600536810004435/ci5026Isup2.hkl
Additional supplementary materials: crystallographic information; 3D view; checkCIF report
Table 1. Hydrogen-bond geometry (Å, °).
| D—H⋯A | D—H | H⋯A | D⋯A | D—H⋯A |
|---|---|---|---|---|
| C12A—H12A⋯F2Ai | 0.93 | 2.53 | 3.294 (3) | 139 |
| C12B—H12B⋯F2Cii | 0.93 | 2.53 | 3.309 (3) | 141 |
| C12C—H12C⋯F2Biii | 0.93 | 2.53 | 3.320 (3) | 143 |
| C17A—H17A⋯F1Civ | 0.96 | 2.53 | 3.276 (8) | 134 |
| C17B—H17D⋯F1Av | 0.96 | 2.47 | 3.309 (4) | 146 |
| C17C—H17G⋯F1Bi | 0.96 | 2.55 | 3.311 (2) | 137 |
| C15A—H15A⋯O1C | 0.93 | 2.32 | 3.225 (3) | 165 |
| C15B—H15B⋯O1Avi | 0.93 | 2.36 | 3.262 (3) | 162 |
| C15C—H15C⋯O1Bvii | 0.93 | 2.42 | 3.281 (3) | 153 |
Symmetry codes: (i) 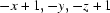 ; (ii)
; (ii) 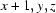 ; (iii)
; (iii) 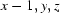 ; (iv)
; (iv) 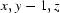 ; (v)
; (v) 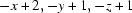 ; (vi)
; (vi) 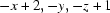 ; (vii)
; (vii) 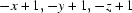 .
.
Acknowledgments
HKF and MH thank the Malaysian Government and Universiti Sains Malaysia for the Research University Golden Goose grant No. 1001/PFIZIK/811012. MH thanks Universiti Sains Malaysia for a post-doctoral research fellowship. SS thanks Mangalore University for the research facilities.
supplementary crystallographic information
Comment
Considerable attention has been focused on substituted pyrazoline compounds, due to their biological activity. In particular, they are used as antitumor, antibacterial, antifungal, antiviral, antiparasitic, anti-tubercular and insecticidal agents (Hes et al., 1978; Manna et al., 2005; Amir et al., 2008). Some of these compounds have also exhibit anti-inflammatory, anti-diabetic, anaesthetic and analgesic properties (Garge et al., 1971; Regaila et al., 1979). Among the pyrazoline derivatives, 1-acetyl-pyrazolines have been identified as one of the most promising scaffold. In the field of medicinal chemistry, 1-acetyl-pyrazoline derivatives have been found to display anticancer and anti-inflammatory activities. In addition, pyrazolines have played a crucial part in the development of the theory of heterocyclic chemistry and are also used extensively in organic synthesis (Klimova et al., 1999; Bhaskarreddy et al., 1997).
The crystal structures of 1-[5-(4-hydroxy-3-methoxyphenyl)-3-methyl-4,5-dihydro-1H-pyrazol-1-yl]ethanone (Wang et al., 2005), 1-[4,5-dihydro-5-phenyl-3-(p-tolyl) pyrazol-1-yl]ethanone (Jian et al., 2006), 1-[3-(4-methoxyphenyl)- 5-phenyl-4,5-dihydro-1H-pyrazol-1-yl]ethanone (Jian & Wang, 2006), 1-[3-(2,4-dichloro-5-fluorophenyl)-5-(3-methyl-2-thienyl)-4,5-dihydro-1H- pyrazol-1-yl]ethanone (Anuradha et al., 2008) and 1-[3-(2-naphthyl)-5-(3,4,5-trimethoxyphenyl)-4,5-dihydro- 1H-pyrazol-1-yl]ethanone (Lu et al., 2008) have been reported. Pyrazolines can be conveniently synthesized by the treatment of α,β-unsaturated carbonyl compounds with hydrazine reagents in basic and acidic media. In this method, hydrazones are formed as intermediates, which can be subsequently cyclized to 2-pyrazolines in the presence of acetic acid. In view of the biological importance of pyrazolines, we report here the crystal structure of a new pyrazoline derivative.
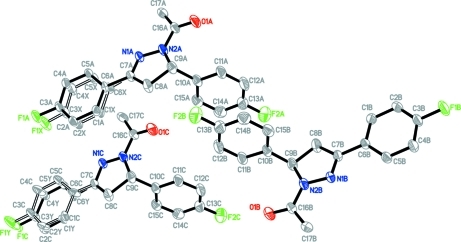
The asymmetric unit of the title compound consists of three crystallographically independent molecules, A, B and C (Fig. 1). In each independent molecule, the pyrazole ring adopts a flattened envelope conformation; the flap atoms C9A, C9B and C9C are displaced by 0.078 (3) and 0.099 (3) and 0.205 (3) Å from the planes of the other four atoms. The dihedral angle between the two fluorophenyl rings are: 70.3 (3) and 72.7 (9)° in the major and minor components of molecule A, 74.73 (10)° in molecule B and 84.0 (3) and 76.1 (9)° in the major and minor components of molecule C.
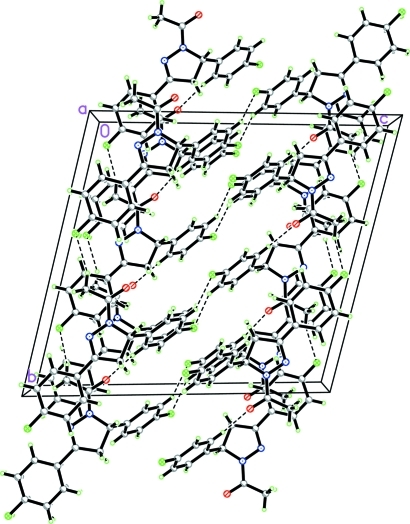
In the crystal structure (Fig. 2), the molecules are linked through intermolecular C—H···O and C—H···F hydrogen bonds (see Table 1) to form three-dimensional network. The crystal structure is further stabilized by weak π···π stacking interactions between C10A–C15A and C10B–C15B phenyl rings at (x, y, z), with a ring centroid-to-centroid distance of 3.7509 (13)Å.
Experimental
A mixture of (2E)-1,3-bis(4-fluorophenyl)prop-2-en-1-one (2.44 g, 0.01 mol) and hydrazine hydrate (0.5ml, 0.01 mol) in ethanol (25 ml) in presence of glacial acetic acid (2 ml) was refluxed for 5 h. The reaction mixture was cooled and poured into 50 ml ice-cold water. The precipitate was collected by filtration and purified by recrystallization from ethanol. Single crystals were grown by slow evaporation of a toluene solution (yield: 84%, m.p. 387 K). Analytical data: Found (Calculated): C 67.86 (67.99), H 4.62 (4.70), N 9.29 (9.33)%.
Refinement
One of the 4-fluorophenyl rings (F1/C1-C6) in molecules A and C are each disordered over two positions with occupancies of 0.72 (2) and 0.28 (2) for molecule A, and 0.67 (2) and 0.33 (2) for molecule C. In all disorder components atoms closer than 1.7 Å were restrained to have the same Uij components and all bonds were subjected to a rigid bond restraint. The 1,2- and 1,3-distances in the major and minor disorder components were restrained to be the same. H atoms were positioned geometrically [C–H = 0.93–0.98 Å] and were refined using a riding model, with Uiso(H) = 1.2 or 1.5 Ueq(C). A rotating group model was used for the methyl groups.
Figures
Fig. 1.
The three independent molecules of the title compound, showing 30% probability displacement ellipsoids and the atom-numbering scheme. Open bonds indicate minor disorder components. H atoms have been omitted for clarity.
Fig. 2.
The crystal packing of the title compound, showing a hydrogen-bonded (dashed lines) network.
