Abstract
In the title compound, C19H15FO3, the dihedral angle between the naphthalene ring system and the benzene ring is 62.93 (5)°. The bridging carbonyl C—C(=O)—C plane makes dihedral angles of 45.55 (6) and 28.62 (7)°, respectively, with the naphthalene ring system and the benzene ring. Weak intermolecular C—H⋯O hydrogen bonds and C—H⋯π interactions stabilize the crystal packing.
Related literature
For general background to the regioselective formation of peri-aroylnaphthalene compounds, see: Okamoto & Yonezawa (2009 ▶). For related structures, see: Hijikata et al. (2010 ▶); Mitsui et al. (2008 ▶); Nakaema et al. (2007 ▶, 2008 ▶); Watanabe et al. (2010a
▶,b
▶).
Experimental
Crystal data
C19H15FO3
M r = 310.31
Monoclinic,
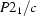
a = 8.3690 (2) Å
b = 19.7603 (5) Å
c = 9.3897 (2) Å
β = 105.126 (2)°
V = 1499.01 (6) Å3
Z = 4
Cu Kα radiation
μ = 0.84 mm−1
T = 193 K
0.55 × 0.50 × 0.45 mm
Data collection
Rigaku R-AXIS RAPID diffractometer
Absorption correction: numerical (NUMABS; Higashi, 1999 ▶) T min = 0.657, T max = 0.705
26258 measured reflections
2738 independent reflections
2530 reflections with I > 2σ(I)
R int = 0.023
Refinement
R[F 2 > 2σ(F 2)] = 0.032
wR(F 2) = 0.090
S = 1.02
2738 reflections
211 parameters
H-atom parameters constrained
Δρmax = 0.20 e Å−3
Δρmin = −0.11 e Å−3
Data collection: PROCESS-AUTO (Rigaku, 1998 ▶); cell refinement: PROCESS-AUTO; data reduction: CrystalStructure (Rigaku/MSC, 2004 ▶); program(s) used to solve structure: SIR2004 (Burla et al., 2005 ▶); program(s) used to refine structure: SHELXL97 (Sheldrick, 2008 ▶); molecular graphics: ORTEPIII (Burnett & Johnson, 1996 ▶); software used to prepare material for publication: SHELXL97.
Supplementary Material
Crystal structure: contains datablocks I, global. DOI: 10.1107/S1600536810006859/bt5198sup1.cif
Structure factors: contains datablocks I. DOI: 10.1107/S1600536810006859/bt5198Isup2.hkl
Additional supplementary materials: crystallographic information; 3D view; checkCIF report
Table 1. Hydrogen-bond geometry (Å, °).
Cg1 is the centroid of the C1–C5/C10 ring.
| D—H⋯A | D—H | H⋯A | D⋯A | D—H⋯A |
|---|---|---|---|---|
| C18—H18B⋯Cg1i | 0.98 | 2.85 | 3.7479 (14) | 152 |
| C17—H17⋯O1ii | 0.95 | 2.55 | 3.2930 (16) | 136 |
| C18—H18A⋯O3iii | 0.98 | 2.39 | 3.3603 (16) | 169 |
Symmetry codes: (i) 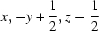 ; (ii)
; (ii) 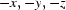 ; (iii)
; (iii) 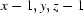 .
.
Acknowledgments
The authors would express their gratitude to professor Keiichi Noguchi for his technical advice. This work was partially supported by the Ogasawara Foundation for the Promotion of Science & Engineering, Tokyo, Japan.
supplementary crystallographic information
Comment
In the course of our study on electrophilic aromatic aroylation of 2,7-dimethoxynaphthalene, peri-aroylnaphthalene compounds have proven to be formed regioselectively with the aid of suitable acidic mediators (Okamoto & Yonezawa, 2009). The aroyl groups at the 1,8-positions of the naphthalene rings in these compounds are twisted almost perpendicularly but the benzene ring moieties of the aroyl groups tilt slightly toward the exo sides of the naphthalene rings.
