Abstract
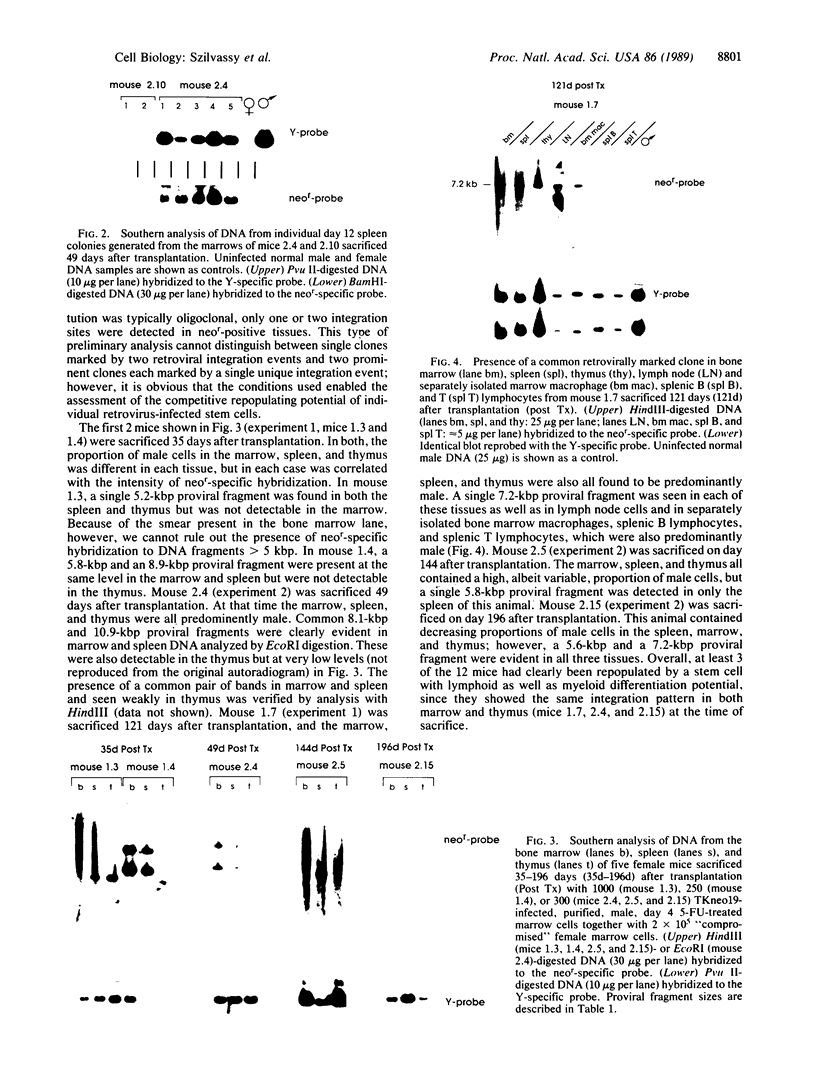
Despite recent advances in marrow stem cell purification, controversy about the nature and heterogeneity of cells with the potential for long-term repopulation of lymphoid and myeloid tissues remains. Essential to the resolution of these questions is the use of strategies to track the progeny produced in vivo from individual hemopoietic stem cells in purified populations. We have used a procedure for obtaining highly enriched populations of stem cells with competitive repopulating ability from male mice (pretreated with 5-fluorouracil), and in this paper we present the results of studies in which small numbers (150-2000) of these cells were exposed to supernatant containing a helper-free recombinant retrovirus carrying the neomycin-resistance gene and then were transplanted together with 2 x 10(5) "compromised" female marrow cells into irradiated female recipients. Male cells--i.e., progeny of purified stem cells--were found in one or more of the tissues examined (peripheral blood, marrow, spleen, and thymus) in 28 of 28 mice evaluated at various times between 35 and 196 days after transplantation. In 20 of these mice (71%), the neomycin-resistance gene was also detected, although not always at a level that correlated with the proportion of male cells. Analysis of spleen colonies (day 12) generated in secondary recipients confirmed that viral integration was confined to male repopulating cells. In three mice direct evidence of a common clone in both lymphoid and myeloid tissues was also obtained. These results show the feasibility of retrovirus-mediated gene transfer to highly purified populations of lympho-myelopoietic stem cells with long-term (6 months) repopulating potential by using a supernatant infection protocol. This approach should facilitate further analysis of hemopoietic stem cell control in vivo and find future applications in the evolving use of bone marrow transplantation for hemopoietic rescue and gene therapy.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abramson S., Miller R. G., Phillips R. A. The identification in adult bone marrow of pluripotent and restricted stem cells of the myeloid and lymphoid systems. J Exp Med. 1977 Jun 1;145(6):1567–1579. doi: 10.1084/jem.145.6.1567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barker J. E., Braun J., McFarland-Starr E. C. Erythrocyte replacement precedes leukocyte replacement during repopulation of W/Wv mice with limiting dilutions of +/+ donor marrow cells. Proc Natl Acad Sci U S A. 1988 Oct;85(19):7332–7335. doi: 10.1073/pnas.85.19.7332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan P. Y., Takei F. Molecular cloning and characterization of a novel murine T cell surface antigen, YE1/48. J Immunol. 1989 Mar 1;142(5):1727–1736. [PubMed] [Google Scholar]
- Cone R. D., Mulligan R. C. High-efficiency gene transfer into mammalian cells: generation of helper-free recombinant retrovirus with broad mammalian host range. Proc Natl Acad Sci U S A. 1984 Oct;81(20):6349–6353. doi: 10.1073/pnas.81.20.6349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dick J. E., Magli M. C., Huszar D., Phillips R. A., Bernstein A. Introduction of a selectable gene into primitive stem cells capable of long-term reconstitution of the hemopoietic system of W/Wv mice. Cell. 1985 Aug;42(1):71–79. doi: 10.1016/s0092-8674(85)80102-1. [DOI] [PubMed] [Google Scholar]
- Gregory C. J., Eaves A. C. Human marrow cells capable of erythropoietic differentiation in vitro: definition of three erythroid colony responses. Blood. 1977 Jun;49(6):855–864. [PubMed] [Google Scholar]