Crystal data
| C17H14F2N2O | Z = 6 |
| Mr = 300.30 | F(000) = 936 |
| Triclinic, P1 | Dx = 1.376 Mg m−3 |
| Hall symbol: -P 1 | Mo Kα radiation, λ = 0.71073 Å |
| a = 7.1447 (1) Å | Cell parameters from 8334 reflections |
| b = 17.2332 (3) Å | θ = 2.9–30.0° |
| c = 18.4871 (4) Å | µ = 0.11 mm−1 |
| α = 102.880 (1)° | T = 100 K |
| β = 97.941 (1)° | Block, colourless |
| γ = 96.373 (1)° | 0.40 × 0.27 × 0.21 mm |
| V = 2173.86 (7) Å3 |
Data collection
| Bruker SMART APEXII CCD area-detector diffractometer | 12544 independent reflections |
| Radiation source: fine-focus sealed tube | 6078 reflections with I > 2σ(I) |
| graphite | Rint = 0.039 |
| φ and ω scans | θmax = 30.0°, θmin = 1.2° |
| Absorption correction: multi-scan (SADABS; Bruker, 2009) | h = −10→10 |
| Tmin = 0.960, Tmax = 0.978 | k = −22→24 |
| 40717 measured reflections | l = −20→25 |
Refinement
| Refinement on F2 | Primary atom site location: structure-invariant direct methods |
| Least-squares matrix: full | Secondary atom site location: difference Fourier map |
| R[F2 > 2σ(F2)] = 0.078 | Hydrogen site location: inferred from neighbouring sites |
| wR(F2) = 0.242 | H-atom parameters constrained |
| S = 1.03 | w = 1/[σ2(Fo2) + (0.1087P)2 + 0.1919P] where P = (Fo2 + 2Fc2)/3 |
| 12544 reflections | (Δ/σ)max = 0.002 |
| 726 parameters | Δρmax = 0.32 e Å−3 |
| 510 restraints | Δρmin = −0.22 e Å−3 |
Special details
| Experimental. The crystal was placed in the cold stream of an Oxford Cryosystems Cobra open-flow nitrogen cryostat (Cosier & Glazer, 1986) operating at 100.0 (1) k. |
| Geometry. All s.u.'s (except the s.u. in the dihedral angle between two l.s. planes) are estimated using the full covariance matrix. The cell s.u.'s are taken into account individually in the estimation of s.u.'s in distances, angles and torsion angles; correlations between s.u.'s in cell parameters are only used when they are defined by crystal symmetry. An approximate (isotropic) treatment of cell s.u.'s is used for estimating s.u.'s involving l.s. planes. |
| Refinement. Refinement of F2 against ALL reflections. The weighted R-factor wR and goodness of fit S are based on F2, conventional R-factors R are based on F, with F set to zero for negative F2. The threshold expression of F2 > 2σ(F2) is used only for calculating R-factors(gt) etc. and is not relevant to the choice of reflections for refinement. R-factors based on F2 are statistically about twice as large as those based on F, and R- factors based on ALL data will be even larger. |
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2)
| x | y | z | Uiso*/Ueq | Occ. (<1) | |
| F2A | 0.4613 (3) | 0.09609 (11) | 0.49187 (11) | 0.1240 (6) | |
| O1A | 0.9145 (2) | −0.05242 (8) | 0.25336 (9) | 0.0730 (5) | |
| N1A | 0.9407 (2) | 0.11515 (8) | 0.17523 (8) | 0.0456 (4) | |
| N2A | 0.9414 (2) | 0.06841 (9) | 0.22753 (8) | 0.0512 (4) | |
| F1A | 1.1097 (13) | 0.42500 (19) | 0.0516 (2) | 0.0741 (14) | 0.72 (2) |
| C1A | 1.1010 (14) | 0.3317 (5) | 0.2059 (4) | 0.0436 (16) | 0.72 (2) |
| H1AA | 1.1266 | 0.3456 | 0.2583 | 0.052* | 0.72 (2) |
| C2A | 1.1175 (13) | 0.3910 (4) | 0.1671 (3) | 0.0483 (15) | 0.72 (2) |
| H2AA | 1.1474 | 0.4451 | 0.1922 | 0.058* | 0.72 (2) |
| C3A | 1.0883 (11) | 0.3676 (3) | 0.0906 (3) | 0.0477 (12) | 0.72 (2) |
| C4A | 1.0423 (10) | 0.2887 (3) | 0.0501 (3) | 0.0489 (11) | 0.72 (2) |
| H4AA | 1.0252 | 0.2754 | −0.0022 | 0.059* | 0.72 (2) |
| C5A | 1.0224 (9) | 0.2300 (4) | 0.0894 (4) | 0.0431 (11) | 0.72 (2) |
| H5AA | 0.9927 | 0.1761 | 0.0636 | 0.052* | 0.72 (2) |
| C6A | 1.0466 (13) | 0.2513 (4) | 0.1680 (4) | 0.0364 (12) | 0.72 (2) |
| F1X | 1.002 (4) | 0.4294 (6) | 0.0552 (7) | 0.097 (5) | 0.28 (2) |
| C1X | 1.055 (3) | 0.3297 (12) | 0.2090 (11) | 0.041 (4) | 0.28 (2) |
| H1XA | 1.0806 | 0.3444 | 0.2614 | 0.050* | 0.28 (2) |
| C2X | 1.062 (3) | 0.3898 (12) | 0.1703 (9) | 0.052 (4) | 0.28 (2) |
| H2XA | 1.1014 | 0.4432 | 0.1964 | 0.062* | 0.28 (2) |
| C3X | 1.011 (3) | 0.3712 (8) | 0.0948 (8) | 0.061 (4) | 0.28 (2) |
| C4X | 0.966 (4) | 0.2924 (8) | 0.0541 (9) | 0.071 (4) | 0.28 (2) |
| H4XA | 0.9397 | 0.2791 | 0.0018 | 0.086* | 0.28 (2) |
| C5X | 0.962 (4) | 0.2339 (10) | 0.0943 (10) | 0.066 (5) | 0.28 (2) |
| H5XA | 0.9226 | 0.1810 | 0.0671 | 0.079* | 0.28 (2) |
| C6X | 1.012 (4) | 0.2471 (11) | 0.1724 (11) | 0.054 (5) | 0.28 (2) |
| C7A | 1.0168 (3) | 0.18737 (10) | 0.21017 (10) | 0.0451 (4) | |
| C8A | 1.0795 (3) | 0.19916 (12) | 0.29317 (11) | 0.0615 (6) | |
| H8AA | 1.0170 | 0.2399 | 0.3218 | 0.074* | |
| H8AB | 1.2168 | 0.2143 | 0.3071 | 0.074* | |
| C9A | 1.0154 (3) | 0.11514 (11) | 0.30573 (10) | 0.0550 (5) | |
| H9AA | 1.1276 | 0.0937 | 0.3258 | 0.066* | |
| C10A | 0.8666 (3) | 0.11110 (11) | 0.35581 (10) | 0.0523 (5) | |
| C11A | 0.8752 (3) | 0.06103 (12) | 0.40491 (12) | 0.0629 (6) | |
| H11A | 0.9747 | 0.0307 | 0.4076 | 0.075* | |
| C12A | 0.7375 (4) | 0.05556 (14) | 0.45006 (14) | 0.0797 (7) | |
| H12A | 0.7432 | 0.0215 | 0.4827 | 0.096* | |
| C13A | 0.5947 (4) | 0.10047 (15) | 0.44597 (15) | 0.0770 (7) | |
