Abstract
The title compound, C20H17NO5, was prepared by the reaction of 3-acetyl-2-oxo-2H-chromen-7-yl acetate with benzyloxyamine. The molecule adopts an E configuration with respect to the C=N double bond. The dihedral angles between the coumarin ring system, the phenyl ring and the C=N—O—C plane of the oxime unit are 35.83 (6), 35.8 (2) and 69.99 (15)°, respectively. In the crystal, a two-dimensional supramolecular network is assembled through weak intermolecular C—H⋯O hydrogen-bonding interactions.
Related literature
For the pharmacological applications of Schiff base compounds derived from coumarins, see: Jolanta et al. (2006 ▶); Kontogiorgis et al. (2006 ▶); Kontogiorgis & Hadjipavlou-Litina (2004 ▶); Nofal et al. (2000 ▶). For their use as dyes, fluorescent agents and as chemosensors, see: Kachkovski et al. (2004 ▶); Turki et al. (2006 ▶); Li et al. (2009 ▶).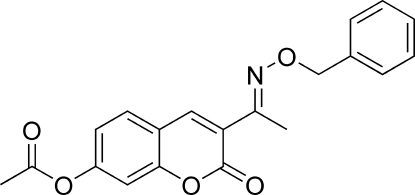
Experimental
Crystal data
C20H17NO5
M r = 351.35
Triclinic,

a = 6.3901 (9) Å
b = 11.2413 (16) Å
c = 12.9298 (18) Å
α = 102.643 (2)°
β = 96.923 (2)°
γ = 101.860 (2)°
V = 873.5 (2) Å3
Z = 2
Mo Kα radiation
μ = 0.10 mm−1
T = 298 K
0.25 × 0.20 × 0.18 mm
Data collection
Bruker APEXII area-detector diffractometer
Absorption correction: multi-scan (SADABS; Bruker, 2004 ▶) T min = 0.977, T max = 0.983
5311 measured reflections
3792 independent reflections
2335 reflections with I > 2σ(I)
R int = 0.025
Refinement
R[F 2 > 2σ(F 2)] = 0.051
wR(F 2) = 0.166
S = 1.09
3792 reflections
238 parameters
H-atom parameters constrained
Δρmax = 0.33 e Å−3
Δρmin = −0.23 e Å−3
Data collection: APEX2 (Bruker, 2004 ▶); cell refinement: SAINT (Bruker, 2004 ▶); data reduction: SAINT; program(s) used to solve structure: SHELXS97 (Sheldrick, 2008 ▶); program(s) used to refine structure: SHELXL97 (Sheldrick, 2008 ▶); molecular graphics: XP in SHELXTL (Sheldrick, 2008 ▶); software used to prepare material for publication: SHELXL97.
Supplementary Material
Crystal structure: contains datablocks global, I. DOI: 10.1107/S1600536810003454/sj2717sup1.cif
Structure factors: contains datablocks I. DOI: 10.1107/S1600536810003454/sj2717Isup2.hkl
Additional supplementary materials: crystallographic information; 3D view; checkCIF report
Table 1. Hydrogen-bond geometry (Å, °).
| D—H⋯A | D—H | H⋯A | D⋯A | D—H⋯A |
|---|---|---|---|---|
| C6—H6⋯O5i | 0.93 | 2.51 | 3.393 (3) | 159 |
| C18—H18⋯O5ii | 0.93 | 2.67 | 3.552 (3) | 160 |
| C8—H8⋯O1iii | 0.93 | 2.65 | 3.397 (2) | 138 |
Symmetry codes: (i)  ; (ii)
; (ii)  ; (iii)
; (iii)  .
.
Acknowledgments
We are grateful to the Science and Technology Plan Project of Guangdong Province (No. 2008B010600008) for financial support.
supplementary crystallographic information
Comment
Coumarin-derived Schiff bases have attracted much attention due to their potential pharmacological applications as antitumor (Jolanta et al., 2006), anti-inflammatory (Kontogiorgis & Hadjipavlou-Litina 2004), antibacterial (Nofal et al., 2000) and antifungal agents (Kontogiorgis et al., 2006). In addition, they can also be used as dyes (Kachkovski et al., 2004), fluorescent agents (Turki et al., 2006) and as colorimetric chemosensors (Li et al., 2009). In our study of bioactive compounds, a series of coumarin-derived Schiff bases have been synthesized. Herein, we report the crystal structure of the title compound, Fig. 1, obtained by the reaction of 3-acetyl-2-oxo-2H-chromen-7-yl acetate with benzyloxy-amine.