Recently, we reported the structures of 1,8-diaroyl-2,7-dimethoxynaphthalenes, i. e., 1,8-bis(4-chlorobenzoyl)-2,7-dimethoxynaphthalene (Nakaema et al., 2007), bis(4-bromobenzoyl)(2,7-dimethoxynaphthalene-1,8-diyl)dimethanone (Watanabe et al., 2010a). In addition, the crystal structural analysis of 1-aroyl-2,7-dimethoxynaphthalenes, i. e., methyl 4-(2,7-dimethoxy-1-naphthoyl)benzoate (Hijikata et al., 2010) and 1-(4-nitorobenzoyl)-2,7-dimethoxynaphthalene (Watanabe et al. 2010b), also has revealed essentially the same non-coplanar structure as the 1,8-diaroylated naphthalenes.
Furthermore, the structure of 3-aroyl-2,7-dimetoxynaphthalenes such as 2-(4-chlorobenzoyl)-3,6-dimethoxynaphthalene (Nakaema et al., 2008), which are generally regarded to be thermodynamically more stable than the corresponding 1-positioned isomeric molecules, 1-(4-chlorobenzoyl)-2,7-dimethoxynaphthalene (Mitsui et al., 2008), has been also studied. As a part of our ongoing work on the formation reaction and the structure of the aroylated naphthalene derivatives, the synthesis and crystal structure of (I), a 3-monoaroylnaphthalene bearing fluoro group, is discussed in this report. (I) was prepared by electrophilic aromatic aroylation reaction of 2,7-dimethoxynaphthalene with 4-fluorobenzoic acid.
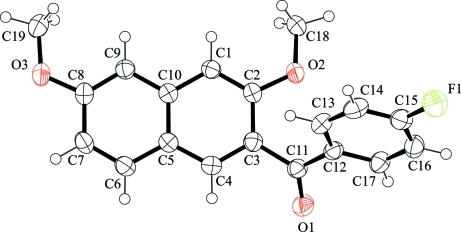
An ORTEPIII (Burnett & Johnson, 1996) plot of (I) is displayed in Fig. 1. The 4-fluorophenyl group is twisted away from the attached naphthalene ring. The dihedral angle between the best planes of the fluorophenyl ring (C12—C17) and the naphthalene ring (C1—C10) is 62.93 (5)°. The bridging carbonyl plane (O1—C11—C3—C12) makes relatively large dihedral angle of 45.55 (6)° with the naphthalene ring (C1—C10) [C4—C3—C11—O1 torsion angle = 43.90 (17)°], whereas it makes rather small one of 28.62 (7)° with 4-fluorophenyl ring (C12—C17) [O1—C11—C12—C17 torsion angle = 28.69 (18)°].
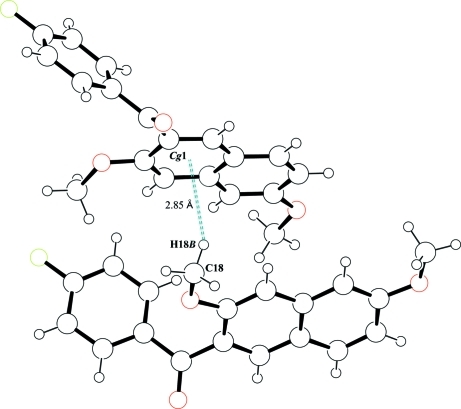
Molecules are linked by C—H···π interactions (Fig. 2). The methyl group acts as an hydrogen-bond donor and π system of the naphthalene ring [C1—C5/C10 ring (with centroid Cg1)] of an adjacent molecule acts as an accepter (C18—H18B···π).