| C14A | 0.5789 (3) | 0.15065 (14) | 0.39909 (13) | 0.0708 (6) | |
| H14A | 0.4783 | 0.1805 | 0.3973 | 0.085* | |
| C15A | 0.7175 (3) | 0.15619 (12) | 0.35381 (11) | 0.0613 (6) | |
| H15A | 0.7102 | 0.1907 | 0.3217 | 0.074* | |
| C16A | 0.8997 (3) | −0.01320 (11) | 0.20599 (11) | 0.0527 (5) | |
| C17A | 0.8353 (3) | −0.04987 (11) | 0.12432 (12) | 0.0621 (6) | |
| H17A | 0.8408 | −0.1066 | 0.1142 | 0.093* | |
| H17B | 0.9171 | −0.0255 | 0.0960 | 0.093* | |
| H17C | 0.7064 | −0.0412 | 0.1100 | 0.093* | |
| F1B | 0.6346 (2) | −0.09751 (7) | 0.94366 (8) | 0.0912 (5) | |
| F2B | 1.2124 (3) | 0.23406 (10) | 0.50335 (11) | 0.1207 (6) | |
| O1B | 0.7863 (3) | 0.38636 (8) | 0.74826 (9) | 0.0774 (5) | |
| N1B | 0.7612 (2) | 0.21708 (8) | 0.82439 (8) | 0.0474 (4) | |
| N2B | 0.7549 (2) | 0.26485 (9) | 0.77306 (9) | 0.0544 (4) | |
| C1B | 0.5996 (3) | 0.00176 (11) | 0.79147 (11) | 0.0512 (5) | |
| H1BA | 0.5638 | −0.0110 | 0.7394 | 0.061* | |
| C2B | 0.5897 (3) | −0.05886 (11) | 0.82958 (12) | 0.0572 (5) | |
| H2BA | 0.5488 | −0.1122 | 0.8038 | 0.069* | |
| C3B | 0.6414 (3) | −0.03835 (11) | 0.90579 (12) | 0.0575 (5) | |
| C4B | 0.6973 (3) | 0.03970 (11) | 0.94695 (12) | 0.0590 (5) | |
| H4BA | 0.7271 | 0.0518 | 0.9992 | 0.071* | |
| C5B | 0.7078 (3) | 0.09941 (11) | 0.90838 (11) | 0.0516 (5) | |
| H5BA | 0.7458 | 0.1526 | 0.9351 | 0.062* | |
| C6B | 0.6628 (2) | 0.08176 (10) | 0.83014 (10) | 0.0437 (4) | |
| C7B | 0.6789 (3) | 0.14553 (11) | 0.78974 (10) | 0.0458 (4) | |
| C8B | 0.6035 (3) | 0.13564 (12) | 0.70780 (11) | 0.0644 (6) | |
| H8BA | 0.4653 | 0.1229 | 0.6973 | 0.077* | |
| H8BB | 0.6576 | 0.0938 | 0.6765 | 0.077* | |
| C9B | 0.6710 (3) | 0.21961 (12) | 0.69536 (11) | 0.0564 (5) | |
| H9BA | 0.5599 | 0.2430 | 0.6773 | 0.068* | |
| C10B | 0.8155 (3) | 0.22149 (11) | 0.64289 (10) | 0.0517 (5) | |
| C11B | 0.8024 (3) | 0.27024 (12) | 0.59256 (11) | 0.0616 (5) | |
| H11B | 0.7022 | 0.3002 | 0.5900 | 0.074* | |
| C12B | 0.9375 (4) | 0.27470 (14) | 0.54602 (13) | 0.0791 (7) | |
| H12B | 0.9295 | 0.3077 | 0.5125 | 0.095* | |
| C13B | 1.0813 (4) | 0.23014 (14) | 0.55029 (14) | 0.0749 (7) | |
| C14B | 1.1007 (3) | 0.18113 (13) | 0.59823 (13) | 0.0694 (6) | |
| H14B | 1.2015 | 0.1514 | 0.5998 | 0.083* | |
| C15B | 0.9645 (3) | 0.17688 (12) | 0.64496 (11) | 0.0596 (5) | |
| H15B | 0.9741 | 0.1435 | 0.6781 | 0.071* | |
| C16B | 0.8064 (3) | 0.34635 (11) | 0.79500 (12) | 0.0595 (5) | |
| C17B | 0.8895 (4) | 0.38121 (11) | 0.87589 (12) | 0.0752 (7) | |
| H17D | 0.9314 | 0.4378 | 0.8837 | 0.113* | |
| H17E | 0.9964 | 0.3551 | 0.8896 | 0.113* | |
| H17F | 0.7944 | 0.3731 | 0.9065 | 0.113* | |
| F2C | 0.1119 (3) | 0.42973 (11) | 0.48513 (12) | 0.1249 (6) | |
| O1C | 0.6321 (3) | 0.28974 (9) | 0.26097 (9) | 0.0863 (6) | |
| N1C | 0.6015 (2) | 0.45434 (8) | 0.18017 (8) | 0.0458 (4) | |
| N2C | 0.6263 (2) | 0.40862 (9) | 0.23277 (9) | 0.0518 (4) | |
| F1C | 0.6000 (10) | 0.7682 (4) | 0.0556 (5) | 0.0767 (13) | 0.67 (2) |
| C1C | 0.712 (2) | 0.6756 (5) | 0.2086 (5) | 0.0469 (16) | 0.67 (2) |
| H1CA | 0.7713 | 0.6896 | 0.2588 | 0.056* | 0.67 (2) |
| C2C | 0.6924 (16) | 0.7348 (5) | 0.1691 (4) | 0.0506 (17) | 0.67 (2) |
| H2CA | 0.7318 | 0.7889 | 0.1926 | 0.061* | 0.67 (2) |
| C3C | 0.6135 (11) | 0.7112 (4) | 0.0953 (4) | 0.0487 (13) | 0.67 (2) |
| C4C | 0.5451 (11) | 0.6328 (3) | 0.0575 (4) | 0.0527 (12) | 0.67 (2) |
| H4CA | 0.4924 | 0.6196 | 0.0067 | 0.063* | 0.67 (2) |
| C5C | 0.5571 (12) | 0.5745 (4) | 0.0975 (4) | 0.0461 (12) | 0.67 (2) |
| H5CA | 0.5068 | 0.5212 | 0.0741 | 0.055* | 0.67 (2) |
| C6C | 0.6446 (13) | 0.5950 (5) | 0.1731 (4) | 0.0395 (14) | 0.67 (2) |
| F1Y | 0.671 (5) | 0.7647 (9) | 0.0556 (10) | 0.123 (6) | 0.33 (2) |
| C1Y | 0.718 (5) | 0.6637 (12) | 0.2093 (10) | 0.057 (4) | 0.33 (2) |
| H1YA | 0.7473 | 0.6776 | 0.2615 | 0.068* | 0.33 (2) |
| C2Y | 0.733 (3) | 0.7245 (11) | 0.1713 (9) | 0.056 (4) | 0.33 (2) |
| H2YA | 0.7820 | 0.7771 | 0.1976 | 0.068* | 0.33 (2) |
| C3Y | 0.676 (3) | 0.7066 (8) | 0.0951 (9) | 0.064 (4) | 0.33 (2) |
| C4Y | 0.623 (4) | 0.6273 (7) | 0.0549 (8) | 0.075 (4) | 0.33 (2) |
| H4YA | 0.5864 | 0.6145 | 0.0029 | 0.090* | 0.33 (2) |
| C5Y | 0.626 (3) | 0.5680 (8) | 0.0937 (8) | 0.056 (3) | 0.33 (2) |
| H5YA | 0.6051 | 0.5148 | 0.0660 | 0.068* | 0.33 (2) |
| C6Y | 0.660 (3) | 0.5826 (11) | 0.1730 (8) | 0.057 (4) | 0.33 (2) |
| C7C | 0.6597 (2) | 0.52828 (10) | 0.21396 (10) | 0.0450 (4) | |
| C8C | 0.7417 (3) | 0.54173 (12) | 0.29583 (11) | 0.0606 (5) | |
| H8CA | 0.6840 | 0.5823 | 0.3270 | 0.073* | |
| H8CB | 0.8791 | 0.5577 | 0.3049 | 0.073* | |
| C9C | 0.6887 (3) | 0.45806 (11) | 0.31055 (11) | 0.0552 (5) | |
| H9CA | 0.8033 | 0.4405 | 0.3337 | 0.066* | |
| C10C | 0.5332 (3) | 0.45217 (11) | 0.35786 (10) | 0.0506 (5) | |
| C11C | 0.5377 (3) | 0.40019 (12) | 0.40562 (11) | 0.0615 (5) | |
| H11C | 0.6378 | 0.3703 | 0.4090 | 0.074* | |
| C12C | 0.3949 (4) | 0.39252 (14) | 0.44809 (14) | 0.0780 (7) | |
| H12C | 0.3976 | 0.3577 | 0.4799 | 0.094* | |
| C13C | 0.2514 (4) | 0.43678 (15) | 0.44227 (15) | 0.0762 (7) | |