- Harrison D. E., Astle C. M., Delaittre J. A. Loss of proliferative capacity in immunohemopoietic stem cells caused by serial transplantation rather than aging. J Exp Med. 1978 May 1;147(5):1526–1531. doi: 10.1084/jem.147.5.1526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones R. J., Celano P., Sharkis S. J., Sensenbrenner L. L. Two phases of engraftment established by serial bone marrow transplantation in mice. Blood. 1989 Feb;73(2):397–401. [PubMed] [Google Scholar]
- Lamar E. E., Palmer E. Y-encoded, species-specific DNA in mice: evidence that the Y chromosome exists in two polymorphic forms in inbred strains. Cell. 1984 May;37(1):171–177. doi: 10.1016/0092-8674(84)90312-x. [DOI] [PubMed] [Google Scholar]
- Lemischka I. R., Raulet D. H., Mulligan R. C. Developmental potential and dynamic behavior of hematopoietic stem cells. Cell. 1986 Jun 20;45(6):917–927. doi: 10.1016/0092-8674(86)90566-0. [DOI] [PubMed] [Google Scholar]
- Mann R., Mulligan R. C., Baltimore D. Construction of a retrovirus packaging mutant and its use to produce helper-free defective retrovirus. Cell. 1983 May;33(1):153–159. doi: 10.1016/0092-8674(83)90344-6. [DOI] [PubMed] [Google Scholar]
- Miller A. D., Trauber D. R., Buttimore C. Factors involved in production of helper virus-free retrovirus vectors. Somat Cell Mol Genet. 1986 Mar;12(2):175–183. doi: 10.1007/BF01560664. [DOI] [PubMed] [Google Scholar]
- Nash R., Storb R., Neiman P. Polyclonal reconstitution of human marrow after allogeneic bone marrow transplantation. Blood. 1988 Dec;72(6):2031–2037. [PubMed] [Google Scholar]
- Ploemacher R. E., Brons R. H. Separation of CFU-S from primitive cells responsible for reconstitution of the bone marrow hemopoietic stem cell compartment following irradiation: evidence for a pre-CFU-S cell. Exp Hematol. 1989 Mar;17(3):263–266. [PubMed] [Google Scholar]
- Snodgrass R., Keller G. Clonal fluctuation within the haematopoietic system of mice reconstituted with retrovirus-infected stem cells. EMBO J. 1987 Dec 20;6(13):3955–3960. doi: 10.1002/j.1460-2075.1987.tb02737.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spangrude G. J., Heimfeld S., Weissman I. L. Purification and characterization of mouse hematopoietic stem cells. Science. 1988 Jul 1;241(4861):58–62. doi: 10.1126/science.2898810. [DOI] [PubMed] [Google Scholar]
- Szilvassy S. J., Lansdorp P. M., Humphries R. K., Eaves A. C., Eaves C. J. Isolation in a single step of a highly enriched murine hematopoietic stem cell population with competitive long-term repopulating ability. Blood. 1989 Aug 15;74(3):930–939. [PubMed] [Google Scholar]
- TILL J. E., McCULLOCH E. A. A direct measurement of the radiation sensitivity of normal mouse bone marrow cells. Radiat Res. 1961 Feb;14:213–222. [PubMed] [Google Scholar]
- Turhan A. G., Humphries R. K., Phillips G. L., Eaves A. C., Eaves C. J. Clonal hematopoiesis demonstrated by X-linked DNA polymorphisms after allogeneic bone marrow transplantation. N Engl J Med. 1989 Jun 22;320(25):1655–1661. doi: 10.1056/NEJM198906223202504. [DOI] [PubMed] [Google Scholar]
- Vennström B., Kahn P., Adkins B., Enrietto P., Hayman M. J., Graf T., Luciw P. Transformation of mammalian fibroblasts and macrophages in vitro by a murine retrovirus encoding an avian v-myc oncogene. EMBO J. 1984 Dec 20;3(13):3223–3229. doi: 10.1002/j.1460-2075.1984.tb02282.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner E. F., Vanek M., Vennström B. Transfer of genes into embryonal carcinoma cells by retrovirus infection: efficient expression from an internal promoter. EMBO J. 1985 Mar;4(3):663–666. doi: 10.1002/j.1460-2075.1985.tb03680.x. [DOI] [PMC free article] [PubMed] [Google Scholar]