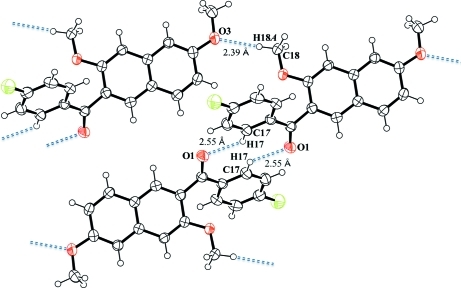
The crystal packing is additionally stabilized by two types of intermolecular weak C—H···O hydrogen bondings: One is between the aromatic hydrogen (H17) at meta position to the F group, and the carbonyl oxygen (O1) (Fig.3, Table 1). The other is between an hydrogen (H18A) of the 2-methoxy group which is situated adjacent to the fluorophenyl group, and the ethereal oxygen (O3) of the 7-methoxy group in a neighboring molecule .
Experimental
The title compound was prepared by treatment of a mixture of 2,7-dimethoxynaphthalene (0.20 mmol) and 4-fluoroobenzoic acid (0.21 mmol) with phosphorus pentoxide–methanesulfonic acid mixture (P2O5–MsOH [1/10 w/w]; 0.44 ml) at 60°C for 24 hours followed by a typical work-up procedure (30% yield; Okamoto & Yonezawa, 2009). Colorless block single crystals suitable for X-ray diffraction were obtained by recrystallization from chloroform.
Refinement
All the H atoms were found in difference maps and were subsequently refined as riding atoms, with C—H = 0.95 (aromatic) and 0.98 (methyl) Å, and Uiso(H) = 1.2Ueq(C).
Figures
Fig. 1.
Molecular structure of (I), with the atom-labeling scheme and displacement ellipsoids drawn at the 50 % probability level.
Fig. 2.
C—H···π interactions (green dotted lines).
Fig. 3.
Two types of intermolecular weak C—H···O interactions (blue dotted lines).
Crystal data
| C19H15FO3 | F(000) = 648 |
| Mr = 310.31 | Dx = 1.375 Mg m−3 |
| Monoclinic, P21/c | Melting point = 409.7–410.3 K |
| Hall symbol: -P 2ybc | Cu Kα radiation, λ = 1.54187 Å |
| a = 8.3690 (2) Å | Cell parameters from 20173 reflections |
| b = 19.7603 (5) Å | θ = 4.5–68.2° |
| c = 9.3897 (2) Å | µ = 0.84 mm−1 |
| β = 105.126 (2)° | T = 193 K |
| V = 1499.01 (6) Å3 | Block, colorless |
| Z = 4 | 0.55 × 0.50 × 0.45 mm |
Data collection
| Rigaku R-AXIS RAPID diffractometer | 2738 independent reflections |
| Radiation source: fine-focus sealed tube | 2530 reflections with I > 2σ(I) |
| graphite | Rint = 0.023 |
| Detector resolution: 10.00 pixels mm-1 | θmax = 68.2°, θmin = 4.5° |
| ω scans | h = −10→9 |
| Absorption correction: numerical (NUMABS; Higashi, 1999) | k = −23→23 |
| Tmin = 0.657, Tmax = 0.705 | l = −11→11 |
| 26258 measured reflections |
Refinement
| Refinement on F2 | Secondary atom site location: difference Fourier map |
| Least-squares matrix: full | Hydrogen site location: inferred from neighbouring sites |