| C14C | 0.2400 (3) | 0.48843 (13) | 0.39656 (13) | 0.0682 (6) | |
| H14C | 0.1389 | 0.5179 | 0.3939 | 0.082* | |
| C15C | 0.3827 (3) | 0.49603 (12) | 0.35406 (11) | 0.0572 (5) | |
| H15C | 0.3775 | 0.5311 | 0.3225 | 0.069* | |
| C16C | 0.6052 (3) | 0.32713 (11) | 0.21266 (12) | 0.0598 (5) | |
| C17C | 0.5497 (4) | 0.28828 (11) | 0.13118 (12) | 0.0732 (7) | |
| H17G | 0.5667 | 0.2327 | 0.1224 | 0.110* | |
| H17H | 0.6285 | 0.3149 | 0.1033 | 0.110* | |
| H17I | 0.4182 | 0.2921 | 0.1151 | 0.110* |
Atomic displacement parameters (Å2)
| U11 | U22 | U33 | U12 | U13 | U23 | |
| F2A | 0.1377 (15) | 0.1098 (12) | 0.1409 (15) | 0.0033 (11) | 0.0792 (13) | 0.0377 (11) |
| O1A | 0.1061 (12) | 0.0531 (8) | 0.0716 (10) | 0.0171 (8) | 0.0127 (9) | 0.0381 (8) |
| N1A | 0.0561 (9) | 0.0378 (8) | 0.0454 (9) | 0.0016 (6) | 0.0047 (7) | 0.0202 (7) |
| N2A | 0.0690 (10) | 0.0413 (8) | 0.0442 (9) | −0.0003 (7) | 0.0014 (7) | 0.0215 (7) |
| F1A | 0.123 (4) | 0.0429 (10) | 0.0600 (13) | −0.0076 (16) | 0.0184 (17) | 0.0272 (9) |
| C1A | 0.044 (4) | 0.042 (2) | 0.039 (2) | −0.0108 (19) | 0.005 (2) | 0.0067 (15) |
| C2A | 0.058 (4) | 0.0319 (16) | 0.051 (2) | −0.010 (2) | 0.0107 (19) | 0.0091 (14) |
| C3A | 0.060 (3) | 0.0363 (15) | 0.0505 (18) | −0.0030 (17) | 0.0139 (19) | 0.0195 (13) |
| C4A | 0.066 (3) | 0.0417 (16) | 0.0386 (17) | −0.0042 (17) | 0.0114 (18) | 0.0132 (13) |
| C5A | 0.055 (3) | 0.0323 (15) | 0.0397 (18) | −0.0034 (15) | 0.0084 (17) | 0.0088 (13) |
| C6A | 0.034 (2) | 0.0368 (19) | 0.040 (2) | −0.0050 (15) | 0.0039 (14) | 0.0207 (17) |
| F1X | 0.151 (13) | 0.049 (3) | 0.090 (5) | −0.012 (5) | 0.008 (6) | 0.037 (3) |
| C1X | 0.041 (9) | 0.035 (4) | 0.051 (5) | −0.006 (4) | 0.024 (5) | 0.014 (3) |
| C2X | 0.056 (9) | 0.037 (4) | 0.059 (5) | −0.011 (5) | 0.020 (5) | 0.008 (4) |
| C3X | 0.073 (9) | 0.043 (4) | 0.065 (5) | −0.013 (5) | 0.012 (6) | 0.022 (4) |
| C4X | 0.104 (12) | 0.051 (5) | 0.056 (5) | −0.017 (7) | 0.016 (7) | 0.019 (4) |
| C5X | 0.099 (12) | 0.039 (5) | 0.055 (5) | −0.012 (7) | 0.016 (7) | 0.013 (4) |
| C6X | 0.064 (11) | 0.041 (5) | 0.044 (5) | −0.018 (5) | 0.007 (6) | −0.003 (5) |
| C7A | 0.0499 (10) | 0.0398 (9) | 0.0447 (11) | −0.0031 (8) | 0.0025 (8) | 0.0165 (8) |
| C8A | 0.0758 (14) | 0.0540 (12) | 0.0491 (12) | −0.0158 (10) | −0.0053 (10) | 0.0227 (10) |
| C9A | 0.0645 (12) | 0.0543 (11) | 0.0447 (11) | −0.0010 (9) | −0.0061 (9) | 0.0230 (9) |
| C10A | 0.0677 (13) | 0.0455 (10) | 0.0398 (10) | −0.0049 (9) | −0.0070 (9) | 0.0182 (9) |
| C11A | 0.0840 (15) | 0.0545 (12) | 0.0549 (13) | 0.0068 (10) | 0.0051 (11) | 0.0287 (10) |
| C12A | 0.117 (2) | 0.0615 (14) | 0.0678 (16) | −0.0007 (14) | 0.0212 (15) | 0.0347 (12) |
| C13A | 0.0909 (18) | 0.0617 (14) | 0.0769 (17) | −0.0080 (13) | 0.0264 (14) | 0.0155 (13) |
| C14A | 0.0716 (15) | 0.0642 (14) | 0.0698 (15) | 0.0057 (11) | 0.0023 (12) | 0.0099 (12) |
| C15A | 0.0786 (15) | 0.0510 (11) | 0.0498 (12) | 0.0030 (10) | −0.0097 (11) | 0.0187 (10) |
| C16A | 0.0621 (12) | 0.0418 (10) | 0.0611 (13) | 0.0092 (8) | 0.0108 (10) | 0.0258 (10) |
| C17A | 0.0827 (15) | 0.0387 (10) | 0.0664 (14) | 0.0049 (9) | 0.0156 (11) | 0.0160 (10) |
| F1B | 0.1521 (14) | 0.0463 (7) | 0.0769 (9) | −0.0046 (7) | 0.0106 (9) | 0.0329 (7) |
| F2B | 0.1402 (15) | 0.1065 (12) | 0.1281 (14) | −0.0002 (10) | 0.0764 (12) | 0.0311 (11) |
| O1B | 0.1182 (13) | 0.0545 (8) | 0.0744 (10) | 0.0239 (8) | 0.0208 (9) | 0.0383 (8) |
| N1B | 0.0634 (10) | 0.0389 (8) | 0.0458 (9) | 0.0084 (7) | 0.0122 (7) | 0.0200 (7) |
| N2B | 0.0790 (11) | 0.0437 (9) | 0.0444 (9) | 0.0061 (8) | 0.0083 (8) | 0.0216 (7) |
| C1B | 0.0632 (12) | 0.0436 (10) | 0.0438 (11) | −0.0027 (8) | 0.0104 (9) | 0.0083 (9) |
| C2B | 0.0758 (14) | 0.0340 (9) | 0.0596 (13) | −0.0017 (9) | 0.0122 (10) | 0.0108 (9) |
| C3B | 0.0749 (14) | 0.0400 (10) | 0.0610 (14) | 0.0002 (9) | 0.0114 (11) | 0.0235 (10) |
| C4B | 0.0797 (14) | 0.0458 (11) | 0.0491 (12) | −0.0032 (9) | 0.0018 (10) | 0.0181 (9) |
| C5B | 0.0676 (12) | 0.0363 (9) | 0.0467 (11) | −0.0037 (8) | 0.0010 (9) | 0.0119 (8) |
| C6B | 0.0463 (10) | 0.0387 (9) | 0.0468 (11) | 0.0009 (7) | 0.0074 (8) | 0.0146 (8) |
| C7B | 0.0499 (10) | 0.0434 (10) | 0.0445 (11) | 0.0005 (8) | 0.0066 (8) | 0.0157 (8) |
| C8B | 0.0770 (14) | 0.0614 (13) | 0.0510 (12) | −0.0118 (10) | −0.0034 (10) | 0.0255 (10) |
| C9B | 0.0654 (13) | 0.0566 (11) | 0.0477 (12) | 0.0016 (9) | −0.0025 (9) | 0.0247 (10) |
| C10B | 0.0679 (12) | 0.0462 (10) | 0.0371 (10) | −0.0036 (9) | −0.0063 (9) | 0.0170 (8) |
| C11B | 0.0828 (15) | 0.0537 (12) | 0.0518 (12) | 0.0065 (10) | 0.0032 (11) | 0.0271 (10) |
| C12B | 0.119 (2) | 0.0616 (14) | 0.0610 (15) | −0.0049 (14) | 0.0171 (14) | 0.0315 (12) |
| C13B | 0.0900 (18) | 0.0596 (14) | 0.0729 (16) | −0.0089 (12) | 0.0261 (14) | 0.0135 (12) |
| C14B | 0.0735 (15) | 0.0636 (14) | 0.0647 (15) | 0.0058 (11) | 0.0044 (12) | 0.0089 (12) |
| C15B | 0.0762 (14) | 0.0528 (11) | 0.0470 (12) | 0.0067 (10) | −0.0060 (10) | 0.0178 (9) |
| C16B | 0.0845 (15) | 0.0431 (11) | 0.0626 (14) | 0.0166 (10) | 0.0238 (11) | 0.0265 (10) |