The title molecule is composed of a coumarin core with acetoxyl and benzyloxyiminoethyl substituents. The dihedral angles between the coumarin ring system, the phenyl ring and the C=N—O—C plane of the oxime unit are 35.83 (6)°, 35.8 (2) ° and 69.99 (15) °, respectively. The benzyloxyiminoethyl and coumarin systems are located on opposite sides of the C=N double bond plane, therefor the molecule presents an E configuration. In the crystal structure, intermolecular C6—H6···O5 hydrogen bonding interactions (Table 1) assemble a two-dimensional supramolecular layer as shown in Fig. 2. Additional weak C8—H8···O1 and C18—H18···O5 contacts are also observed. The overall crystal packing is shown in Fig. 3.
Experimental
A solution of benzyloxy-amine hydrochloride (2 mmol) in ethanol (10 ml) was added to a solution of 3-acetyl-2-oxo-2H-chromen-7-yl acetate (1 mmol) in ethanol (10 ml) at room temperature, the solution pH was then maintained at a value of 7 by the addition of sodium hydroxide. The reaction mixture was refluxed for 5 h at 353 K (monitored by TLC). After completion of the reaction, the reaction solution was purified by column chromatography (ethyl acetate: petroleum ether = 2:3). The eluate was evaporated to give the title compound (286 mg, yield: 81.5%). Single crystals suitable for X-ray analysis were obtained by recrystallization from DMSO, m.p. 426 K. ESI-MS (m/z): 352 (M+1); Analysis calculated for C20H17NO5: C 68.37%, H 4.88%, N 3.99%; Found: C 68.54%, H 4.32%, N 3.78%.
Refinement
All H-atoms were positioned geometrically and refined using a riding model with d(C—H) = 0.93 Å, Uiso=1.2Ueq (C) for aromatic 0.97 Å, Uiso = 1.2Ueq (C) for CH2 and 0.96 Å, Uiso = 1.5Ueq (C) for CH3 atoms.
Figures
Fig. 1.
The molecular structure of the title compound showing the atom numbering scheme. Displacement ellipsoids are drawn at the 50% probability level.
Fig. 2.
Part of the crystal structure of the title compound showing weak C—H···O hydrogen bonds as dashed lines.
Fig. 3.
Crystal packing in the title compound.
Crystal data
| C20H17NO5 | Z = 2 |
| Mr = 351.35 | F(000) = 368 |
| Triclinic, P1 | Dx = 1.336 Mg m−3 |
| Hall symbol: -P 1 | Mo Kα radiation, λ = 0.71073 Å |
| a = 6.3901 (9) Å | Cell parameters from 1332 reflections |
| b = 11.2413 (16) Å | θ = 2.8–24.5° |
| c = 12.9298 (18) Å | µ = 0.10 mm−1 |
| α = 102.643 (2)° | T = 298 K |
| β = 96.923 (2)° | Block, colorless |
| γ = 101.860 (2)° | 0.25 × 0.20 × 0.18 mm |
| V = 873.5 (2) Å3 |
Data collection
| Bruker APEXII area-detector diffractometer | 3792 independent reflections |