| R[F2 > 2σ(F2)] = 0.032 | H-atom parameters constrained |
| wR(F2) = 0.090 | w = 1/[σ2(Fo2) + (0.0489P)2 + 0.3826P] where P = (Fo2 + 2Fc2)/3 |
| S = 1.02 | (Δ/σ)max < 0.001 |
| 2738 reflections | Δρmax = 0.20 e Å−3 |
| 211 parameters | Δρmin = −0.11 e Å−3 |
| 0 restraints | Extinction correction: SHELXL97 (Sheldrick, 2008), Fc*=kFc[1+0.001xFc2λ3/sin(2θ)]-1/4 |
| Primary atom site location: structure-invariant direct methods | Extinction coefficient: 0.0068 (5) |
Special details
| Geometry. All esds (except the esd in the dihedral angle between two l.s. planes) are estimated using the full covariance matrix. The cell esds are taken into account individually in the estimation of esds in distances, angles and torsion angles; correlations between esds in cell parameters are only used when they are defined by crystal symmetry. An approximate (isotropic) treatment of cell esds is used for estimating esds involving l.s. planes. |
| Refinement. Refinement of F2 against ALL reflections. The weighted R-factor wR and goodness of fit S are based on F2, conventional R-factors R are based on F, with F set to zero for negative F2. The threshold expression of F2 > σ(F2) is used only for calculating R-factors(gt) etc. and is not relevant to the choice of reflections for refinement. R-factors based on F2 are statistically about twice as large as those based on F, and R- factors based on ALL data will be even larger. |
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2)
| x | y | z | Uiso*/Ueq | ||
| F1 | 0.23655 (12) | 0.05261 (4) | −0.47975 (8) | 0.0590 (3) | |
| O1 | 0.24071 (11) | 0.01533 (5) | 0.18556 (10) | 0.0455 (2) | |
| O2 | 0.27326 (11) | 0.19750 (4) | 0.05828 (9) | 0.0398 (2) | |
| O3 | 0.94318 (11) | 0.28084 (5) | 0.70653 (10) | 0.0440 (2) | |
| C1 | 0.49935 (15) | 0.22971 (6) | 0.26543 (13) | 0.0323 (3) | |
| H1 | 0.4904 | 0.2759 | 0.2362 | 0.039* | |
| C2 | 0.39672 (14) | 0.18266 (6) | 0.18062 (12) | 0.0320 (3) | |
| C3 | 0.41037 (14) | 0.11285 (6) | 0.22130 (12) | 0.0324 (3) | |
| C4 | 0.52148 (14) | 0.09433 (6) | 0.35119 (13) | 0.0336 (3) | |
| H4 | 0.5262 | 0.0483 | 0.3813 | 0.040* | |
| C5 | 0.62836 (14) | 0.14138 (6) | 0.44084 (12) | 0.0318 (3) | |
| C6 | 0.74594 (15) | 0.12300 (6) | 0.57403 (13) | 0.0365 (3) | |
| H6 | 0.7542 | 0.0771 | 0.6052 | 0.044* | |
| C7 | 0.84681 (15) | 0.17022 (6) | 0.65743 (13) | 0.0384 (3) | |