| C17B | 0.130 (2) | 0.0381 (10) | 0.0620 (14) | 0.0134 (12) | 0.0289 (14) | 0.0122 (10) |
| F2C | 0.1355 (15) | 0.1059 (12) | 0.1501 (16) | 0.0022 (10) | 0.0853 (13) | 0.0360 (11) |
| O1C | 0.1375 (16) | 0.0627 (9) | 0.0793 (11) | 0.0351 (10) | 0.0268 (10) | 0.0446 (9) |
| N1C | 0.0557 (9) | 0.0376 (8) | 0.0477 (9) | 0.0036 (6) | 0.0081 (7) | 0.0195 (7) |
| N2C | 0.0712 (11) | 0.0433 (8) | 0.0447 (9) | 0.0048 (7) | 0.0063 (8) | 0.0222 (7) |
| F1C | 0.117 (3) | 0.0422 (15) | 0.081 (2) | 0.0150 (16) | 0.021 (2) | 0.0325 (15) |
| C1C | 0.059 (3) | 0.030 (3) | 0.046 (3) | −0.006 (2) | 0.006 (2) | 0.005 (2) |
| C2C | 0.072 (4) | 0.025 (2) | 0.060 (3) | 0.007 (2) | 0.022 (2) | 0.0142 (17) |
| C3C | 0.059 (3) | 0.0343 (18) | 0.060 (2) | 0.0096 (17) | 0.020 (2) | 0.0203 (16) |
| C4C | 0.072 (3) | 0.0406 (18) | 0.047 (2) | 0.011 (2) | 0.006 (2) | 0.0161 (15) |
| C5C | 0.059 (3) | 0.0294 (17) | 0.0475 (19) | 0.0037 (18) | 0.004 (2) | 0.0092 (14) |
| C6C | 0.055 (3) | 0.025 (2) | 0.044 (2) | 0.0003 (18) | 0.018 (2) | 0.0181 (19) |
| F1Y | 0.238 (17) | 0.042 (4) | 0.075 (6) | −0.013 (7) | −0.019 (9) | 0.031 (4) |
| C1Y | 0.093 (9) | 0.035 (6) | 0.050 (6) | −0.001 (6) | 0.027 (6) | 0.023 (4) |
| C2Y | 0.078 (9) | 0.023 (5) | 0.059 (5) | 0.007 (5) | −0.004 (5) | 0.001 (4) |
| C3Y | 0.098 (11) | 0.035 (4) | 0.058 (5) | −0.002 (5) | 0.003 (6) | 0.020 (4) |
| C4Y | 0.119 (11) | 0.036 (4) | 0.054 (5) | −0.002 (6) | −0.022 (7) | 0.007 (3) |
| C5Y | 0.081 (9) | 0.033 (4) | 0.051 (4) | 0.001 (5) | −0.007 (5) | 0.015 (3) |
| C6Y | 0.073 (7) | 0.030 (5) | 0.053 (5) | −0.002 (5) | −0.019 (5) | −0.003 (4) |
| C7C | 0.0490 (10) | 0.0410 (10) | 0.0445 (11) | −0.0046 (8) | 0.0043 (8) | 0.0166 (8) |
| C8C | 0.0690 (13) | 0.0567 (12) | 0.0504 (12) | −0.0149 (9) | −0.0031 (10) | 0.0211 (10) |
| C9C | 0.0603 (12) | 0.0562 (12) | 0.0493 (12) | −0.0026 (9) | −0.0034 (9) | 0.0260 (10) |
| C10C | 0.0608 (12) | 0.0488 (10) | 0.0387 (10) | −0.0041 (9) | −0.0053 (8) | 0.0174 (8) |
| C11C | 0.0801 (15) | 0.0575 (12) | 0.0516 (12) | 0.0080 (10) | 0.0051 (11) | 0.0277 (10) |
| C12C | 0.111 (2) | 0.0615 (14) | 0.0675 (16) | −0.0037 (14) | 0.0241 (14) | 0.0316 (12) |
| C13C | 0.0844 (17) | 0.0630 (14) | 0.0797 (17) | −0.0094 (12) | 0.0307 (14) | 0.0134 (13) |
| C14C | 0.0655 (14) | 0.0614 (13) | 0.0707 (15) | 0.0018 (10) | 0.0034 (12) | 0.0099 (12) |
| C15C | 0.0665 (13) | 0.0533 (11) | 0.0484 (12) | 0.0012 (9) | −0.0063 (10) | 0.0187 (9) |
| C16C | 0.0784 (14) | 0.0439 (11) | 0.0679 (14) | 0.0164 (10) | 0.0197 (11) | 0.0281 (10) |
| C17C | 0.1102 (19) | 0.0383 (10) | 0.0728 (16) | 0.0099 (11) | 0.0195 (14) | 0.0147 (11) |
Geometric parameters (Å, °)
| F2A—C13A | 1.368 (3) | C8B—C9B | 1.550 (3) |
| O1A—C16A | 1.220 (2) | C8B—H8BA | 0.97 |
| N1A—C7A | 1.287 (2) | C8B—H8BB | 0.97 |
| N1A—N2A | 1.3895 (18) | C9B—C10B | 1.514 (3) |
| N2A—C16A | 1.360 (2) | C9B—H9BA | 0.98 |
| N2A—C9A | 1.481 (2) | C10B—C15B | 1.382 (3) |
| F1A—C3A | 1.355 (4) | C10B—C11B | 1.386 (2) |
| C1A—C2A | 1.379 (6) | C11B—C12B | 1.386 (3) |
| C1A—C6A | 1.390 (6) | C11B—H11B | 0.93 |
| C1A—H1AA | 0.93 | C12B—C13B | 1.353 (4) |
| C2A—C3A | 1.360 (5) | C12B—H12B | 0.93 |
| C2A—H2AA | 0.93 | C13B—C14B | 1.358 (3) |
| C3A—C4A | 1.377 (5) | C14B—C15B | 1.394 (3) |
| C4A—C5A | 1.376 (5) | C14B—H14B | 0.93 |
| C4A—H4AA | 0.93 | C15B—H15B | 0.93 |
| C5A—C6A | 1.399 (6) | C16B—C17B | 1.492 (3) |
| C5A—H5AA | 0.93 | C17B—H17D | 0.96 |
| C6A—C7A | 1.497 (6) | C17B—H17E | 0.96 |
| F1X—C3X | 1.369 (11) | C17B—H17F | 0.96 |
| C1X—C2X | 1.385 (13) | F2C—C13C | 1.368 (3) |
| C1X—C6X | 1.413 (13) | O1C—C16C | 1.219 (2) |
| C1X—H1XA | 0.93 | N1C—C7C | 1.282 (2) |
| C2X—C3X | 1.348 (13) | N1C—N2C | 1.3867 (18) |
| C2X—H2XA | 0.93 | N2C—C16C | 1.356 (2) |
| C3X—C4X | 1.377 (12) | N2C—C9C | 1.478 (2) |
| C4X—C5X | 1.379 (13) | F1C—C3C | 1.355 (6) |
| C4X—H4XA | 0.93 | C1C—C2C | 1.390 (7) |
| C5X—C6X | 1.399 (14) | C1C—C6C | 1.396 (6) |
| C5X—H5XA | 0.93 | C1C—H1CA | 0.93 |
| C6X—C7A | 1.367 (18) | C2C—C3C | 1.354 (7) |
| C7A—C8A | 1.499 (2) | C2C—H2CA | 0.93 |
| C8A—C9A | 1.547 (2) | C3C—C4C | 1.375 (6) |
| C8A—H8AA | 0.97 | C4C—C5C | 1.376 (6) |
| C8A—H8AB | 0.97 | C4C—H4CA | 0.93 |
| C9A—C10A | 1.509 (3) | C5C—C6C | 1.400 (7) |
| C9A—H9AA | 0.98 | C5C—H5CA | 0.93 |
| C10A—C11A | 1.385 (2) | C6C—C7C | 1.516 (7) |
| C10A—C15A | 1.388 (3) | F1Y—C3Y | 1.365 (11) |
| C11A—C12A | 1.384 (3) | C1Y—C2Y | 1.388 (13) |
| C11A—H11A | 0.93 | C1Y—C6Y | 1.396 (12) |
| C12A—C13A | 1.351 (4) | C1Y—H1YA | 0.93 |
| C12A—H12A | 0.93 | C2Y—C3Y | 1.367 (12) |
| C13A—C14A | 1.357 (3) | C2Y—H2YA | 0.93 |
| C14A—C15A | 1.391 (3) | C3Y—C4Y | 1.384 (11) |
| C14A—H14A | 0.93 | C4Y—C5Y | 1.373 (11) |
| C15A—H15A | 0.93 | C4Y—H4YA | 0.93 |
| C16A—C17A | 1.486 (3) | C5Y—C6Y | 1.412 (12) |
| C17A—H17A | 0.96 | C5Y—H5YA | 0.93 |
| C17A—H17B | 0.96 | C6Y—C7C | 1.329 (16) |
| C17A—H17C | 0.96 | C7C—C8C | 1.502 (2) |
| F1B—C3B | 1.360 (2) | C8C—C9C | 1.542 (2) |
| F2B—C13B | 1.368 (3) | C8C—H8CA | 0.97 |
| O1B—C16B | 1.223 (2) | C8C—H8CB | 0.97 |