| Radiation source: fine-focus sealed tube | 2335 reflections with I > 2σ(I) |
| graphite | Rint = 0.025 |
| φ and ω scans | θmax = 27.3°, θmin = 2.2° |
| Absorption correction: multi-scan (SADABS; Bruker, 2004) | h = −8→6 |
| Tmin = 0.977, Tmax = 0.983 | k = −12→14 |
| 5311 measured reflections | l = −16→16 |
Refinement
| Refinement on F2 | Secondary atom site location: difference Fourier map |
| Least-squares matrix: full | Hydrogen site location: inferred from neighbouring sites |
| R[F2 > 2σ(F2)] = 0.051 | H-atom parameters constrained |
| wR(F2) = 0.166 | w = 1/[σ2(Fo2) + (0.076P)2 + 0.0611P] where P = (Fo2 + 2Fc2)/3 |
| S = 1.09 | (Δ/σ)max < 0.001 |
| 3792 reflections | Δρmax = 0.33 e Å−3 |
| 238 parameters | Δρmin = −0.23 e Å−3 |
| 0 restraints | Extinction correction: SHELXL97 (Sheldrick, 2008), Fc*=kFc[1+0.001xFc2λ3/sin(2θ)]-1/4 |
| Primary atom site location: structure-invariant direct methods | Extinction coefficient: 0.010 (4) |
Special details
| Experimental. FT—IR (KBr): 1763, 1717, 1615, 1426, 1365, 1200, 1030 cm-1; 1H NMR δ (400 Hz, DMSO, TMS): 8.08 (s, 1H), 7.83 (d, J = 8.4 Hz, 1H), 7.36–7.28 (m, 5H) 7.26 (d, J = 2.0 Hz, 1H), 7.14 (dd, J = 2.0, 8.4 Hz, 1H), 5.17 (s, 2H), 2.27 (s, 3H), 2.11 (s, 3H). |
| Geometry. All e.s.d.'s (except the e.s.d. in the dihedral angle between two l.s. planes) are estimated using the full covariance matrix. The cell e.s.d.'s are taken into account individually in the estimation of e.s.d.'s in distances, angles and torsion angles; correlations between e.s.d.'s in cell parameters are only used when they are defined by crystal symmetry. An approximate (isotropic) treatment of cell e.s.d.'s is used for estimating e.s.d.'s involving l.s. planes. |
| Refinement. Refinement of F2 against ALL reflections. The weighted R-factor wR and goodness of fit S are based on F2, conventional R-factors R are based on F, with F set to zero for negative F2. The threshold expression of F2 > σ(F2) is used only for calculating R-factors(gt) etc. and is not relevant to the choice of reflections for refinement. R-factors based on F2 are statistically about twice as large as those based on F, and R- factors based on ALL data will be even larger. |
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2)
| x | y | z | Uiso*/Ueq | ||
| O1 | −0.33761 (19) | 0.48915 (12) | 0.62680 (10) | 0.0488 (4) | |
| C2 | −0.2517 (3) | 0.56481 (18) | 0.72817 (15) | 0.0499 (5) | |
| O2 | −0.3700 (2) | 0.62098 (15) | 0.77276 (12) | 0.0710 (5) | |
| C3 | −0.0274 (3) | 0.56859 (17) | 0.77165 (15) | 0.0472 (5) | |
| C4 | 0.0814 (3) | 0.49479 (17) | 0.71383 (15) | 0.0478 (5) | |