| H7 | 0.9247 | 0.1571 | 0.7461 | 0.046* | |
| C8 | 0.83613 (14) | 0.23878 (6) | 0.61265 (13) | 0.0349 (3) | |
| C9 | 0.72528 (14) | 0.25877 (6) | 0.48456 (13) | 0.0329 (3) | |
| H9 | 0.7199 | 0.3049 | 0.4551 | 0.039* | |
| C10 | 0.61869 (14) | 0.21041 (6) | 0.39610 (12) | 0.0306 (3) | |
| C11 | 0.30651 (14) | 0.05923 (6) | 0.12869 (13) | 0.0342 (3) | |
| C12 | 0.29002 (14) | 0.05826 (6) | −0.03341 (13) | 0.0325 (3) | |
| C13 | 0.41406 (15) | 0.08347 (6) | −0.09276 (14) | 0.0377 (3) | |
| H13 | 0.5108 | 0.1027 | −0.0294 | 0.045* | |
| C14 | 0.39778 (17) | 0.08070 (6) | −0.24310 (15) | 0.0428 (3) | |
| H14 | 0.4831 | 0.0969 | −0.2839 | 0.051* | |
| C15 | 0.25461 (17) | 0.05384 (6) | −0.33183 (13) | 0.0409 (3) | |
| C16 | 0.13017 (16) | 0.02778 (7) | −0.27807 (14) | 0.0420 (3) | |
| H16 | 0.0334 | 0.0090 | −0.3424 | 0.050* | |
| C17 | 0.14990 (15) | 0.02967 (6) | −0.12746 (14) | 0.0382 (3) | |
| H17 | 0.0663 | 0.0111 | −0.0874 | 0.046* | |
| C18 | 0.23490 (16) | 0.26755 (6) | 0.02792 (14) | 0.0402 (3) | |
| H18A | 0.1387 | 0.2715 | −0.0575 | 0.048* | |
| H18B | 0.3300 | 0.2904 | 0.0065 | 0.048* | |
| H18C | 0.2098 | 0.2887 | 0.1140 | 0.048* | |
| C19 | 0.92919 (19) | 0.35155 (7) | 0.67676 (16) | 0.0505 (4) | |
| H19A | 1.0101 | 0.3760 | 0.7539 | 0.061* | |
| H19B | 0.8173 | 0.3668 | 0.6752 | 0.061* | |
| H19C | 0.9506 | 0.3606 | 0.5808 | 0.061* |
Atomic displacement parameters (Å2)
| U11 | U22 | U33 | U12 | U13 | U23 | |
| F1 | 0.0822 (6) | 0.0620 (5) | 0.0351 (4) | −0.0059 (4) | 0.0196 (4) | −0.0056 (4) |
| O1 | 0.0478 (5) | 0.0476 (5) | 0.0395 (5) | −0.0152 (4) | 0.0088 (4) | 0.0038 (4) |
| O2 | 0.0411 (5) | 0.0351 (5) | 0.0353 (4) | 0.0021 (4) | −0.0042 (4) | −0.0002 (3) |
| O3 | 0.0421 (5) | 0.0433 (5) | 0.0387 (5) | −0.0027 (4) | −0.0039 (4) | −0.0046 (4) |
| C1 | 0.0351 (6) | 0.0297 (6) | 0.0313 (6) | 0.0017 (5) | 0.0071 (5) | 0.0016 (4) |
| C2 | 0.0311 (6) | 0.0352 (6) | 0.0284 (5) | 0.0017 (5) | 0.0056 (4) | 0.0004 (5) |
| C3 | 0.0319 (6) | 0.0340 (6) | 0.0315 (6) | −0.0009 (5) | 0.0085 (5) | −0.0006 (5) |
| C4 | 0.0359 (6) | 0.0310 (6) | 0.0346 (6) | 0.0009 (5) | 0.0104 (5) | 0.0021 (5) |
| C5 | 0.0314 (6) | 0.0334 (6) | 0.0310 (6) | 0.0023 (4) | 0.0090 (5) | 0.0009 (4) |
| C6 | 0.0382 (6) | 0.0353 (6) | 0.0348 (6) | 0.0044 (5) | 0.0073 (5) | 0.0043 (5) |
| C7 | 0.0368 (6) | 0.0433 (7) | 0.0311 (6) | 0.0059 (5) | 0.0015 (5) | 0.0031 (5) |