| N1B—C7B | 1.288 (2) | C9C—C10C | 1.514 (3) |
| N1B—N2B | 1.3873 (18) | C9C—H9CA | 0.98 |
| N2B—C16B | 1.365 (2) | C10C—C15C | 1.384 (3) |
| N2B—C9B | 1.480 (2) | C10C—C11C | 1.391 (2) |
| C1B—C2B | 1.385 (2) | C11C—C12C | 1.383 (3) |
| C1B—C6B | 1.396 (2) | C11C—H11C | 0.93 |
| C1B—H1BA | 0.93 | C12C—C13C | 1.351 (4) |
| C2B—C3B | 1.361 (3) | C12C—H12C | 0.93 |
| C2B—H2BA | 0.93 | C13C—C14C | 1.358 (3) |
| C3B—C4B | 1.374 (3) | C14C—C15C | 1.383 (3) |
| C4B—C5B | 1.377 (2) | C14C—H14C | 0.93 |
| C4B—H4BA | 0.93 | C15C—H15C | 0.93 |
| C5B—C6B | 1.394 (3) | C16C—C17C | 1.483 (3) |
| C5B—H5BA | 0.93 | C17C—H17G | 0.96 |
| C6B—C7B | 1.463 (2) | C17C—H17H | 0.96 |
| C7B—C8B | 1.501 (3) | C17C—H17I | 0.96 |
| C7A—N1A—N2A | 108.01 (14) | N2B—C9B—C8B | 101.28 (14) |
| C16A—N2A—N1A | 121.67 (15) | C10B—C9B—C8B | 115.55 (17) |
| C16A—N2A—C9A | 124.59 (15) | N2B—C9B—H9BA | 109.5 |
| N1A—N2A—C9A | 113.27 (13) | C10B—C9B—H9BA | 109.5 |
| C2A—C1A—C6A | 121.0 (5) | C8B—C9B—H9BA | 109.5 |
| C2A—C1A—H1AA | 119.5 | C15B—C10B—C11B | 118.6 (2) |
| C6A—C1A—H1AA | 119.5 | C15B—C10B—C9B | 121.48 (16) |
| C3A—C2A—C1A | 117.5 (5) | C11B—C10B—C9B | 119.88 (18) |
| C3A—C2A—H2AA | 121.2 | C12B—C11B—C10B | 120.5 (2) |
| C1A—C2A—H2AA | 121.2 | C12B—C11B—H11B | 119.7 |
| F1A—C3A—C2A | 118.2 (4) | C10B—C11B—H11B | 119.7 |
| F1A—C3A—C4A | 117.7 (4) | C13B—C12B—C11B | 118.7 (2) |
| C2A—C3A—C4A | 124.1 (4) | C13B—C12B—H12B | 120.6 |
| C5A—C4A—C3A | 117.9 (5) | C11B—C12B—H12B | 120.6 |
| C5A—C4A—H4AA | 121.0 | C12B—C13B—C14B | 123.3 (2) |
| C3A—C4A—H4AA | 121.0 | C12B—C13B—F2B | 118.3 (2) |
| C4A—C5A—C6A | 120.1 (5) | C14B—C13B—F2B | 118.4 (2) |
| C4A—C5A—H5AA | 119.9 | C13B—C14B—C15B | 117.8 (2) |
| C6A—C5A—H5AA | 119.9 | C13B—C14B—H14B | 121.1 |
| C1A—C6A—C5A | 119.2 (5) | C15B—C14B—H14B | 121.1 |
| C1A—C6A—C7A | 121.0 (5) | C10B—C15B—C14B | 121.08 (19) |
| C5A—C6A—C7A | 119.8 (5) | C10B—C15B—H15B | 119.5 |
| C2X—C1X—C6X | 122.8 (15) | C14B—C15B—H15B | 119.5 |
| C2X—C1X—H1XA | 118.6 | O1B—C16B—N2B | 119.4 (2) |
| C6X—C1X—H1XA | 118.6 | O1B—C16B—C17B | 123.72 (18) |
| C3X—C2X—C1X | 120.2 (15) | N2B—C16B—C17B | 116.87 (16) |
| C3X—C2X—H2XA | 119.9 | C16B—C17B—H17D | 109.5 |
| C1X—C2X—H2XA | 119.9 | C16B—C17B—H17E | 109.5 |
| C2X—C3X—F1X | 121.8 (12) | H17D—C17B—H17E | 109.5 |
| C2X—C3X—C4X | 121.1 (12) | C16B—C17B—H17F | 109.5 |
| F1X—C3X—C4X | 117.1 (12) | H17D—C17B—H17F | 109.5 |
| C3X—C4X—C5X | 117.1 (13) | H17E—C17B—H17F | 109.5 |
| C3X—C4X—H4XA | 121.4 | C7C—N1C—N2C | 108.00 (14) |
| C5X—C4X—H4XA | 121.4 | C16C—N2C—N1C | 122.17 (16) |
| C4X—C5X—C6X | 125.9 (14) | C16C—N2C—C9C | 124.71 (15) |
| C4X—C5X—H5XA | 117.1 | N1C—N2C—C9C | 113.01 (13) |
| C6X—C5X—H5XA | 117.1 | C2C—C1C—C6C | 120.1 (6) |
| C7A—C6X—C5X | 124.4 (15) | C2C—C1C—H1CA | 119.9 |
| C7A—C6X—C1X | 123.0 (14) | C6C—C1C—H1CA | 119.9 |
| C5X—C6X—C1X | 112.6 (14) | C3C—C2C—C1C | 117.9 (6) |
| N1A—C7A—C6X | 119.1 (8) | C3C—C2C—H2CA | 121.1 |
| N1A—C7A—C6A | 120.7 (3) | C1C—C2C—H2CA | 121.1 |
| N1A—C7A—C8A | 114.60 (15) | C2C—C3C—F1C | 118.3 (6) |
| C6X—C7A—C8A | 125.8 (8) | C2C—C3C—C4C | 124.4 (6) |
| C6A—C7A—C8A | 124.6 (3) | F1C—C3C—C4C | 117.4 (6) |
| C7A—C8A—C9A | 102.63 (15) | C3C—C4C—C5C | 117.8 (6) |
| C7A—C8A—H8AA | 111.2 | C3C—C4C—H4CA | 121.1 |
| C9A—C8A—H8AA | 111.2 | C5C—C4C—H4CA | 121.1 |
| C7A—C8A—H8AB | 111.2 | C4C—C5C—C6C | 120.3 (5) |
| C9A—C8A—H8AB | 111.2 | C4C—C5C—H5CA | 119.9 |
| H8AA—C8A—H8AB | 109.2 | C6C—C5C—H5CA | 119.9 |
| N2A—C9A—C10A | 111.54 (15) | C1C—C6C—C5C | 119.5 (6) |
| N2A—C9A—C8A | 101.25 (13) | C1C—C6C—C7C | 122.2 (6) |
| C10A—C9A—C8A | 116.02 (17) | C5C—C6C—C7C | 118.2 (5) |
| N2A—C9A—H9AA | 109.2 | C2Y—C1Y—C6Y | 123.4 (14) |
| C10A—C9A—H9AA | 109.2 | C2Y—C1Y—H1YA | 118.3 |
| C8A—C9A—H9AA | 109.2 | C6Y—C1Y—H1YA | 118.3 |
| C11A—C10A—C15A | 118.2 (2) | C3Y—C2Y—C1Y | 119.5 (13) |
| C11A—C10A—C9A | 120.21 (18) | C3Y—C2Y—H2YA | 120.2 |
| C15A—C10A—C9A | 121.60 (17) | C1Y—C2Y—H2YA | 120.2 |
| C12A—C11A—C10A | 120.8 (2) | F1Y—C3Y—C2Y | 122.2 (13) |
| C12A—C11A—H11A | 119.6 | F1Y—C3Y—C4Y | 117.5 (12) |
| C10A—C11A—H11A | 119.6 | C2Y—C3Y—C4Y | 120.2 (11) |
| C13A—C12A—C11A | 118.9 (2) | C5Y—C4Y—C3Y | 118.4 (11) |
| C13A—C12A—H12A | 120.6 | C5Y—C4Y—H4YA | 120.8 |
| C11A—C12A—H12A | 120.6 | C3Y—C4Y—H4YA | 120.8 |
| C12A—C13A—C14A | 123.0 (2) | C4Y—C5Y—C6Y | 124.3 (12) |
| C12A—C13A—F2A | 118.6 (2) | C4Y—C5Y—H5YA | 117.9 |
| C14A—C13A—F2A | 118.4 (3) | C6Y—C5Y—H5YA | 117.9 |
| C13A—C14A—C15A | 118.1 (2) | C7C—C6Y—C1Y | 118.9 (13) |
| C13A—C14A—H14A | 121.0 | C7C—C6Y—C5Y | 127.3 (13) |
| C15A—C14A—H14A | 121.0 | C1Y—C6Y—C5Y | 113.4 (13) |
| C10A—C15A—C14A | 121.08 (19) | N1C—C7C—C6Y | 118.2 (7) |
| C10A—C15A—H15A | 119.5 | N1C—C7C—C8C | 114.27 (15) |
| C14A—C15A—H15A | 119.5 | C6Y—C7C—C8C | 127.2 (7) |
| O1A—C16A—N2A | 119.64 (18) | N1C—C7C—C6C | 121.6 (3) |
| O1A—C16A—C17A | 123.35 (17) | C8C—C7C—C6C | 124.1 (3) |
| N2A—C16A—C17A | 117.00 (15) | C7C—C8C—C9C | 102.18 (15) |
| C16A—C17A—H17A | 109.5 | C7C—C8C—H8CA | 111.3 |
| C16A—C17A—H17B | 109.5 | C9C—C8C—H8CA | 111.3 |