| H4 | 0.2234 | 0.4970 | 0.7421 | 0.057* | |
| C5 | 0.0893 (3) | 0.33485 (18) | 0.54693 (16) | 0.0504 (5) | |
| H5 | 0.2301 | 0.3320 | 0.5725 | 0.060* | |
| C6 | −0.0133 (3) | 0.26229 (17) | 0.44758 (16) | 0.0503 (5) | |
| H6 | 0.0568 | 0.2097 | 0.4062 | 0.060* | |
| C7 | −0.2229 (3) | 0.26729 (17) | 0.40849 (15) | 0.0448 (4) | |
| O4 | −0.3080 (2) | 0.19654 (12) | 0.30368 (10) | 0.0542 (4) | |
| C20 | −0.5180 (4) | 0.12835 (19) | 0.27716 (18) | 0.0591 (6) | |
| O5 | −0.6377 (3) | 0.12328 (15) | 0.34060 (14) | 0.0791 (5) | |
| C21 | −0.5679 (4) | 0.0639 (2) | 0.1608 (2) | 0.0821 (8) | |
| H21C | −0.7167 | 0.0173 | 0.1424 | 0.123* | |
| H21A | −0.4749 | 0.0076 | 0.1455 | 0.123* | |
| H21B | −0.5445 | 0.1251 | 0.1194 | 0.123* | |
| C8 | −0.3309 (3) | 0.34359 (17) | 0.46779 (14) | 0.0450 (4) | |
| H8 | −0.4706 | 0.3471 | 0.4410 | 0.054* | |
| C9 | −0.2257 (3) | 0.41476 (16) | 0.56822 (14) | 0.0415 (4) | |
| C10 | −0.0149 (3) | 0.41373 (16) | 0.61081 (14) | 0.0427 (4) | |
| C11 | 0.0738 (3) | 0.65509 (19) | 0.87771 (16) | 0.0539 (5) | |
| C12 | 0.0328 (4) | 0.7832 (2) | 0.90913 (19) | 0.0729 (7) | |
| H12C | 0.1607 | 0.8404 | 0.9536 | 0.109* | |
| H12B | −0.0857 | 0.7804 | 0.9484 | 0.109* | |
| H12A | −0.0030 | 0.8111 | 0.8456 | 0.109* | |
| N1 | 0.2041 (3) | 0.61121 (16) | 0.93402 (13) | 0.0644 (5) | |
| O3 | 0.3083 (3) | 0.70165 (14) | 1.03000 (11) | 0.0744 (5) | |
| C13 | 0.4579 (5) | 0.6475 (3) | 1.0870 (2) | 0.0940 (9) | |
| H13B | 0.5690 | 0.6295 | 1.0452 | 0.113* | |
| H13A | 0.3816 | 0.5700 | 1.1006 | 0.113* | |
| C14 | 0.5568 (4) | 0.74251 (19) | 1.19015 (17) | 0.0609 (6) | |
| C15 | 0.4399 (4) | 0.7731 (2) | 1.26843 (19) | 0.0677 (6) | |
| H15 | 0.2937 | 0.7326 | 1.2573 | 0.081* | |
| C16 | 0.5268 (5) | 0.8596 (2) | 1.3618 (2) | 0.0812 (8) | |
| H16 | 0.4415 | 0.8772 | 1.4139 | 0.097* | |
| C17 | 0.7365 (5) | 0.9205 (2) | 1.3795 (2) | 0.0820 (8) | |
| H17 | 0.7944 | 0.9811 | 1.4437 | 0.098* | |
| C18 | 0.8661 (4) | 0.8957 (3) | 1.3058 (2) | 0.0842 (8) | |
| H18 | 1.0115 | 0.9384 | 1.3194 | 0.101* | |
| C19 | 0.7778 (4) | 0.8050 (3) | 1.2092 (2) | 0.0817 (7) | |
| H19 | 0.8644 | 0.7862 | 1.1579 | 0.098* |
Atomic displacement parameters (Å2)
| U11 | U22 | U33 | U12 | U13 | U23 | |
| O1 | 0.0384 (7) | 0.0550 (8) | 0.0484 (8) | 0.0149 (6) | 0.0013 (6) | 0.0035 (6) |
| C2 | 0.0446 (11) | 0.0558 (11) | 0.0473 (11) | 0.0116 (9) | 0.0061 (9) | 0.0102 (9) |
| O2 | 0.0508 (9) | 0.0869 (11) | 0.0646 (10) | 0.0236 (8) | 0.0070 (7) | −0.0079 (8) |
| C3 | 0.0426 (10) | 0.0514 (11) | 0.0448 (10) | 0.0066 (8) | 0.0044 (8) | 0.0123 (8) |