| C8 | 0.0314 (6) | 0.0400 (7) | 0.0319 (6) | 0.0005 (5) | 0.0058 (5) | −0.0045 (5) |
| C9 | 0.0335 (6) | 0.0321 (6) | 0.0325 (6) | 0.0012 (5) | 0.0078 (5) | −0.0004 (5) |
| C10 | 0.0298 (6) | 0.0333 (6) | 0.0295 (6) | 0.0017 (4) | 0.0092 (4) | −0.0001 (4) |
| C11 | 0.0316 (6) | 0.0320 (6) | 0.0383 (6) | −0.0011 (5) | 0.0081 (5) | 0.0018 (5) |
| C12 | 0.0336 (6) | 0.0273 (5) | 0.0364 (6) | −0.0008 (4) | 0.0085 (5) | −0.0014 (4) |
| C13 | 0.0374 (6) | 0.0334 (6) | 0.0427 (7) | −0.0064 (5) | 0.0111 (5) | −0.0051 (5) |
| C14 | 0.0520 (8) | 0.0349 (6) | 0.0479 (7) | −0.0058 (5) | 0.0247 (6) | −0.0038 (5) |
| C15 | 0.0562 (8) | 0.0339 (6) | 0.0336 (6) | 0.0034 (5) | 0.0136 (6) | −0.0033 (5) |
| C16 | 0.0415 (7) | 0.0423 (7) | 0.0389 (7) | −0.0020 (5) | 0.0048 (5) | −0.0063 (5) |
| C17 | 0.0356 (6) | 0.0387 (6) | 0.0403 (6) | −0.0061 (5) | 0.0103 (5) | −0.0026 (5) |
| C18 | 0.0400 (7) | 0.0370 (6) | 0.0376 (7) | 0.0032 (5) | −0.0007 (5) | 0.0047 (5) |
| C19 | 0.0513 (8) | 0.0409 (7) | 0.0509 (8) | −0.0057 (6) | −0.0018 (6) | −0.0071 (6) |
Geometric parameters (Å, °)
| F1—C15 | 1.3575 (14) | C8—C9 | 1.3715 (16) |
| O1—C11 | 1.2224 (15) | C9—C10 | 1.4186 (16) |
| O2—C2 | 1.3615 (14) | C9—H9 | 0.9500 |
| O2—C18 | 1.4327 (15) | C11—C12 | 1.4922 (16) |
| O3—C8 | 1.3632 (14) | C12—C17 | 1.3906 (17) |
| O3—C19 | 1.4238 (16) | C12—C13 | 1.3922 (17) |
| C1—C2 | 1.3709 (16) | C13—C14 | 1.3835 (18) |
| C1—C10 | 1.4174 (16) | C13—H13 | 0.9500 |
| C1—H1 | 0.9500 | C14—C15 | 1.3750 (19) |
| C2—C3 | 1.4279 (16) | C14—H14 | 0.9500 |
| C3—C4 | 1.3763 (16) | C15—C16 | 1.3708 (19) |
| C3—C11 | 1.4965 (16) | C16—C17 | 1.3808 (18) |
| C4—C5 | 1.4072 (16) | C16—H16 | 0.9500 |
| C4—H4 | 0.9500 | C17—H17 | 0.9500 |
| C5—C6 | 1.4219 (16) | C18—H18A | 0.9800 |
| C5—C10 | 1.4232 (16) | C18—H18B | 0.9800 |
| C6—C7 | 1.3608 (18) | C18—H18C | 0.9800 |
| C6—H6 | 0.9500 | C19—H19A | 0.9800 |
| C7—C8 | 1.4144 (17) | C19—H19B | 0.9800 |
| C7—H7 | 0.9500 | C19—H19C | 0.9800 |
| C2—O2—C18 | 117.22 (9) | O1—C11—C3 | 120.54 (11) |
| C8—O3—C19 | 117.70 (10) | C12—C11—C3 | 119.14 (10) |
| C2—C1—C10 | 120.89 (11) | C17—C12—C13 | 118.98 (11) |
| C2—C1—H1 | 119.6 | C17—C12—C11 | 119.42 (10) |
| C10—C1—H1 | 119.6 | C13—C12—C11 | 121.57 (11) |
| O2—C2—C1 | 124.50 (11) | C14—C13—C12 | 120.60 (12) |
| O2—C2—C3 | 115.09 (10) | C14—C13—H13 | 119.7 |
| C1—C2—C3 | 120.37 (10) | C12—C13—H13 | 119.7 |