| H17A—C17A—H17B | 109.5 | C7C—C8C—H8CB | 111.3 |
| C16A—C17A—H17C | 109.5 | C9C—C8C—H8CB | 111.3 |
| H17A—C17A—H17C | 109.5 | H8CA—C8C—H8CB | 109.2 |
| H17B—C17A—H17C | 109.5 | N2C—C9C—C10C | 111.47 (15) |
| C7B—N1B—N2B | 108.18 (15) | N2C—C9C—C8C | 100.87 (13) |
| C16B—N2B—N1B | 121.94 (16) | C10C—C9C—C8C | 115.30 (17) |
| C16B—N2B—C9B | 124.58 (15) | N2C—C9C—H9CA | 109.6 |
| N1B—N2B—C9B | 113.25 (14) | C10C—C9C—H9CA | 109.6 |
| C2B—C1B—C6B | 120.88 (18) | C8C—C9C—H9CA | 109.6 |
| C2B—C1B—H1BA | 119.6 | C15C—C10C—C11C | 118.38 (19) |
| C6B—C1B—H1BA | 119.6 | C15C—C10C—C9C | 121.91 (16) |
| C3B—C2B—C1B | 118.29 (17) | C11C—C10C—C9C | 119.70 (18) |
| C3B—C2B—H2BA | 120.9 | C12C—C11C—C10C | 120.7 (2) |
| C1B—C2B—H2BA | 120.9 | C12C—C11C—H11C | 119.7 |
| F1B—C3B—C2B | 118.77 (17) | C10C—C11C—H11C | 119.7 |
| F1B—C3B—C4B | 117.96 (18) | C13C—C12C—C11C | 118.5 (2) |
| C2B—C3B—C4B | 123.26 (17) | C13C—C12C—H12C | 120.7 |
| C3B—C4B—C5B | 117.89 (18) | C11C—C12C—H12C | 120.7 |
| C3B—C4B—H4BA | 121.1 | C12C—C13C—C14C | 123.2 (2) |
| C5B—C4B—H4BA | 121.1 | C12C—C13C—F2C | 118.2 (2) |
| C4B—C5B—C6B | 121.40 (17) | C14C—C13C—F2C | 118.5 (2) |
| C4B—C5B—H5BA | 119.3 | C13C—C14C—C15C | 118.2 (2) |
| C6B—C5B—H5BA | 119.3 | C13C—C14C—H14C | 120.9 |
| C5B—C6B—C1B | 118.20 (16) | C15C—C14C—H14C | 120.9 |
| C5B—C6B—C7B | 120.86 (16) | C14C—C15C—C10C | 120.96 (19) |
| C1B—C6B—C7B | 120.94 (17) | C14C—C15C—H15C | 119.5 |
| N1B—C7B—C6B | 120.47 (16) | C10C—C15C—H15C | 119.5 |
| N1B—C7B—C8B | 114.47 (15) | O1C—C16C—N2C | 119.7 (2) |
| C6B—C7B—C8B | 125.06 (16) | O1C—C16C—C17C | 123.49 (18) |
| C7B—C8B—C9B | 102.45 (15) | N2C—C16C—C17C | 116.85 (16) |
| C7B—C8B—H8BA | 111.3 | C16C—C17C—H17G | 109.5 |
| C9B—C8B—H8BA | 111.3 | C16C—C17C—H17H | 109.5 |
| C7B—C8B—H8BB | 111.3 | H17G—C17C—H17H | 109.5 |
| C9B—C8B—H8BB | 111.3 | C16C—C17C—H17I | 109.5 |
| H8BA—C8B—H8BB | 109.2 | H17G—C17C—H17I | 109.5 |
| N2B—C9B—C10B | 111.14 (16) | H17H—C17C—H17I | 109.5 |
| C7A—N1A—N2A—C16A | −168.87 (17) | C16B—N2B—C9B—C8B | −168.37 (19) |
| C7A—N1A—N2A—C9A | 3.5 (2) | N1B—N2B—C9B—C8B | 6.1 (2) |
| C6A—C1A—C2A—C3A | −3.3 (11) | C7B—C8B—C9B—N2B | −5.5 (2) |
| C1A—C2A—C3A—F1A | −178.0 (6) | C7B—C8B—C9B—C10B | 114.70 (18) |
| C1A—C2A—C3A—C4A | 0.5 (9) | N2B—C9B—C10B—C15B | 73.5 (2) |
| F1A—C3A—C4A—C5A | 179.3 (4) | C8B—C9B—C10B—C15B | −41.2 (2) |
| C2A—C3A—C4A—C5A | 0.8 (7) | N2B—C9B—C10B—C11B | −104.97 (19) |
| C3A—C4A—C5A—C6A | 0.7 (7) | C8B—C9B—C10B—C11B | 140.37 (18) |
| C2A—C1A—C6A—C5A | 4.8 (12) | C15B—C10B—C11B—C12B | −0.8 (3) |
| C2A—C1A—C6A—C7A | −176.1 (7) | C9B—C10B—C11B—C12B | 177.75 (19) |
| C4A—C5A—C6A—C1A | −3.4 (11) | C10B—C11B—C12B—C13B | 0.5 (3) |
| C4A—C5A—C6A—C7A | 177.5 (5) | C11B—C12B—C13B—C14B | −0.2 (4) |
| C6X—C1X—C2X—C3X | 5(3) | C11B—C12B—C13B—F2B | 179.1 (2) |
| C1X—C2X—C3X—F1X | 175.1 (15) | C12B—C13B—C14B—C15B | 0.2 (4) |
| C1X—C2X—C3X—C4X | −5(2) | F2B—C13B—C14B—C15B | −179.1 (2) |
| C2X—C3X—C4X—C5X | 5(2) | C11B—C10B—C15B—C14B | 0.7 (3) |
| F1X—C3X—C4X—C5X | −175.6 (12) | C9B—C10B—C15B—C14B | −177.75 (18) |
| C3X—C4X—C5X—C6X | −5(2) | C13B—C14B—C15B—C10B | −0.4 (3) |
| C4X—C5X—C6X—C7A | −176.1 (18) | N1B—N2B—C16B—O1B | −175.16 (17) |
| C4X—C5X—C6X—C1X | 5(3) | C9B—N2B—C16B—O1B | −1.1 (3) |
| C2X—C1X—C6X—C7A | 176 (2) | N1B—N2B—C16B—C17B | 6.2 (3) |
| C2X—C1X—C6X—C5X | −5(3) | C9B—N2B—C16B—C17B | −179.76 (19) |
| N2A—N1A—C7A—C6X | −172.3 (15) | C7C—N1C—N2C—C16C | −169.85 (17) |
| N2A—N1A—C7A—C6A | 176.6 (4) | C7C—N1C—N2C—C9C | 6.5 (2) |
| N2A—N1A—C7A—C8A | −0.3 (2) | C6C—C1C—C2C—C3C | −2.9 (18) |
| C5X—C6X—C7A—N1A | −12 (3) | C1C—C2C—C3C—F1C | −177.5 (10) |
| C1X—C6X—C7A—N1A | 166.6 (18) | C1C—C2C—C3C—C4C | 2.7 (14) |
| C5X—C6X—C7A—C6A | 90 (7) | C2C—C3C—C4C—C5C | 0.2 (9) |
| C1X—C6X—C7A—C6A | −91 (7) | F1C—C3C—C4C—C5C | −179.6 (5) |
| C5X—C6X—C7A—C8A | 176.6 (16) | C3C—C4C—C5C—C6C | −2.9 (8) |
| C1X—C6X—C7A—C8A | −4(3) | C2C—C1C—C6C—C5C | 0.3 (18) |
| C1A—C6A—C7A—N1A | 170.0 (6) | C2C—C1C—C6C—C7C | −178.9 (10) |
| C5A—C6A—C7A—N1A | −10.9 (9) | C4C—C5C—C6C—C1C | 2.7 (12) |
| C1A—C6A—C7A—C6X | 87 (6) | C4C—C5C—C6C—C7C | −178.1 (5) |
| C5A—C6A—C7A—C6X | −94 (6) | C6Y—C1Y—C2Y—C3Y | 5(4) |
| C1A—C6A—C7A—C8A | −13.4 (10) | C1Y—C2Y—C3Y—F1Y | 174 (2) |
| C5A—C6A—C7A—C8A | 165.7 (5) | C1Y—C2Y—C3Y—C4Y | −7(3) |
| N1A—C7A—C8A—C9A | −2.7 (2) | F1Y—C3Y—C4Y—C5Y | −179.6 (12) |
| C6X—C7A—C8A—C9A | 168.6 (16) | C2Y—C3Y—C4Y—C5Y | 1(2) |
| C6A—C7A—C8A—C9A | −179.4 (5) | C3Y—C4Y—C5Y—C6Y | 7(2) |
| C16A—N2A—C9A—C10A | −68.8 (2) | C2Y—C1Y—C6Y—C7C | 176 (3) |
| N1A—N2A—C9A—C10A | 119.06 (16) | C2Y—C1Y—C6Y—C5Y | 3(4) |
| C16A—N2A—C9A—C8A | 167.22 (18) | C4Y—C5Y—C6Y—C7C | 178.1 (15) |
| N1A—N2A—C9A—C8A | −4.9 (2) | C4Y—C5Y—C6Y—C1Y | −9(3) |
| C7A—C8A—C9A—N2A | 4.23 (19) | N2C—N1C—C7C—C6Y | 176.0 (12) |
| C7A—C8A—C9A—C10A | −116.68 (18) | N2C—N1C—C7C—C8C | 2.6 (2) |
| N2A—C9A—C10A—C11A | 102.35 (19) | N2C—N1C—C7C—C6C | −177.3 (4) |
| C8A—C9A—C10A—C11A | −142.43 (18) | C1Y—C6Y—C7C—N1C | 178 (2) |
| N2A—C9A—C10A—C15A | −77.0 (2) | C5Y—C6Y—C7C—N1C | −9(3) |
| C8A—C9A—C10A—C15A | 38.2 (2) | C1Y—C6Y—C7C—C8C | −9(3) |