| C4 | 0.0359 (10) | 0.0543 (11) | 0.0510 (11) | 0.0074 (8) | 0.0004 (8) | 0.0153 (9) |
| C5 | 0.0354 (10) | 0.0531 (11) | 0.0627 (12) | 0.0126 (8) | 0.0064 (9) | 0.0138 (9) |
| C6 | 0.0430 (11) | 0.0463 (10) | 0.0618 (12) | 0.0125 (8) | 0.0130 (9) | 0.0103 (9) |
| C7 | 0.0427 (10) | 0.0419 (9) | 0.0480 (11) | 0.0059 (8) | 0.0076 (8) | 0.0118 (8) |
| O4 | 0.0472 (8) | 0.0580 (8) | 0.0494 (8) | 0.0078 (6) | 0.0067 (6) | 0.0023 (6) |
| C20 | 0.0505 (13) | 0.0480 (11) | 0.0688 (14) | 0.0118 (9) | 0.0011 (11) | −0.0009 (10) |
| O5 | 0.0550 (10) | 0.0705 (10) | 0.0932 (12) | −0.0003 (8) | 0.0205 (9) | −0.0079 (9) |
| C21 | 0.0753 (16) | 0.0762 (16) | 0.0731 (16) | 0.0155 (13) | −0.0072 (13) | −0.0128 (13) |
| C8 | 0.0374 (10) | 0.0488 (10) | 0.0481 (11) | 0.0125 (8) | 0.0029 (8) | 0.0109 (8) |
| C9 | 0.0377 (10) | 0.0428 (9) | 0.0464 (10) | 0.0126 (7) | 0.0081 (8) | 0.0128 (8) |
| C10 | 0.0364 (10) | 0.0432 (9) | 0.0472 (10) | 0.0058 (7) | 0.0054 (8) | 0.0134 (8) |
| C11 | 0.0495 (12) | 0.0610 (12) | 0.0456 (11) | 0.0079 (9) | 0.0042 (9) | 0.0091 (9) |
| C12 | 0.0735 (16) | 0.0703 (15) | 0.0628 (14) | 0.0169 (12) | 0.0027 (12) | −0.0030 (11) |
| N1 | 0.0720 (12) | 0.0609 (11) | 0.0453 (10) | 0.0069 (9) | −0.0095 (9) | 0.0015 (8) |
| O3 | 0.0890 (12) | 0.0662 (10) | 0.0523 (9) | 0.0159 (8) | −0.0181 (8) | 0.0014 (7) |
| C13 | 0.126 (2) | 0.0769 (17) | 0.0661 (16) | 0.0340 (16) | −0.0280 (15) | 0.0053 (13) |
| C14 | 0.0722 (15) | 0.0542 (12) | 0.0487 (12) | 0.0134 (11) | −0.0092 (11) | 0.0092 (9) |
| C15 | 0.0686 (15) | 0.0641 (14) | 0.0688 (15) | 0.0136 (11) | 0.0064 (12) | 0.0186 (12) |
| C16 | 0.109 (2) | 0.0721 (16) | 0.0676 (16) | 0.0310 (16) | 0.0149 (15) | 0.0201 (13) |
| C17 | 0.099 (2) | 0.0624 (15) | 0.0700 (17) | 0.0142 (15) | −0.0147 (16) | 0.0070 (13) |
| C18 | 0.0612 (16) | 0.0781 (17) | 0.098 (2) | −0.0013 (13) | −0.0165 (15) | 0.0231 (15) |
| C19 | 0.0776 (18) | 0.0987 (19) | 0.0770 (17) | 0.0287 (15) | 0.0165 (14) | 0.0307 (15) |
Geometric parameters (Å, °)
| O1—C9 | 1.372 (2) | C9—C10 | 1.396 (2) |
| O1—C2 | 1.375 (2) | C11—N1 | 1.283 (3) |
| C2—O2 | 1.202 (2) | C11—C12 | 1.494 (3) |
| C2—C3 | 1.464 (3) | C12—H12C | 0.9600 |
| C3—C4 | 1.350 (3) | C12—H12B | 0.9600 |
| C3—C11 | 1.479 (3) | C12—H12A | 0.9600 |
| C4—C10 | 1.423 (2) | N1—O3 | 1.410 (2) |
| C4—H4 | 0.9300 | O3—C13 | 1.447 (3) |
| C5—C6 | 1.365 (3) | C13—C14 | 1.489 (3) |
| C5—C10 | 1.403 (3) | C13—H13B | 0.9700 |
| C5—H5 | 0.9300 | C13—H13A | 0.9700 |
| C6—C7 | 1.390 (3) | C14—C15 | 1.355 (3) |
| C6—H6 | 0.9300 | C14—C19 | 1.408 (3) |