| C4—C3—C2 | 118.84 (11) | C15—C14—C13 | 118.15 (12) |
| C4—C3—C11 | 118.84 (11) | C15—C14—H14 | 120.9 |
| C2—C3—C11 | 122.32 (10) | C13—C14—H14 | 120.9 |
| C3—C4—C5 | 122.10 (11) | F1—C15—C16 | 118.53 (12) |
| C3—C4—H4 | 118.9 | F1—C15—C14 | 118.26 (12) |
| C5—C4—H4 | 118.9 | C16—C15—C14 | 123.20 (12) |
| C4—C5—C6 | 122.91 (11) | C15—C16—C17 | 117.88 (12) |
| C4—C5—C10 | 118.57 (10) | C15—C16—H16 | 121.1 |
| C6—C5—C10 | 118.52 (11) | C17—C16—H16 | 121.1 |
| C7—C6—C5 | 120.93 (11) | C16—C17—C12 | 121.13 (11) |
| C7—C6—H6 | 119.5 | C16—C17—H17 | 119.4 |
| C5—C6—H6 | 119.5 | C12—C17—H17 | 119.4 |
| C6—C7—C8 | 120.28 (11) | O2—C18—H18A | 109.5 |
| C6—C7—H7 | 119.9 | O2—C18—H18B | 109.5 |
| C8—C7—H7 | 119.9 | H18A—C18—H18B | 109.5 |
| O3—C8—C9 | 124.86 (11) | O2—C18—H18C | 109.5 |
| O3—C8—C7 | 114.32 (10) | H18A—C18—H18C | 109.5 |
| C9—C8—C7 | 120.82 (11) | H18B—C18—H18C | 109.5 |
| C8—C9—C10 | 119.84 (11) | O3—C19—H19A | 109.5 |
| C8—C9—H9 | 120.1 | O3—C19—H19B | 109.5 |
| C10—C9—H9 | 120.1 | H19A—C19—H19B | 109.5 |
| C1—C10—C9 | 121.26 (11) | O3—C19—H19C | 109.5 |
| C1—C10—C5 | 119.10 (10) | H19A—C19—H19C | 109.5 |
| C9—C10—C5 | 119.61 (10) | H19B—C19—H19C | 109.5 |
| O1—C11—C12 | 120.27 (11) | ||
| C18—O2—C2—C1 | −8.47 (17) | C8—C9—C10—C5 | −0.31 (17) |
| C18—O2—C2—C3 | 169.07 (10) | C4—C5—C10—C1 | 1.65 (16) |
| C10—C1—C2—O2 | 176.29 (10) | C6—C5—C10—C1 | −178.51 (11) |
| C10—C1—C2—C3 | −1.12 (18) | C4—C5—C10—C9 | −179.93 (10) |
| O2—C2—C3—C4 | −173.95 (10) | C6—C5—C10—C9 | −0.08 (16) |
| C1—C2—C3—C4 | 3.70 (17) | C4—C3—C11—O1 | 43.90 (16) |
| O2—C2—C3—C11 | 5.21 (16) | C2—C3—C11—O1 | −135.26 (12) |
| C1—C2—C3—C11 | −177.14 (11) | C4—C3—C11—C12 | −133.52 (12) |
| C2—C3—C4—C5 | −3.63 (17) | C2—C3—C11—C12 | 47.32 (16) |
| C11—C3—C4—C5 | 177.18 (10) | O1—C11—C12—C17 | 28.70 (17) |
| C3—C4—C5—C6 | −178.86 (11) | C3—C11—C12—C17 | −153.88 (11) |
| C3—C4—C5—C10 | 0.98 (17) | O1—C11—C12—C13 | −149.31 (12) |
| C4—C5—C6—C7 | −179.94 (11) | C3—C11—C12—C13 | 28.11 (16) |
| C10—C5—C6—C7 | 0.22 (18) | C17—C12—C13—C14 | 0.55 (18) |
| C5—C6—C7—C8 | 0.02 (19) | C11—C12—C13—C14 | 178.57 (11) |
| C19—O3—C8—C9 | 5.92 (18) | C12—C13—C14—C15 | 1.42 (19) |
| C19—O3—C8—C7 | −174.07 (11) | C13—C14—C15—F1 | 178.38 (11) |
| C6—C7—C8—O3 | 179.56 (11) | C13—C14—C15—C16 | −2.2 (2) |
| C6—C7—C8—C9 | −0.42 (18) | F1—C15—C16—C17 | −179.69 (11) |
| O3—C8—C9—C10 | −179.42 (10) | C14—C15—C16—C17 | 0.9 (2) |