| C15A—C10A—C11A—C12A | 0.7 (3) | C5Y—C6Y—C7C—C8C | 163.3 (15) |
| C9A—C10A—C11A—C12A | −178.70 (19) | C1Y—C6Y—C7C—C6C | 56 (8) |
| C10A—C11A—C12A—C13A | −0.5 (4) | C5Y—C6Y—C7C—C6C | −131 (10) |
| C11A—C12A—C13A—C14A | 0.4 (4) | C1C—C6C—C7C—N1C | −178.1 (9) |
| C11A—C12A—C13A—F2A | −178.6 (2) | C5C—C6C—C7C—N1C | 2.8 (9) |
| C12A—C13A—C14A—C15A | −0.5 (4) | C1C—C6C—C7C—C6Y | −117 (9) |
| F2A—C13A—C14A—C15A | 178.6 (2) | C5C—C6C—C7C—C6Y | 64 (8) |
| C11A—C10A—C15A—C14A | −0.8 (3) | C1C—C6C—C7C—C8C | 2.0 (12) |
| C9A—C10A—C15A—C14A | 178.59 (18) | C5C—C6C—C7C—C8C | −177.1 (5) |
| C13A—C14A—C15A—C10A | 0.7 (3) | N1C—C7C—C8C—C9C | −9.8 (2) |
| N1A—N2A—C16A—O1A | 175.02 (16) | C6Y—C7C—C8C—C9C | 177.4 (13) |
| C9A—N2A—C16A—O1A | 3.5 (3) | C6C—C7C—C8C—C9C | 170.1 (4) |
| N1A—N2A—C16A—C17A | −5.7 (3) | C16C—N2C—C9C—C10C | −72.8 (2) |
| C9A—N2A—C16A—C17A | −177.21 (18) | N1C—N2C—C9C—C10C | 110.90 (16) |
| C7B—N1B—N2B—C16B | 170.59 (18) | C16C—N2C—C9C—C8C | 164.28 (18) |
| C7B—N1B—N2B—C9B | −4.1 (2) | N1C—N2C—C9C—C8C | −12.0 (2) |
| C6B—C1B—C2B—C3B | 0.6 (3) | C7C—C8C—C9C—N2C | 11.94 (19) |
| C1B—C2B—C3B—F1B | −179.15 (18) | C7C—C8C—C9C—C10C | −108.26 (18) |
| C1B—C2B—C3B—C4B | 2.1 (3) | N2C—C9C—C10C—C15C | −80.1 (2) |
| F1B—C3B—C4B—C5B | 178.73 (18) | C8C—C9C—C10C—C15C | 34.1 (2) |
| C2B—C3B—C4B—C5B | −2.5 (3) | N2C—C9C—C10C—C11C | 98.58 (19) |
| C3B—C4B—C5B—C6B | 0.2 (3) | C8C—C9C—C10C—C11C | −147.21 (17) |
| C4B—C5B—C6B—C1B | 2.3 (3) | C15C—C10C—C11C—C12C | 0.1 (3) |
| C4B—C5B—C6B—C7B | −178.54 (18) | C9C—C10C—C11C—C12C | −178.56 (19) |
| C2B—C1B—C6B—C5B | −2.7 (3) | C10C—C11C—C12C—C13C | −0.2 (4) |
| C2B—C1B—C6B—C7B | 178.12 (17) | C11C—C12C—C13C—C14C | 0.2 (4) |
| N2B—N1B—C7B—C6B | −179.56 (15) | C11C—C12C—C13C—F2C | −179.2 (2) |
| N2B—N1B—C7B—C8B | −0.1 (2) | C12C—C13C—C14C—C15C | −0.1 (4) |
| C5B—C6B—C7B—N1B | 10.3 (3) | F2C—C13C—C14C—C15C | 179.2 (2) |
| C1B—C6B—C7B—N1B | −170.53 (17) | C13C—C14C—C15C—C10C | 0.1 (3) |
| C5B—C6B—C7B—C8B | −169.09 (19) | C11C—C10C—C15C—C14C | −0.1 (3) |
| C1B—C6B—C7B—C8B | 10.1 (3) | C9C—C10C—C15C—C14C | 178.58 (18) |
| N1B—C7B—C8B—C9B | 3.8 (2) | N1C—N2C—C16C—O1C | 178.61 (17) |
| C6B—C7B—C8B—C9B | −176.74 (18) | C9C—N2C—C16C—O1C | 2.7 (3) |
| C16B—N2B—C9B—C10B | 68.4 (2) | N1C—N2C—C16C—C17C | −1.3 (3) |
| N1B—N2B—C9B—C10B | −117.15 (16) | C9C—N2C—C16C—C17C | −177.25 (19) |
Hydrogen-bond geometry (Å, °)
| D—H···A | D—H | H···A | D···A | D—H···A |
| C12A—H12A···F2Ai | 0.93 | 2.53 | 3.294 (3) | 139 |
| C12B—H12B···F2Cii | 0.93 | 2.53 | 3.309 (3) | 141 |
| C12C—H12C···F2Biii | 0.93 | 2.53 | 3.320 (3) | 143 |
| C17A—H17A···F1Civ | 0.96 | 2.53 | 3.276 (8) | 134 |
| C17B—H17D···F1Av | 0.96 | 2.47 | 3.309 (4) | 146 |
| C17C—H17G···F1Bi | 0.96 | 2.55 | 3.311 (2) | 137 |
| C15A—H15A···O1C | 0.93 | 2.32 | 3.225 (3) | 165 |
| C15B—H15B···O1Avi | 0.93 | 2.36 | 3.262 (3) | 162 |
| C15C—H15C···O1Bvii | 0.93 | 2.42 | 3.281 (3) | 153 |
Symmetry codes: (i) −x+1, −y, −z+1; (ii) x+1, y, z; (iii) x−1, y, z; (iv) x, y−1, z; (v) −x+2, −y+1, −z+1; (vi) −x+2, −y, −z+1; (vii) −x+1, −y+1, −z+1.
Footnotes
Supplementary data and figures for this paper are available from the IUCr electronic archives (Reference: CI5026).
References
- Amir, M., Kumar, H. & Khan, S. A. (2008). Bioorg. Med. Chem. Lett.18, 918–922. [DOI] [PubMed]
- Anuradha, N., Thiruvalluvar, A., Mahalinga, M. & Butcher, R. J. (2008). Acta Cryst. E64, o2160. [DOI] [PMC free article] [PubMed]
- Bhaskarreddy, D., Chandrasekhar, B. N., Padmavathi, V. & Sumathi, R. P. (1997). Synthesis, 3, 491–494.
- Bruker (2009). APEX2, SAINT and SADABS Bruker AXS Inc., Madison, Wisconsin, USA.
- Cosier, J. & Glazer, A. M. (1986). J. Appl. Cryst.19, 105–107.
- Garge, H. G. & Chandraprakash (1971). J. Pharm. Sci.60, 323–325.
- Hes, R. V., Wellinga, K. & Grosscurt, A. C. (1978). J. Agric. Food Chem.26, 915–918.
- Jian, F.-F. & Wang, J. (2006). Acta Cryst. E62, o5303–o5304.
- Jian, F.-F., Wang, J. & Xiao, H.-L. (2006). Acta Cryst. E62, o4771–o4772.
- Klimova, E. I., Marcos, M., Klimova, T. B., Cecilio, A. T., Ruben, A. T. & Lena, R. R. (1999). J. Organomet. Chem.585, 106–111.
- Lu, Z.-K., Diao, H.-L., Li, S. & He, B. (2008). Acta Cryst. E64, o1638. [DOI] [PMC free article] [PubMed]
- Manna, F., Chimenti, F., Fioravanti, R., Bolasco, A., Secci, D., Chimenti, P., Ferlini, C. & Scambia, G. (2005). Bioorg. Med. Chem. Lett.15, 4632–4635. [DOI] [PubMed]
- Regaila, H. A., El-Bayonk, A. K. & Hammad, M. (1979). Egypt. J. Chem.20, 197–202.
- Sheldrick, G. M. (2008). Acta Cryst. A64, 112–122. [DOI] [PubMed]
- Spek, A. L. (2009). Acta Cryst. D65, 148–155. [DOI] [PMC free article] [PubMed]
- Wang, S.-F., Zhu, W., Yang, X. & Zhou, L.-J. (2005). Acta Cryst. E61, o3985–o3986.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Crystal structure: contains datablocks global, I. DOI: 10.1107/S1600536810004435/ci5026sup1.cif
Structure factors: contains datablocks I. DOI: 10.1107/S1600536810004435/ci5026Isup2.hkl
Additional supplementary materials: crystallographic information; 3D view; checkCIF report