| C7—C8 | 1.372 (3) | C15—C16 | 1.347 (3) |
| C7—O4 | 1.392 (2) | C15—H15 | 0.9300 |
| O4—C20 | 1.363 (2) | C16—C17 | 1.340 (4) |
| C20—O5 | 1.190 (2) | C16—H16 | 0.9300 |
| C20—C21 | 1.483 (3) | C17—C18 | 1.356 (4) |
| C21—H21C | 0.9600 | C17—H17 | 0.9300 |
| C21—H21A | 0.9600 | C18—C19 | 1.398 (4) |
| C21—H21B | 0.9600 | C18—H18 | 0.9300 |
| C8—C9 | 1.375 (2) | C19—H19 | 0.9300 |
| C8—H8 | 0.9300 | ||
| C9—O1—C2 | 122.88 (14) | C5—C10—C4 | 124.61 (17) |
| O2—C2—O1 | 116.29 (17) | N1—C11—C3 | 113.44 (18) |
| O2—C2—C3 | 126.37 (18) | N1—C11—C12 | 125.35 (19) |
| O1—C2—C3 | 117.34 (17) | C3—C11—C12 | 121.10 (18) |
| C4—C3—C2 | 119.42 (17) | C11—C12—H12C | 109.5 |
| C4—C3—C11 | 122.05 (18) | C11—C12—H12B | 109.5 |
| C2—C3—C11 | 118.53 (17) | H12C—C12—H12B | 109.5 |
| C3—C4—C10 | 121.90 (17) | C11—C12—H12A | 109.5 |
| C3—C4—H4 | 119.0 | H12C—C12—H12A | 109.5 |
| C10—C4—H4 | 119.0 | H12B—C12—H12A | 109.5 |
| C6—C5—C10 | 120.80 (17) | C11—N1—O3 | 110.70 (17) |
| C6—C5—H5 | 119.6 | N1—O3—C13 | 107.54 (16) |
| C10—C5—H5 | 119.6 | O3—C13—C14 | 106.20 (19) |
| C5—C6—C7 | 119.80 (18) | O3—C13—H13B | 110.5 |
| C5—C6—H6 | 120.1 | C14—C13—H13B | 110.5 |
| C7—C6—H6 | 120.1 | O3—C13—H13A | 110.5 |
| C8—C7—C6 | 121.47 (17) | C14—C13—H13A | 110.5 |
| C8—C7—O4 | 122.92 (16) | H13B—C13—H13A | 108.7 |
| C6—C7—O4 | 115.48 (16) | C15—C14—C19 | 117.5 (2) |
| C20—O4—C7 | 120.76 (15) | C15—C14—C13 | 122.1 (2) |
| O5—C20—O4 | 123.2 (2) | C19—C14—C13 | 120.4 (2) |
| O5—C20—C21 | 127.0 (2) | C16—C15—C14 | 122.7 (2) |
| O4—C20—C21 | 109.8 (2) | C16—C15—H15 | 118.7 |
| C20—C21—H21C | 109.5 | C14—C15—H15 | 118.7 |
| C20—C21—H21A | 109.5 | C17—C16—C15 | 119.9 (3) |
| H21C—C21—H21A | 109.5 | C17—C16—H16 | 120.0 |
| C20—C21—H21B | 109.5 | C15—C16—H16 | 120.0 |
| H21C—C21—H21B | 109.5 | C16—C17—C18 | 121.5 (3) |
| H21A—C21—H21B | 109.5 | C16—C17—H17 | 119.3 |
| C7—C8—C9 | 117.84 (17) | C18—C17—H17 | 119.3 |
| C7—C8—H8 | 121.1 | C17—C18—C19 | 119.0 (3) |
| C9—C8—H8 | 121.1 | C17—C18—H18 | 120.5 |
| O1—C9—C8 | 116.92 (15) | C19—C18—H18 | 120.5 |
| O1—C9—C10 | 120.20 (16) | C18—C19—C14 | 119.4 (2) |
| C8—C9—C10 | 122.89 (16) | C18—C19—H19 | 120.3 |
| C9—C10—C5 | 117.19 (17) | C14—C19—H19 | 120.3 |
| C9—C10—C4 | 118.17 (16) | ||
| C9—O1—C2—O2 | −176.76 (17) | C8—C9—C10—C4 | 177.57 (16) |
| C9—O1—C2—C3 | 2.8 (3) | C6—C5—C10—C9 | −0.2 (3) |
| O2—C2—C3—C4 | 176.5 (2) | C6—C5—C10—C4 | −178.29 (17) |
| O1—C2—C3—C4 | −3.0 (3) | C3—C4—C10—C9 | 2.0 (3) |
| O2—C2—C3—C11 | −4.3 (3) | C3—C4—C10—C5 | −179.96 (18) |
| O1—C2—C3—C11 | 176.28 (16) | C4—C3—C11—N1 | −36.2 (3) |
| C2—C3—C4—C10 | 0.6 (3) | C2—C3—C11—N1 | 144.62 (19) |