| C7—C8—C9—C10 | 0.56 (17) | C15—C16—C17—C12 | 1.21 (19) |
| C2—C1—C10—C9 | −179.95 (10) | C13—C12—C17—C16 | −1.91 (18) |
| C2—C1—C10—C5 | −1.55 (17) | C11—C12—C17—C16 | −179.97 (11) |
| C8—C9—C10—C1 | 178.08 (11) |
Hydrogen-bond geometry (Å, °)
| Cg1 is the centroid of the C1–C5/C10 ring. |
| D—H···A | D—H | H···A | D···A | D—H···A |
| C18—H18B···Cg1i | 0.98 | 2.85 | 3.7479 (14) | 152 |
| C17—H17···O1ii | 0.95 | 2.55 | 3.2930 (16) | 136 |
| C18—H18A···O3iii | 0.98 | 2.39 | 3.3603 (16) | 169 |
Symmetry codes: (i) x, −y+1/2, z−1/2; (ii) −x, −y, −z; (iii) x−1, y, z−1.
Footnotes
Supplementary data and figures for this paper are available from the IUCr electronic archives (Reference: BT5198).
References
- Burla, M. C., Caliandro, R., Camalli, M., Carrozzini, B., Cascarano, G. L., De Caro, L., Giacovazzo, C., Polidori, G. & Spagna, R. (2005). J. Appl. Cryst.38, 381–388.
- Burnett, M. N. & Johnson, C. K. (1996). ORTEPIII Report ORNL-6895. Oak Ridge National Laboratory. Tennessee, USA.
- Higashi, T. (1999). NUMABS Rigaku Corporation, Tokyo, Japan.
- Hijikata, D., Nakaema, K., Watanabe, S., Okamoto, A. & Yonezawa, N. (2010). Acta Cryst. E66, o554. [DOI] [PMC free article] [PubMed]
- Mitsui, R., Nakaema, K., Noguchi, K., Okamoto, A. & Yonezawa, N. (2008). Acta Cryst. E64, o1278. [DOI] [PMC free article] [PubMed]
- Nakaema, K., Okamoto, A., Imaizumi, M., Noguchi, K. & Yonezawa, N. (2008). Acta Cryst. E64, o612. [DOI] [PMC free article] [PubMed]
- Nakaema, K., Okamoto, A., Noguchi, K. & Yonezawa, N. (2007). Acta Cryst. E63, o4120.
- Okamoto, A. & Yonezawa, N. (2009). Chem. Lett 38, 914–915.
- Rigaku (1998). PROCESS-AUTO Rigaku Corporation, Tokyo, Japan.
- Rigaku/MSC (2004). CrystalStructure Rigaku/MSC, The Woodlands, Texas, USA.
- Sheldrick, G. M. (2008). Acta Cryst. A64, 112–122. [DOI] [PubMed]
- Watanabe, S., Nakaema, K., Muto, T., Okamoto, A. & Yonezawa, N. (2010a). Acta Cryst. E66, o403. [DOI] [PMC free article] [PubMed]
- Watanabe, S., Nakaema, K., Nishijima, T., Okamoto, A. & Yonezawa, N. (2010b). Acta Cryst. E66, o615. [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Crystal structure: contains datablocks I, global. DOI: 10.1107/S1600536810006859/bt5198sup1.cif
Structure factors: contains datablocks I. DOI: 10.1107/S1600536810006859/bt5198Isup2.hkl
Additional supplementary materials: crystallographic information; 3D view; checkCIF report