| C11—C3—C4—C10 | −178.60 (16) | C4—C3—C11—C12 | 140.3 (2) |
| C10—C5—C6—C7 | 0.7 (3) | C2—C3—C11—C12 | −38.9 (3) |
| C5—C6—C7—C8 | −0.3 (3) | C3—C11—N1—O3 | 175.21 (16) |
| C5—C6—C7—O4 | 175.76 (16) | C12—C11—N1—O3 | −1.1 (3) |
| C8—C7—O4—C20 | −45.7 (2) | C11—N1—O3—C13 | −177.84 (19) |
| C6—C7—O4—C20 | 138.31 (18) | N1—O3—C13—C14 | −177.2 (2) |
| C7—O4—C20—O5 | −1.4 (3) | O3—C13—C14—C15 | 67.9 (3) |
| C7—O4—C20—C21 | 179.04 (17) | O3—C13—C14—C19 | −111.6 (3) |
| C6—C7—C8—C9 | −0.6 (3) | C19—C14—C15—C16 | 0.1 (3) |
| O4—C7—C8—C9 | −176.27 (15) | C13—C14—C15—C16 | −179.4 (2) |
| C2—O1—C9—C8 | 179.99 (16) | C14—C15—C16—C17 | 0.8 (4) |
| C2—O1—C9—C10 | −0.1 (3) | C15—C16—C17—C18 | −1.0 (4) |
| C7—C8—C9—O1 | −179.15 (15) | C16—C17—C18—C19 | 0.4 (4) |
| C7—C8—C9—C10 | 1.0 (3) | C17—C18—C19—C14 | 0.6 (4) |
| O1—C9—C10—C5 | 179.54 (15) | C15—C14—C19—C18 | −0.8 (3) |
| C8—C9—C10—C5 | −0.6 (3) | C13—C14—C19—C18 | 178.8 (2) |
| O1—C9—C10—C4 | −2.3 (2) |
Hydrogen-bond geometry (Å, °)
| D—H···A | D—H | H···A | D···A | D—H···A |
| C6—H6···O5i | 0.93 | 2.51 | 3.393 (3) | 159 |
| C18—H18···O5ii | 0.93 | 2.67 | 3.552 (3) | 160 |
| C8—H8···O1iii | 0.93 | 2.65 | 3.397 (2) | 138 |
Symmetry codes: (i) x+1, y, z; (ii) x+2, y+1, z+1; (iii) −x−1, −y+1, −z+1.
Footnotes
Supplementary data and figures for this paper are available from the IUCr electronic archives (Reference: SJ2717).
References
- Bruker (2004). APEX2, SAINT and SADABS Bruker AXS Inc., Madison, Wisconsin, USA.
- Jolanta, N. M., Ewa, N. & Julita, G. (2006). Eur. J. Med. Chem.41, 1301–1309.
- Kachkovski, O. D., Tolmachev, O. I., Kobryn, L. O., Bila, E. E. & Ganushchak, M. I. (2004). Dyes Pigments, 63, 203–211.
- Kontogiorgis, C. A. & Hadjipavlou-Litina, D. J. (2004). Bioorg. Med. Chem. Lett.14, 611–614. [DOI] [PubMed]
- Kontogiorgis, C. A., Savvoglou, K. & Hadjipavlou-Litina, D. J. (2006). J. Enzyme Inhib. Med. Chem.21, 21–29. [DOI] [PubMed]
- Li, H. Y., Gao, S. & Xi, Z. (2009). Inorg. Chem. Commun.12, 300–303.
- Nofal, Z. M., El-Zahar, M. I. & Abd El-Karim, S. S. (2000). Molecules, 5, 99–113.
- Sheldrick, G. M. (2008). Acta Cryst. A64, 112–122. [DOI] [PubMed]
- Turki, H., Abid, S., El Gharbi, R. & Fery-Forgues, S. (2006). C. R. Chim.9, 1252–1259.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Crystal structure: contains datablocks global, I. DOI: 10.1107/S1600536810003454/sj2717sup1.cif
Structure factors: contains datablocks I. DOI: 10.1107/S1600536810003454/sj2717Isup2.hkl
Additional supplementary materials: crystallographic information; 3D view; checkCIF report





